Synergistic Photoantimicrobial Chemotherapy of Methylene Blue-Encapsulated Chitosan on Biofilm-Contaminated Titanium
Abstract
:1. Introduction
2. Results
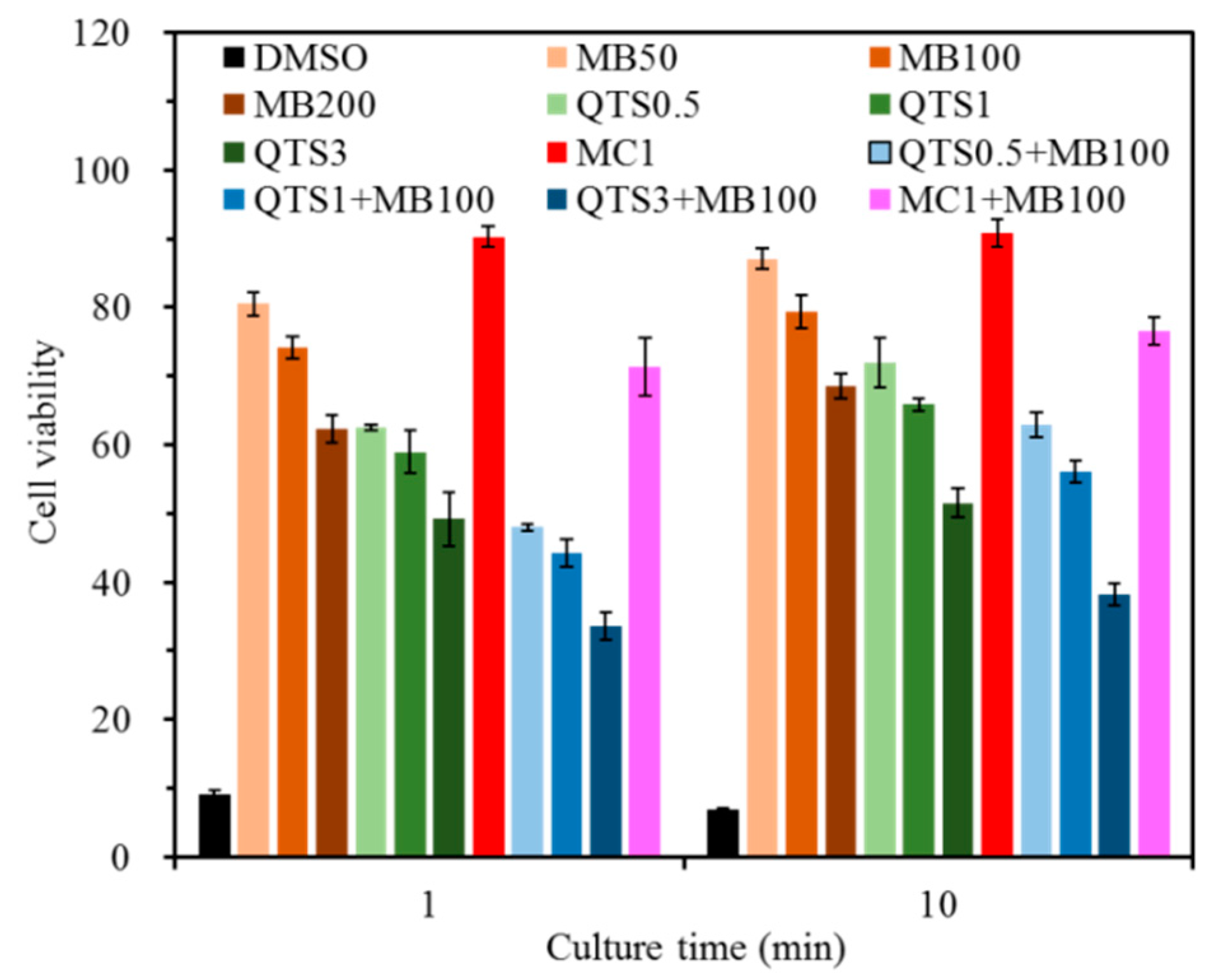
2.1. Cytotoxicity
2.2. Viscosity
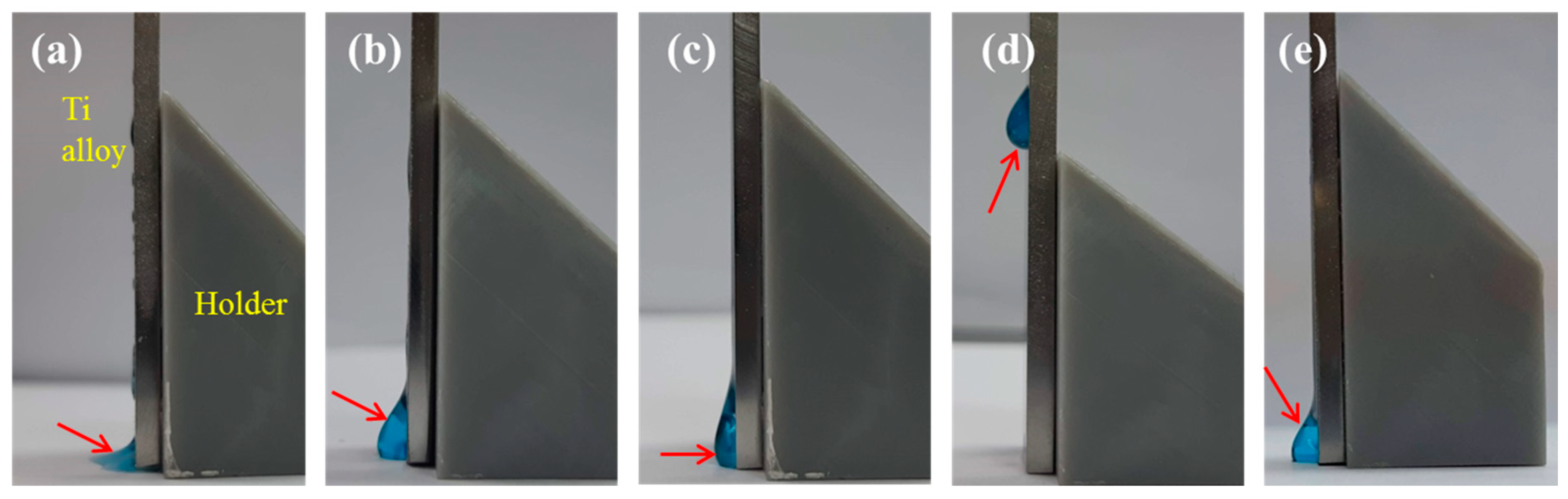
2.3. Retention Efficacy
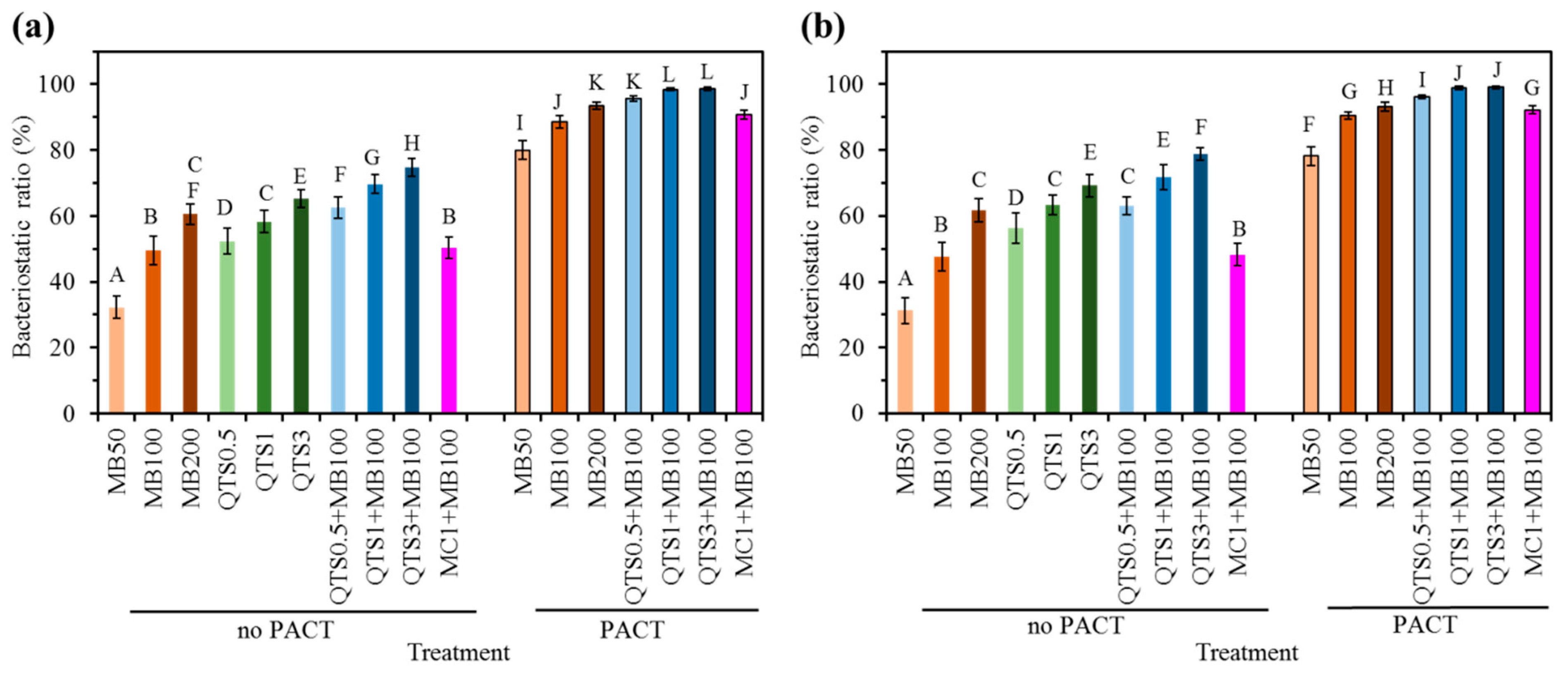
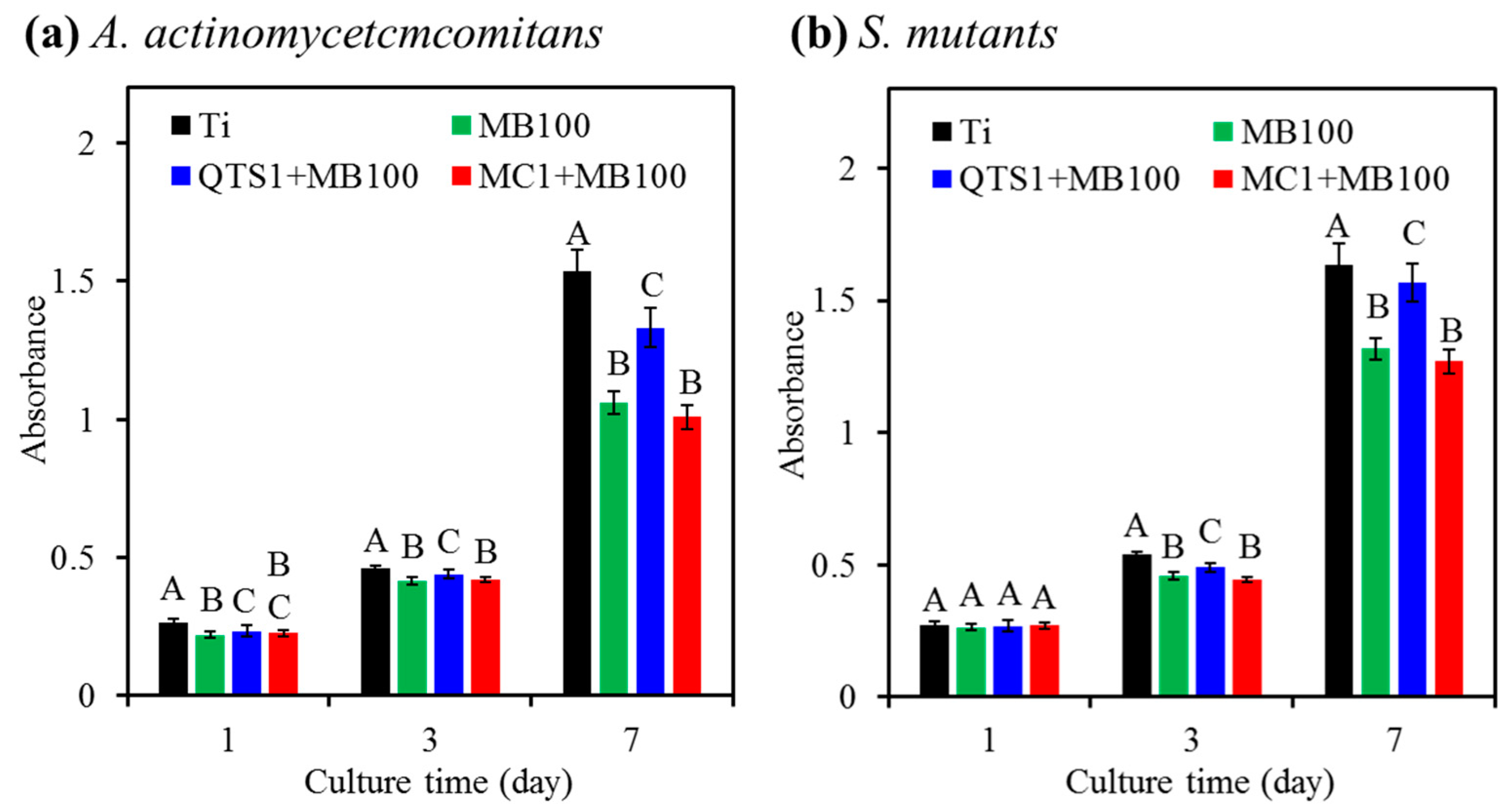
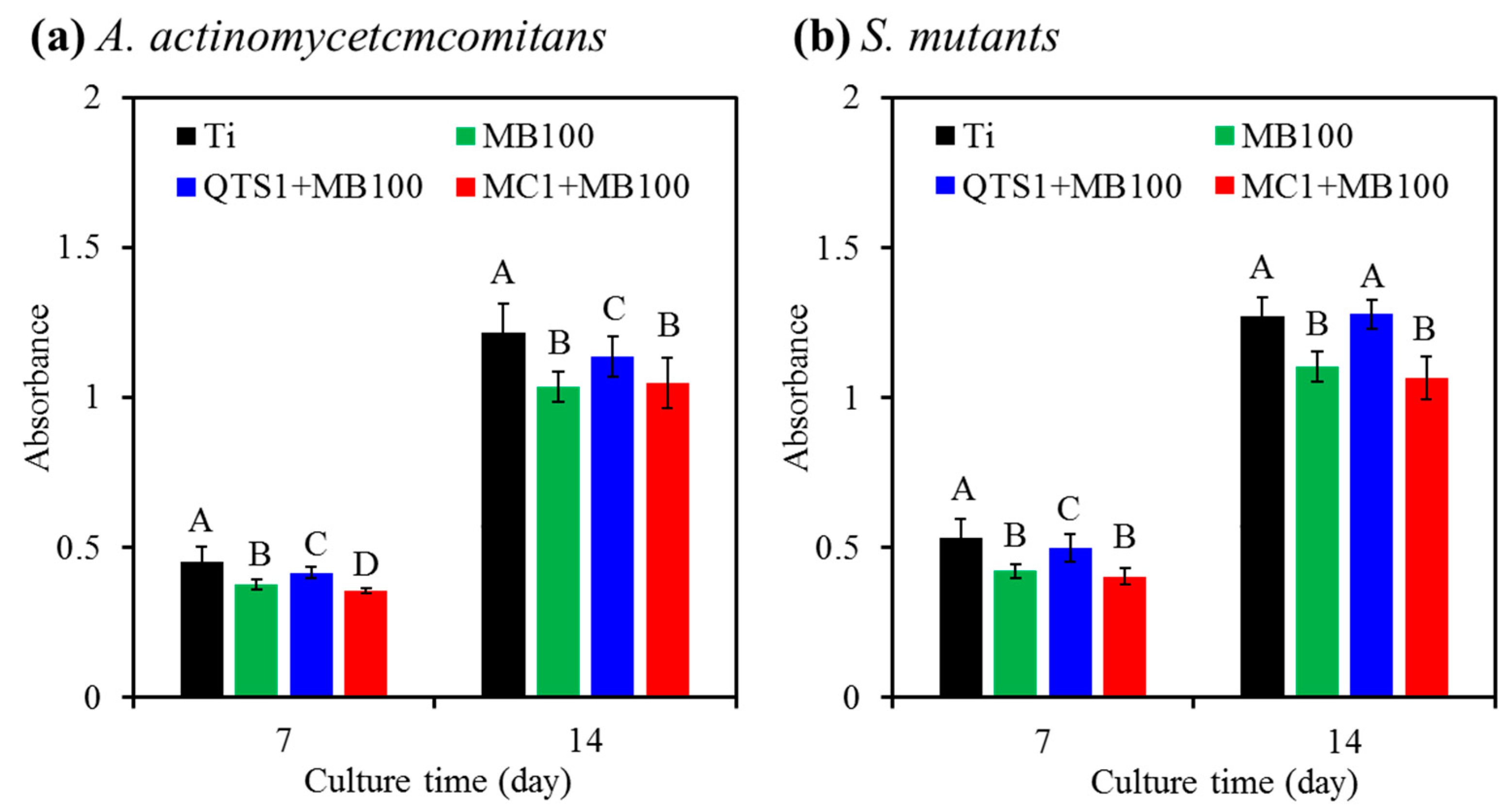
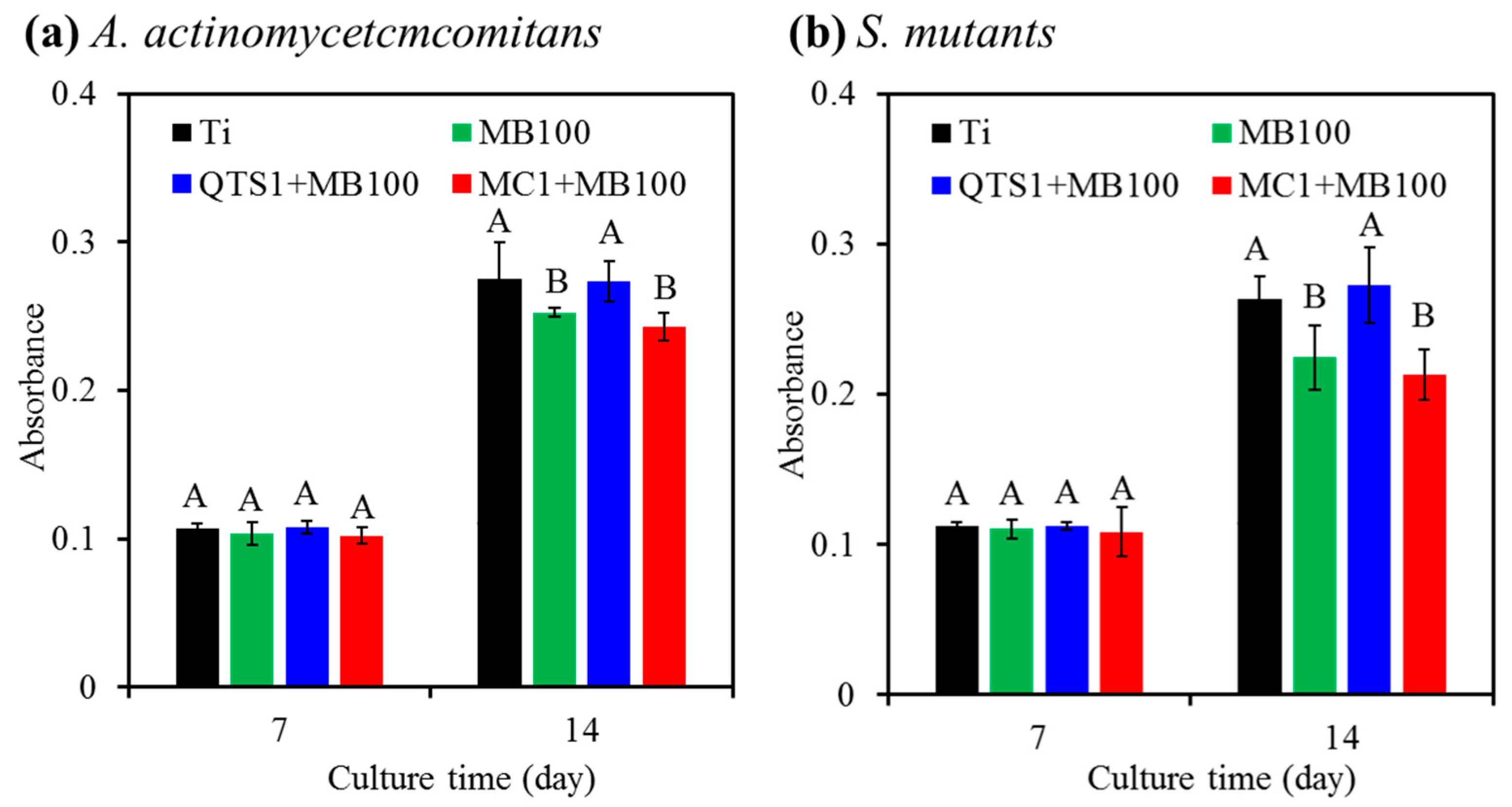
2.4. Bacteriostatic Ratio
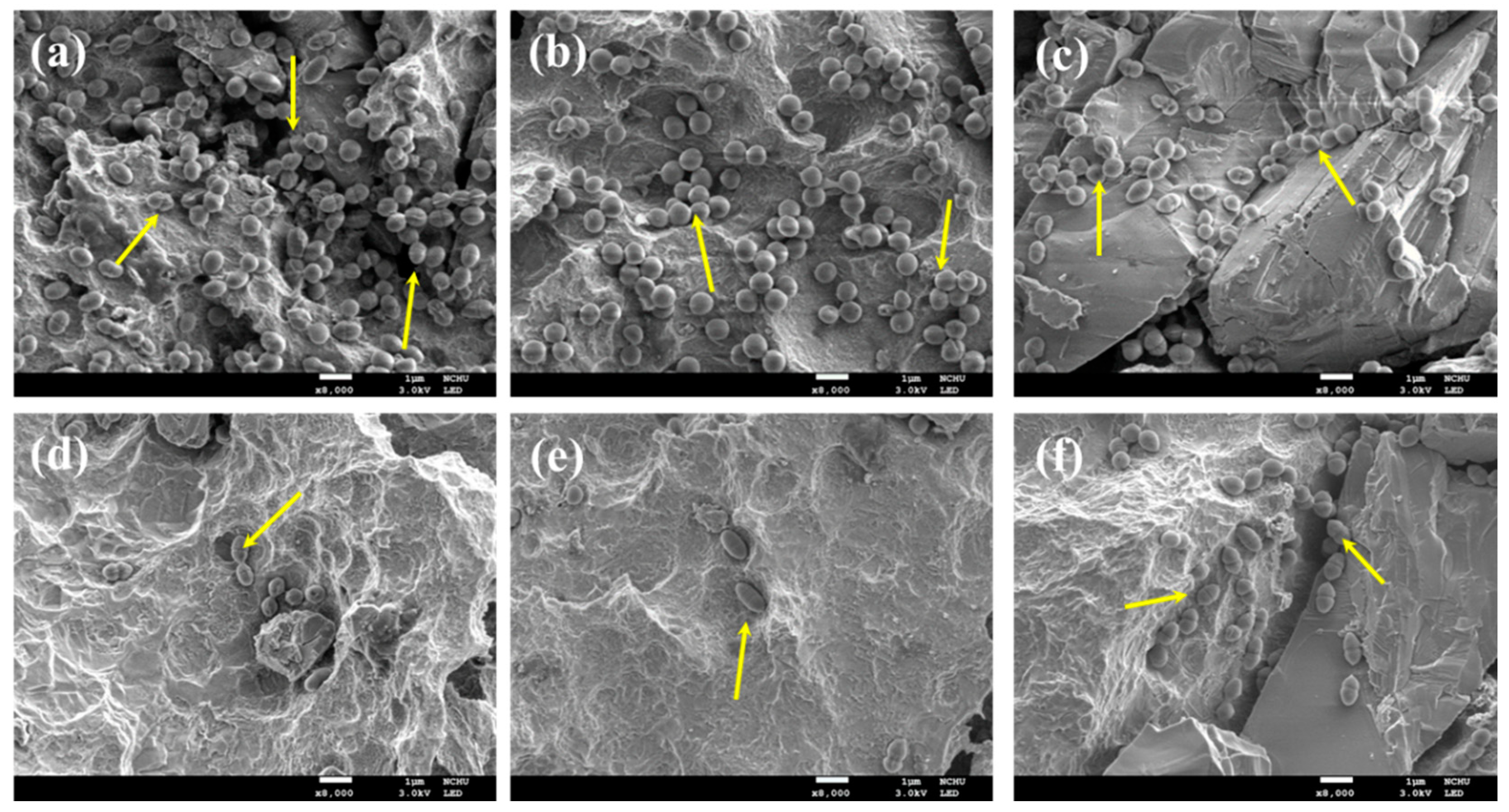
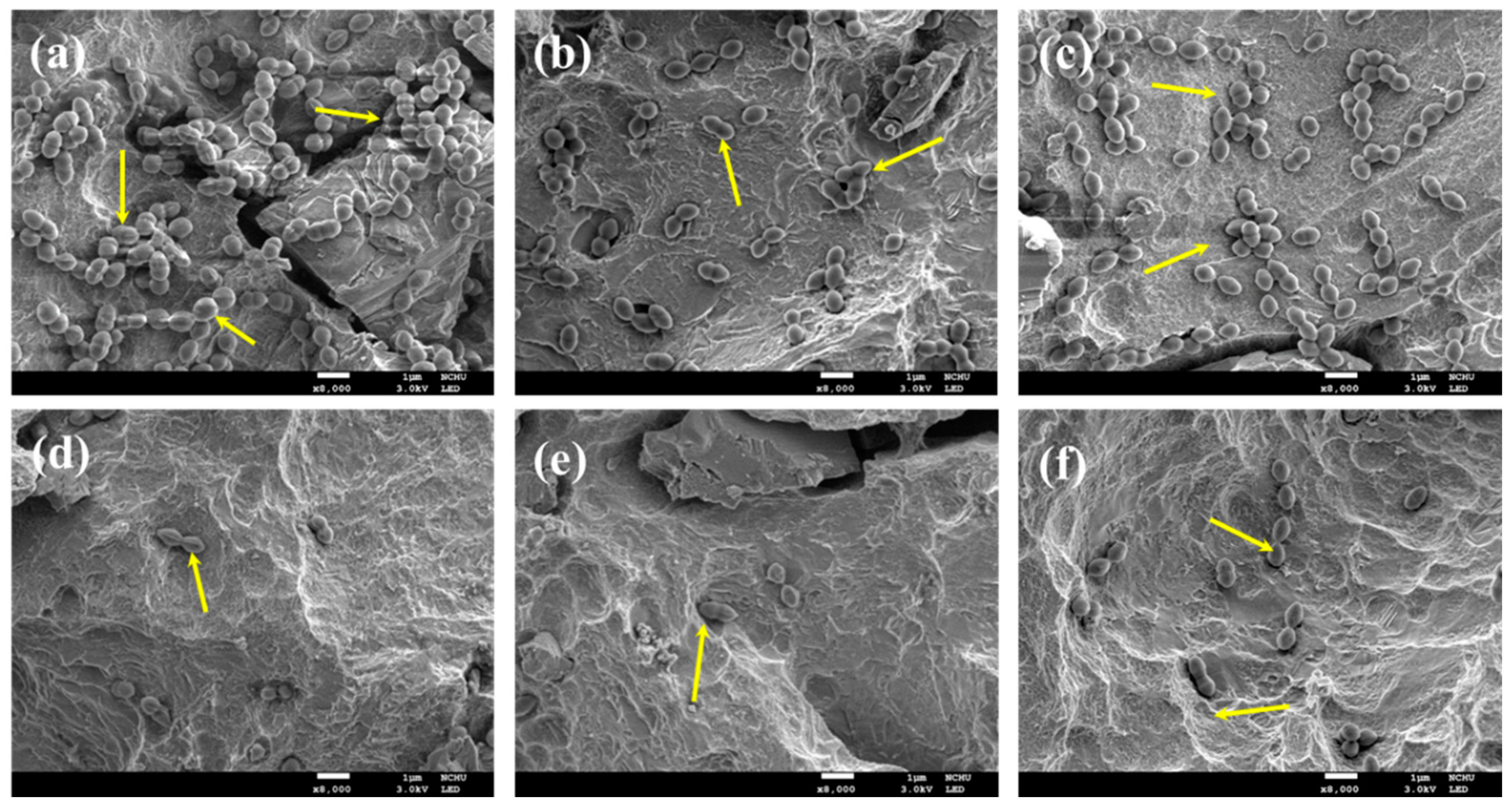
2.5. Bacterial Colonies
2.6. Residual LPS Amount
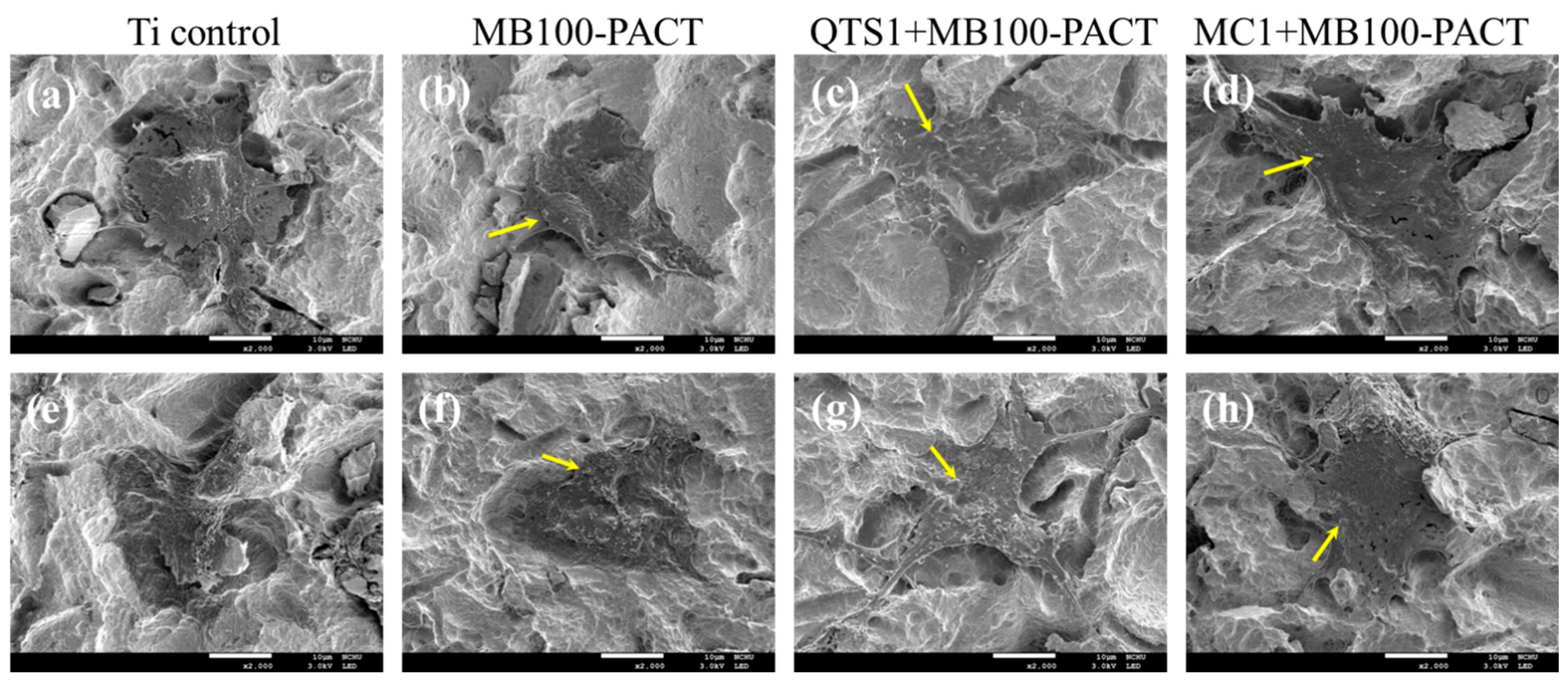
2.7. Cell Morphology
2.8. Cell Proliferation
2.9. Alkaline Phosphatase (ALP) Activity
2.10. Mineralization
3. Discussion
4. Materials and Methods
4.1. Preparation of QTS
4.2. Preparation of Solution
4.3. L929 Cytotoxicity
4.4. Viscosity
4.5. Preparation of Titanium Alloy
4.6. Solution Flowability
4.7. Bacteria Seeding
4.8. Photodynamic Treatment
4.9. Antibacterial Efficacy
4.9.1. Bacterial Counting
4.9.2. Bacterial Colony Observation
4.9.3. LPS Detection
4.10. MG63 Cell Culture
4.10.1. Cell Attachment
4.10.2. Cell Proliferation
4.10.3. Alkaline Phosphatase Activity
4.10.4. Calcium Deposit Quantification
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charalampakis, G.; Ramberg, P.; Dahlén, G.; Berglundh, T.; Abrahamsson, I. Effect of Cleansing of Biofilm Formed on Titanium Discs. Clin. Oral Impl. Res. 2015, 26, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, J.; Bradley, J.W.; McKay, K.; McBride, F.; Donaghy, D.; Raval, R.; D’Sa, R.A. Linker-Free Covalent Immobilization of Nisin Using Atmospheric Pressure Plasma Induced Grafting. J. Mater. Chem. B 2017, 5, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chan, Y.J.; Kung, K.C.; Lee, T.M. Comparison of Osseointegration on Various Implant Surfaces after Bacterial Contamination and Cleaning: A Rabbit Study. Int. J. Oral Maxillofac. Impl. 2014, 29, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.C.; Chen, C.J.; Chen, C.C.; Ding, S.J. Enhancing Osteoblast Functions on Biofilm-Contaminated Titanium Alloy by Concentration-Dependent Use of Methylene Blue-Mediated Antimicrobial Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2019, 27, 7–18. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine-to Fourteen-Year Follow-Up of Implant Treatment. Part II: Presence of Peri-Implant Lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, F.J.J.; Ofec, R.; Schulten, E.A.J.M.; ten Bruggenkate, C.M. 10-Year Survival Rate and the Incidence of Peri-Implant Disease of 374 Titanium Dental Implants with a SLA Surface: A Prospective Cohort Study in 177 fully and Partially Edentulous Patients. Clin. Oral Impl. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Fransson, C.; Lekholm, U.; Jemt, T.; Berglundh, T. Prevalence of Subjects with Progressive Bone Loss at Implants. Clin. Oral Impl. Res. 2005, 16, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Lang, N.P. The Diagnosis and Treatment of Peri-Implantitis. Periodontology 2000 1998, 17, 63–76. [Google Scholar] [CrossRef]
- Pier-Francesco, A.; Adams, R.J.; Waters, M.G.J.; Williams, D.W. Titanium Surface Modification and its Effect on the Adherence of Porphyromonas Gingivalis: An in Vitro Study. Clin. Oral Impl. Res. 2006, 17, 633–637. [Google Scholar] [CrossRef]
- Chen, C.J.; Ding, S.J.; Chen, C.C. Effects of Surface Conditions of Titanium Dental Implants on Bacterial Adhesion. Photomed. Laser Surg. 2016, 34, 379–388. [Google Scholar] [CrossRef]
- Kao, H.; Chen, C.C.; Huang, Y.R.; Chu, Y.H.; Csík, A.; Ding, S.J. Metal Ion-Dependent Tailored Antibacterial Activity and Biological Properties of Polydopamine-Coated Titanium Implants. Surf. Coat. Technol. 2019, 378, 124998. [Google Scholar] [CrossRef]
- Slots, J.; Ting, M. Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Human Periodontal Disease: Occurrence and Treatment. Periodontology 2000 1999, 20, 82–121. [Google Scholar] [CrossRef]
- Bonito, A.J.; Lux, L.; Lohs, K.N. Impact of Local Adjuncts to Scaling and Root Planing the Periodontal Disease Therapy: A Systematic Review. J. Periodontol. 2005, 76, 1227–1236. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, J.M. Photodynamic Therapy in the Control of Oral Biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Aoki, A.; Romanos, G.; Schwarz, F.; Miron, R.J.; Cosgarea, R. Is Photodynamic Therapy an Effective Treatment for Periodontal and Peri-Implant Infections? Dent. Clin. N. Am. 2015, 59, 831–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial Photodynamic Therapy—a Promising Treatment for Prosthetic Joint Infections. Lasers Med. Sci. 2018, 33, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.C.E.; Lacava, Z.G.M.; Kuckelhaus, S.; Silva, L.P.; Neto, L.F.M.; Sauro, E.E.; Tedesco, A.C. Evaluation of the Use of Low Level Laser and Photosensitizer Drugs in Healing. Lasers Surg. Med. 2004, 34, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Baham, P.; Herron, C.; Street, C.; Darveau, R. Antimicrobial Photodynamic Therapy May Promote Periodontal Healing Through Multiple Mechanisms. J. Periodontol. 2009, 80, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.Y.; Koshy, G.; Romanos, G.; Ishikawa, I.; Izumi, Y. Application of Antimicrobial Photodynamic Therapy in Periodontal and Peri-Implant Diseases. Periodontol. 2000 2009, 51, 109–140. [Google Scholar] [CrossRef]
- Wainwright, M. Photoantimicrobials and PACT: What’s in an Abbreviation? Photochem Photobiol Sci. 2019, 18, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and Molecular Actions of Methylene Blue in the Nervous System. Med. Res. Rev. 2009, 31, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khdair, A.; Gerard, B.; Handa, H.; Mao, G.; Shekhar, M.P.V.; Panyam, J. Surfactant-Polymer Nanoparticles Enhance the Effectiveness of Anticancer Photodynamic Therapy. Mol. Pharm. 2008, 5, 795–807. [Google Scholar] [CrossRef]
- Wilson, M. Lethal Photosensitisation of Oral Bacteria and its Potential Application in the Photodynamic Therapy of Oral Infections. Photochem. Photobiol. Sci. 2004, 3, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Bürgers, R.; Witecy, C.; Hahnel, S.; Gosau, M. The Effect of Various Topical Peri-Implantitis Antiseptics on Staphylococcus Epidermidis, Candida Albicans, and Streptococcus Sanguinis. Arch. Oral Biol. 2012, 57, 940–947. [Google Scholar] [CrossRef]
- Longer, M.A.; Robinson, J.R. Fundamental Aspects of Bioadhesion. Pharm. Int. 1986, 7, 114–117. [Google Scholar]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Donnelly, R.F. Mucoadhesive Drug Delivery Systems. J. Pharma. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar]
- Ahuja, A.; Ali, J.; Khar, K.R. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1997, 23, 489–515. [Google Scholar] [CrossRef]
- López-Jiménez, L.; Fusté, E.; Martínez-Garriga, B.; Arnabat-Domínguez, J.; Vinuesa, T.; Viñas, M. Effects of Photodynamic Therapy on Enterococcus Faecalis Biofilms. Lasers Med. Sci. 2015, 30, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.K. GAG-Augmented Polysaccharide Hydrogel: A Novel Biocompatible and Biodegradable Material to Support Chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Ding, S.J. Biodegradation Behavior of Chitosan/Calcium Phosphate Composites. J. Non-Crystal. Solids 2007, 353, 2367–2373. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Y.; Zhao, L. Chitosan Nanoparticles Loaded with Aspirin and 5-Fluororacil Enable Synergistic Antitumour Activity Through the Modulation of NF-κB/COX-2 Signaling Pathway. IET Nanobiotechnol. 2020, 14, 479–484. [Google Scholar] [CrossRef]
- Uzair, B.; Akhtar, N.; Sajjad, S.; Bano, A.; Fasim, F.; Zafar, N.; Leghari, S.A.K. Targeting Microbial Biofilms: By Arctium Lappa l. Synthesised Biocompatible CeO2-NPs Encapsulated in Nano-Chitosan. IET Nanobiotechnol. 2020, 14, 217–223. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, C.C.; Wu, I.T.; Ding, S.J. Enhanced Antibacterial Activity of Calcium Silicate-Based Hybrid Cements for Bone Repair. Mater. Sci. Eng. C 2020, 110, 110727. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its Antimicrobial Potential—A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chien, H.F.; Lin, M.H.; Chen, C.P.; Shen, M.; Chen, C.T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int. J. Mol. Sci. 2018, 19, 2598. [Google Scholar]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.F.; Chiu, R.J. Investigation of Charge Effects on Drug Release Behavior for Ionic Thermosensitive Hydrogels. Mater. Sci. Eng. C 2002, 20, 161–166. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a Conjugate between Rose Bengal and Chitosan for Targeted Antibiofilm and Tissue Stabilization Effects as a Potential Treatment of Infected Dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.S.; Lee, H.K.; Chae, H.S. Synergistic in Vitro Photodynamic Antimicrobial Activity of Methylene Blue and Chitosan Against Helicobacter Pylori 26695. Photodiagn. Photodyn. Ther. 2014, 11, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Nardi, G.M.; Libotte, F.; Sabatini, S.; Palaia, G.; Grassi, F.R. The Antimicrobial Photodynamic Therapy in the Treatment of Peri-Implantitis. Int. J. Dent. 2016, 7692387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright new Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akilov, O.E.; O’Riordan, K.; Kosaka, S.; Hasan, T. Photodynamic Therapy against Intracellular Pathogens: Problems and Potentials. Med. Lasers Appl. 2006, 21, 251–260. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.M.; Bäumler, W. The Role of Singlet Oxygen and Oxygen Concentration in Photodynamic Inactivation of Bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalling, S.S.; Akintoye, S.O.; Nicoll, S.B. Development of Photocrosslinked Methylcellulose Hydrogels for Soft Tissue Reconstruction. Acta Biomater. 2009, 5, 1911–1918. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Dai, T.; Kharkwal, G.B.; Huang, Y.Y.; Huang, L.; Bil de Arce, V.J.; Tegos, G.P.; Hamblin, M.R. Drug Discovery of Antimicrobial Photosensitizers Using Animal Models. Curr. Pharm. Des. 2011, 17, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.K.; Ding, S.J. Dual-Functional Bone Implants with Antibacterial Ability and Osteogenic Activity. J. Mater. Chem. B 2017, 5, 1943–1953. [Google Scholar] [CrossRef]
- Tate, M.C.; Shear, D.A.; Hoffman, S.W.; Stein, D.G.; LaPlaca, M.C. Biocompatibility of Methylcellulose-Based Constructs Designed for Intracerebral Gelation Following Experimental Traumatic Brain Injury. Biomaterials 2001, 22, 1113–1123. [Google Scholar] [CrossRef]
- Rolim, J.P.; de-Melo, M.A.; Guedes, S.F.; Albuquerque-Filho, F.B.; de Souza, J.R.; Nogueira, N.A.; Zanin, I.C.; Rodrigues, L.K. The Antimicrobial Activity of Photodynamic Therapy Against Streptococcus Mutans Using Different Photosensitizers. J. Photochem. Photobiol. B 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wurster, R.D. Methylene Blue Induces Cytotoxicity in Human Brain Tumor Cells. Cancer Lett. 1995, 88, 141–145. [Google Scholar] [CrossRef]
- Jung, J.; Wen, J.; Sun, Y. Amphiphilic Quaternary Ammonium Chitosans Self-Assemble Onto Bacterial and Fungal Biofilms and Kill Adherent Microorganisms. Colloids Surf. B Biointerfaces 2019, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm Formation by Pseudomonas Aeruginosa wild Type, Flagella and Type IV Pili Mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Abernethy, A.D.; Som, S.; Ruggiero, K.; Doucette, S.; Marcantonio, R.C.; Boussios, C.I.; Kent, R.; Goodson, J.M.; Tanner, A.C.; et al. The Antibacterial Effect of Photodynamic Therapy in Dental Plaque-Derived Biofilms. J. Periodontal. Res. 2009, 44, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial Photodynamic Activity and Cytocompatibility of Au25(Capt)18 Clusters Photoexcited by Blue LE D Light Irradiation. Int. J. Nanomed. 2017, 12, 2703–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Park, S.H.; Chang, B.S.; Lee, S.Y.; Lee, J.K.; Um, H.S. Antimicrobial Effect of Photodynamic Therapy Using Methylene Blue and Red Color Diode Laser on Biofilm Attached to Sandblasted and Acid-Etched Surface of Titanium. Laser Dent. Sci. 2017, 1, 83–90. [Google Scholar] [CrossRef]
- Huang, T.C.; Chen, C.J.; Ding, S.J.; Chen, C.C. Antimicrobial Efficacy of Methylene Blue-Mediated Photodynamic Therapy on Titanium Alloy Surfaces in Vitro. Photodiagn. Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Alonso, F.; Julián-Belmonte, E.; Chiva-García, F.; Martínez-Beneyto, Y. Bactericidal Efficacy of Photodynamic Therapy and Chitosan in Root Canals Experimentally Infected with Enterococcus Faecalis: An in Vitro Study. Photomed. Laser Surg. 2017, 35, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan Nanoparticles Enhance the Efficiency of Methylene Blue-Mediated Antimicrobial Photodynamic Inactivation of Bacterial Biofilms: An in Vitro Study. Photodiagn. Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- del Carpio-Perochena, A.; Kishen, A.; Shrestha, A.; Bramante, C.M. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; O’Toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [Green Version]
- Kreisler, M.; Kohnen, W.; Christoffers, A.B.; Götz, H.; Jansen, B.; Duschner, H.; d’Hoedt, B. In Vitro Evaluation of the Biocompatibility of Contaminated Implant Surfaces Treated with an Er:YAG Laser and an Air Powder System. Clin. Oral Impl. Res. 2005, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, C.C.; Ding, S.J. Effectiveness of Hypochlorous Acid to Reduce the Biofilms on Titanium Alloy Surfaces in Vitro. Int. J. Mol. Sci. 2016, 17, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Cordova, M.; Kishen, A. Photoactivated Polycationic Bioactive Chitosan Nanoparticles Inactivate Bacterial Endotoxins. J. Endod. 2015, 41, 686–691. [Google Scholar] [CrossRef]
- Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Oshiiwa, M.; Garcia, V.G. In Vivo Effect of Photodynamic Therapy on Periodontal Bone Loss in Dental Furcations. J. Periodontal. 2008, 79, 1081–1088. [Google Scholar] [CrossRef]
- Ding, S.J.; Chu, Y.H.; Wang, D.Y. Enhanced Properties of Novel Zirconia-Based Osteo-Implant Systems. Appl. Mater. Today 2017, 9, 622–632. [Google Scholar] [CrossRef]
- Eick, S.; Meier, I.; Spoerlé, F.; Bender, P.; Aoki, A.; Izumi, Y.; Salvi, G.E.; Sculean, A. In Vitro-Activity of Er:YAG Laser in Comparison with other Treatment Modalities on Biofilm Ablation from Implant and Tooth Surfaces. PLoS ONE 2017, 12, e0171086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, I.T.; Kao, P.F.; Huang, Y.R.; Ding, S.J. In Vitro and in Vivo Osteogenesis of Gelatin-Modified Calcium Silicate Cement with Washout Resistance. Mater. Sci. Eng. C 2020, 117, 111297. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-Centered Infection: Microbial Adhesion Versus Tissue Integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Wu, B.C.; Wei, C.K.; Hsueh, N.S.; Ding, S.J. Comparative Cell Attachment, Cytotoxicity and Antibacterial Activity of Radiopaque Dicalcium Silicate Cement and White-Coloured Mineral Trioxide Aggregate. Int. Endod. J. 2015, 48, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Wu, I.T.; Chen, C.C.; Ding, S.J. In Vitro Comparisons of Microscale and Nanoscale Calcium Silicate Particles. J. Mater. Chem. B 2020, 8, 6034–6047. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-N.; Ding, S.-J.; Chen, C.-C. Synergistic Photoantimicrobial Chemotherapy of Methylene Blue-Encapsulated Chitosan on Biofilm-Contaminated Titanium. Pharmaceuticals 2021, 14, 346. https://doi.org/10.3390/ph14040346
Lin C-N, Ding S-J, Chen C-C. Synergistic Photoantimicrobial Chemotherapy of Methylene Blue-Encapsulated Chitosan on Biofilm-Contaminated Titanium. Pharmaceuticals. 2021; 14(4):346. https://doi.org/10.3390/ph14040346
Chicago/Turabian StyleLin, Chiu-Nan, Shinn-Jyh Ding, and Chun-Cheng Chen. 2021. "Synergistic Photoantimicrobial Chemotherapy of Methylene Blue-Encapsulated Chitosan on Biofilm-Contaminated Titanium" Pharmaceuticals 14, no. 4: 346. https://doi.org/10.3390/ph14040346
APA StyleLin, C.-N., Ding, S.-J., & Chen, C.-C. (2021). Synergistic Photoantimicrobial Chemotherapy of Methylene Blue-Encapsulated Chitosan on Biofilm-Contaminated Titanium. Pharmaceuticals, 14(4), 346. https://doi.org/10.3390/ph14040346






