Relationship between CYP3A5 Polymorphism and Tacrolimus Blood Concentration Changes in Allogeneic Hematopoietic Stem Cell Transplant Recipients during Continuous Infusion
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
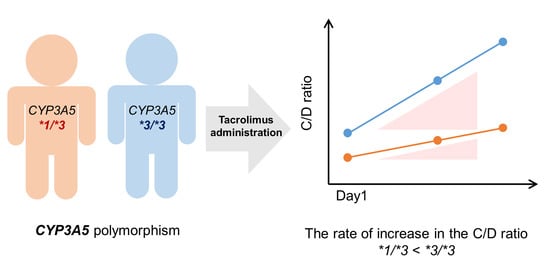
2.2. Effect of the CYP3A5 Polymorphism on Tacrolimus Pharmacokinetics
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Study Design
4.3. CYP3A5 Polymorphism Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (fk506): Molecular and cellular mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L.; Foster, R.H. Tacrolimus: A further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs 2000, 59, 323–389. [Google Scholar] [CrossRef]
- Przepiorka, D.; Nash, R.A.; Wingard, J.R.; Zhu, J.; Maher, R.M.; Fitzsimmons, W.E.; Fay, J.W. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol. Blood Marrow Transplant. 1999, 5, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ma, J. Tacrolimus in adult hematopoietic stem cell transplantation. Expert Opin. Drug Metab. Toxicol. 2019, 15, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R.J. Intrapatient variability of tacrolimus exposure in solid organ transplantation: A novel marker for clinical outcome. Clin. Pharmacol. Ther. 2000, 107, 347–358. [Google Scholar] [CrossRef]
- Piekoszewski, W.; Chow, F.S.; Jusko, W.J. Disposition of tacrolimus (FK 506) in rabbits. Role of red blood cell binding in hepatic clearance. Drug Metab. Dispos. 1993, 21, 690–698. [Google Scholar] [PubMed]
- Chow, F.S.; Piekoszewski, W.; Jusko, W.J. Effect of hematocrit and albumin concentration on hepatic clearance of tacrolimus (FK506) during rabbit liver perfusion. Drug Metab. Dispos. 1997, 25, 610–616. [Google Scholar]
- Yoshikawa, N.; Urata, S.; Yasuda, K.; Sekiya, H.; Hirabara, Y.; Okumura, M.; Ikeda, R. Retrospective analysis of the correlation between tacrolimus concentrations measured in whole blood and variations of blood cell counts in patients undergoing allogeneic haematopoietic stem cell transplantation. Eur. J. Hosp. Pharm. 2020, 27, e7–e11. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Yokota, T.; Matsuo, A.; Matsumoto, N.; Iwakiri, T.; Ikeda, R. Role of FK506 binding protein on tacrolimus distribution in red blood cells. Pharm. Res. 2020, 37, 143. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: Second consensus report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- McCune, J.S.; Bemer, M.J. Pharmacokinetics, pharmacodynamics and pharmacogenomics of immunosuppressants in allogeneic haematopoietic cell transplantation: Part I. Clin. Pharmacokinet. 2016, 55, 525–550. [Google Scholar] [CrossRef] [Green Version]
- Picard, N.; Bergan, S.; Marquet, P.; van Gelder, T.; Wallemacq, P.; Hesselink, D.A.; Haufroid, V. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther. Drug Monit. 2016, 38, S57–S69. [Google Scholar] [CrossRef]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef]
- Ota, T.; Kamada, Y.; Hayashida, M.; Iwao-Koizumi, K.; Murata, S.; Kinoshita, K. Combination analysis in genetic polymorphisms of drug-metabolizing enzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A5 in the Japanese population. Int. J. Med. Sci. 2015, 12, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Suetsugu, K.; Mori, Y.; Yamamoto, N.; Shigematsu, T.; Miyamoto, T.; Egashira, N.; Akashi, K.; Masuda, S. Impact of CYP3A5, POR, and CYP2C19 polymorphisms on trough concentration to dose ratio of tacrolimus in allogeneic hematopoietic stem cell transplantation. Int. J. Mol. Sci. 2019, 20, 2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamadeh, I.S.; Zhang, Q.; Steuerwald, N.; Hamilton, A.; Druhan, L.J.; McSwain, M.; Diez, Y.; Rusin, S.; Han, Y.; Symanowski, J.; et al. Effect of CYP3A4, CYP3A5, and ABCB1 polymorphisms on intravenous tacrolimus exposure and adverse events in adult allogeneic stem cell transplant patients. Biol. Blood Marrow Transplant. 2019, 25, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Venkataramanan, R.; Swaminathan, A.; Prasad, T.; Jain, A.; Zuckerman, S.; Warty, V.; McMichael, J.; Lever, J.; Burckart, G.; Starzl, T. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 1995, 29, 404–430. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Goodman, L.K.; Tett, S.E. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part i. Clin. Pharmacokinet 2010, 49, 141–175. [Google Scholar] [CrossRef]
- Hu, L.; Zhuo, W.; He, Y.J.; Zhou, H.H.; Fan, L. Pharmacogenetics of P450 oxidoreductase: Implications in drug metabolism and therapy. Pharmacogenet. Genomics 2012, 22, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, C.M.; Lv, J.F.; Zhou, H.H.; Fan, L. Polymorphisms in cytochrome P450 oxidoreductase and its effect on drug metabolism and efficacy. Pharmacogenet. Genomics 2017, 27, 337–346. [Google Scholar] [CrossRef]
- Chen, X.; Pan, L.Q.; Naranmandura, H.; Zeng, S.; Chen, S.Q. Influence of various polymorphic variants of Cytochrome P450 Oxidoreductase (POR) on drug metabolic activity of CYP3A4 and CYP2B6. PLoS ONE 2012, 7, e38495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayani, S.; Palmer, J.M.; Stiller, T.; Chan, H.; Keuylian, S.; Parker, P.; Thomas, S.; Pullarkat, V.; Nademanee, A.; Forman, S.J.; et al. Aprepitant (Emend) significantly increases sirolimus levels in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012, 47, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, Y.; Nakagome, S.; Ando, N.; Igarashi, T.; Harada, D.; Kitamura, M.; Kawakubo, T. Effect of aprepitant on concentration of tacrolimus induced by pretreatment for hematopoietic stem cell transplantation. J. Hematop. Cell Transplant. 2020, 9, 32–39. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuen, S.; Fukuda, T.; Maune, H.; Ikenaga, Y.; Yamamoto, I.; Inaba, T.; Azuma, J. Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5* 3 and * 6, in a Japanese population. Pharmacogenetics 2002, 12, 331–334. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | *1/*3 | *3/*3 | p Value |
|---|---|---|---|
| Number of patients (male/female) | 9 (4/5) | 11 (9/2) | 0.160 (a) |
| Median age (min–max) (years) | 55 (38–63) | 61 (38–98) | 0.102 (b) |
| Body weight (kg) | 58.6 ± 6.1 | 59.7 ± 10.3 | 0.732 (b) |
| Disease diagnosis | 0.610 (a) | ||
| AML | 1 | 3 | |
| ALL | 1 | 2 | |
| CML | 1 | 1 | |
| MDS | 1 | 3 | |
| NHL | 0 | 1 | |
| ATL | 3 | 1 | |
| DLBCL | 1 | 0 | |
| MF | 1 | 0 | |
| Stem cell source | 0.070 (a) | ||
| BMT | 8 | 5 | |
| CBT | 0 | 4 | |
| PBSCT | 1 | 2 | |
| Conditioning regimen | 0.281 (a) | ||
| Flu/Mel/TBI | 4 | 7 | |
| Cy/TBI | 4 | 1 | |
| Bu/Cy | 1 | 3 | |
| Combination of aprepitant | 6 | 7 | 1.000 (a) |
| GVHD prophylaxis | 0.056 (a) | ||
| Tacrolimus + sMTX | 9 | 6 | |
| Tacrolimus + MMF | 0 | 4 | |
| PTCY + tacrolimus + MMF | 0 | 1 | |
| Laboratory data | |||
| ALT (U/L) | 12.9 ± 5.3 | 27.4 ± 21.5 | 0.063 (b) |
| AST (U/L) | 13.7 ± 5.5 | 19.8 ± 6.6 | 0.055 (b) |
| GGTP (U/L) | 39.3 ± 16.4 | 46.0 ± 42.9 | 0.788 (b) |
| Serum creatinine (mg/dL) | 0.54 ± 0.16 | 0.70 ± 0.19 | 0.083 (b) |
| Urea nitrogen (mg/dL) | 15.5 ± 2.9 | 16.6 ± 8.3 | 0.968 (b) |
| RBC (106/µL) | 2.61 ± 0.47 | 2.76 ± 0.67 | 0.941 (b) |
| Hematocrit (%) | 24.6 ± 4.3 | 26.2 ± 6.2 | 0.824 (b) |
| Receipt of amlodipine | 1 | 2 | 1.000 (a) |
| Genotypes | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| *1/*3 (n = 9) | 540.1 ± 157.2 | 798.4 ± 283.8 | 869.3 ± 280.3 | 988.5 ± 316.3 | 1000.9 ± 339.7 |
| *3/*3 (n = 11) | 608.9 ± 166.7 | 1222.4 ± 676.3 | 1773.9 ± 1148.8 | 2018.1 ± 1494.8 | 2007.6 ± 1367.2 |
| p Value | 0.356 | 0.080 | 0.028 * | 0.048 * | 0.037 * |
| Drug | Genotype | (C/D)After/(C/D)Before | Average | p Value |
|---|---|---|---|---|
| Itraconazole | *1/*3 | 1.11 | 1.12 | - |
| Itraconazole | *1/*3 | 1.13 | ||
| Itraconazole | *3/*3 | 1.45 | 1.55 | |
| Itraconazole | *3/*3 | 1.59 | ||
| Itraconazole | *3/*3 | 1.61 | ||
| Lansoprazole | *1/*3 | 0.86 | - | - |
| Lansoprazole | *3/*3 | 1.93 | - | |
| Letermovir | *1/*3 | 1.29 | 1.71 | 0.345 (a) |
| Letermovir | *1/*3 | 1.44 | ||
| Letermovir | *1/*3 | 1.73 | ||
| Letermovir | *1/*3 | 1.82 | ||
| Letermovir | *1/*3 | 2.24 | ||
| Letermovir | *3/*3 | 1.41 | 2.28 | |
| Letermovir | *3/*3 | 1.52 | ||
| Letermovir | *3/*3 | 2.05 | ||
| Letermovir | *3/*3 | 2.12 | ||
| Letermovir | *3/*3 | 4.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, N.; Takeshima, H.; Sekine, M.; Akizuki, K.; Hidaka, T.; Shimoda, K.; Ikeda, R. Relationship between CYP3A5 Polymorphism and Tacrolimus Blood Concentration Changes in Allogeneic Hematopoietic Stem Cell Transplant Recipients during Continuous Infusion. Pharmaceuticals 2021, 14, 353. https://doi.org/10.3390/ph14040353
Yoshikawa N, Takeshima H, Sekine M, Akizuki K, Hidaka T, Shimoda K, Ikeda R. Relationship between CYP3A5 Polymorphism and Tacrolimus Blood Concentration Changes in Allogeneic Hematopoietic Stem Cell Transplant Recipients during Continuous Infusion. Pharmaceuticals. 2021; 14(4):353. https://doi.org/10.3390/ph14040353
Chicago/Turabian StyleYoshikawa, Naoki, Hidemi Takeshima, Masaaki Sekine, Keiichi Akizuki, Tomonori Hidaka, Kazuya Shimoda, and Ryuji Ikeda. 2021. "Relationship between CYP3A5 Polymorphism and Tacrolimus Blood Concentration Changes in Allogeneic Hematopoietic Stem Cell Transplant Recipients during Continuous Infusion" Pharmaceuticals 14, no. 4: 353. https://doi.org/10.3390/ph14040353
APA StyleYoshikawa, N., Takeshima, H., Sekine, M., Akizuki, K., Hidaka, T., Shimoda, K., & Ikeda, R. (2021). Relationship between CYP3A5 Polymorphism and Tacrolimus Blood Concentration Changes in Allogeneic Hematopoietic Stem Cell Transplant Recipients during Continuous Infusion. Pharmaceuticals, 14(4), 353. https://doi.org/10.3390/ph14040353