Decorating sdAbs with Chelators: Effect of Conjugation on Biodistribution and Functionality
Abstract
:1. Introduction
2. Results
2.1. AEX and Product Characterization
2.2. In Vivo Biodistribution
2.2.1. [68Ga]Ga-NOTA-anti-HER2
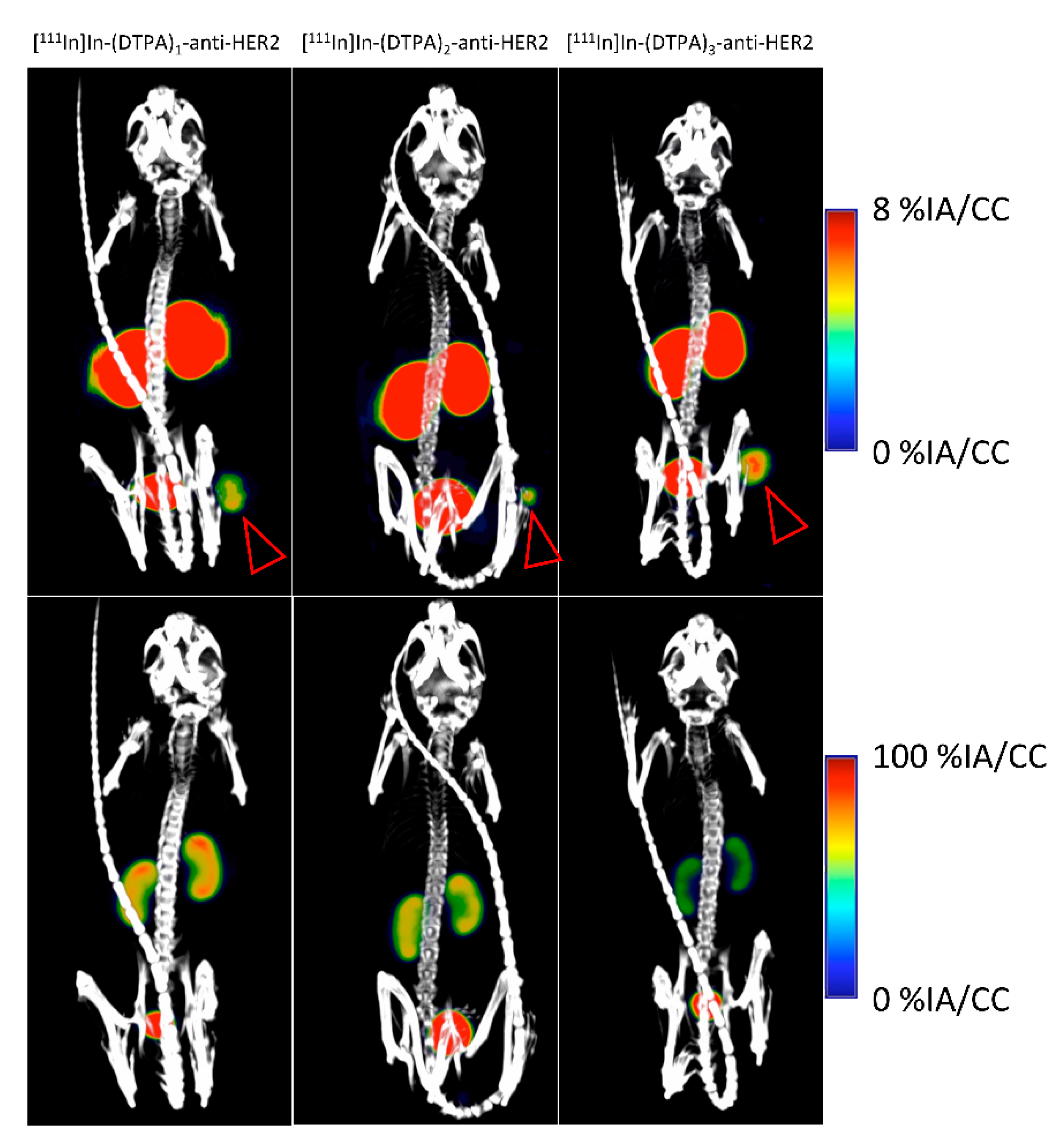
2.2.2. [111In]In-DTPA-anti-HER2
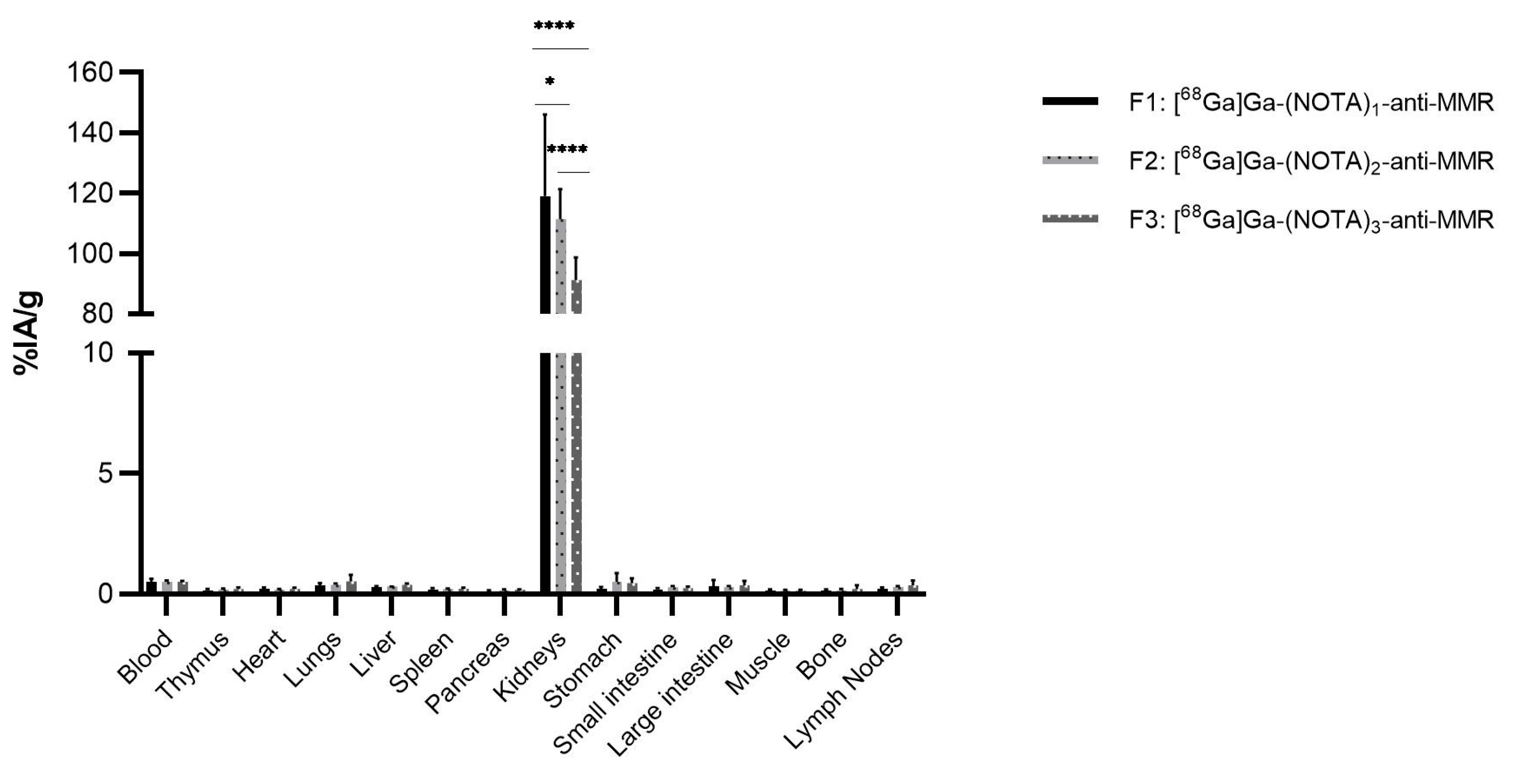
2.2.3. [68Ga]Ga-NOTA-anti-MMR
2.2.4. [111In]In-DTPA-anti-MMR
2.2.5. [68Ga]Ga-NOTA-anti-MMR in MMR KO Mice
3. Discussion
4. Materials and Methods
4.1. NOTA/DTPA-Conjugation
4.1.1. SEC Purification
4.1.2. AEX Purification
4.2. Mass Spectrometry Analysis
4.3. Surface Plasmon Resonance Analysis
4.4. Iso-Electric Focusing Electrophoresis
4.5. Radiolabeling
4.6. Cell Culturing
4.7. In Vivo Biodistribution Study
4.8. SPECT/CT
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, A.M.; Olafsen, T. Antibodies for molecular imaging of cancer. Cancer J. 2008, 14, 191–197. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Mestel, R. Cancer: Imaging with antibodies. Nature 2017, 543, 743–746. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-human imaging with 89 Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: Preliminary pharmacokinetics, biodistribution, and lesion targeting. J. Nucl. Med. 2019. [Google Scholar] [CrossRef]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Prince, D.; Rossouw, D.; Rubow, S. Optimization of a labeling and kit preparation method for Ga-68 labeled dotatate, using cation exchange resin purified Ga-68 eluates obtained from a tin dioxide. Mol. Imaging Biol. 2018, 20, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Könning, D.; Zielonka, S.; Grzeschik, J.; Empting, M.; Valldorf, B.; Krah, S.; Schröter, C.; Sellmann, C.; Hock, B.; Kolmar, H. Camelid and shark single domain antibodies: Structural features and therapeutic potential. Curr. Opin. Struct. Biol. 2016, 45, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Vincke, C.; Gutiérrez, C.; Wernery, U.; Devoogdt, N.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S. Generation of single domain antibody fragments derived from camelids and generation of manifold constructs. Methods Mol. Biol. 2012, 907, 145–176. [Google Scholar] [CrossRef]
- Muyldermans, S.; Atarhouch, T.; Saldanha, J.; Barbosa, J.A.; Hamers, R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994, 7, 1129–1135. [Google Scholar] [CrossRef]
- Vaneycken, I.; D’Huyvetter, M.; Hernot, S.; De Vos, J.; Xavier, C.; Devoogdt, N.; Caveliers, V.; Lahoutte, T. Immuno-imaging using nanobodies. Curr. Opin. Biotechnol. 2011, 22, 877–881. [Google Scholar] [CrossRef]
- Devoogdt, N.; Xavier, C.; Hernot, S.; Vaneycken, I.; D’Huyvetter, M.; De Vos, J.; Massa, S.; De Baetselier, P.; Caveliers, V.; Lahoutte, T. Molecular imaging using Nanobodies: A case study. Methods Mol. Biol. 2012, 911, 559–567. [Google Scholar] [CrossRef] [PubMed]
- de Vos, J.; Devoogdt, N.; Lahoutte, T.; Muyldermans, S. Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: Adjusting the bullet to its target. Expert Opin. Biol. Ther. 2013, 13, 1149–1160. [Google Scholar] [CrossRef]
- Debie, P.; Devoogdt, N.; Hernot, S. Targeted nanobody-based molecular tracers for nuclear imaging and image-guided surgery. Antibodies 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Blykers, A.; Schoonooghe, S.; Xavier, C.; D’Hoe, K.; Laoui, D.; D’Huyvetter, M.; Vaneycken, I.; Cleeren, F.; Bormans, G.; Heemskerk, J.; et al. PET imaging of macrophage mannose receptor-expressing macrophages in tumor stroma using 18F-radiolabeled camelid single-domain antibody fragments. J. Nucl. Med. 2015, 56, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Debie, P.; Vanhoeij, M.; Poortmans, N.; Puttemans, J.; Gillis, K.; Devoogdt, N.; Lahoutte, T.; Hernot, S. Improved debulking of peritoneal tumor implants by near-infrared fluorescent nanobody image guidance in an experimental mouse model. Mol. Imaging Biol. 2018, 20, 361–367. [Google Scholar] [CrossRef]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van Der Jeught, K.; Muyldermans, S.; Van Der Heyden, J.; Lahoutte, T.; Tavernier, J.; Devoogdt, N. Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-hodgkin lymphoma. Mol. Cancer Ther. 2017, 16, 2828–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017. [Google Scholar] [CrossRef] [Green Version]
- Lecocq, Q.; Zeven, K.; De Vlaeminck, Y.; Martens, S.; Massa, S.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; Breckpot, K.; Devoogdt, N. Noninvasive imaging of the immune checkpoint LAG-3 using nanobodies, from development to pre-clinical use. Biomolecules 2019, 9, 548. [Google Scholar] [CrossRef] [Green Version]
- Bala, G.; Blykers, A.; Xavier, C.; Descamps, B.; Broisat, A.; Ghezzi, C.; Fagret, D.; Van Camp, G.; Caveliers, V.; Vanhove, C.; et al. Targeting of vascular cell adhesion molecule-1 by 18F-labelled nanobodies for PET/CT imaging of inflamed atherosclerotic plaques. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Bala, G.; Baudhuin, H.; Remory, I.; Gillis, K.; Debie, P.; Krasniqi, A.; Lahoutte, T.; Raes, G.; Devoogdt, N.; Cosyns, B.; et al. Evaluation of [99m Tc] radiolabeled macrophage mannose receptor-specific nanobodies for targeting of atherosclerotic lesions in mice. Mol. Imaging Biol. 2018, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ. Res. 2012, 110, 927–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Put, S.; Schoonooghe, S.; Devoogdt, N.; Schurgers, E.; Avau, A.; Mitera, T.; D’Huyvetter, M.; De Baetselier, P.; Raes, G.; Lahoutte, T.; et al. SPECT imaging of joint inflammation with Nanobodies targeting the macrophage mannose receptor in a mouse model for rheumatoid arthritis. J. Nucl. Med. 2013, 54, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 Expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, C.; Blykers, A.; Laoui, D.; Bolli, E.; Vaneyken, I.; Bridoux, J.; Baudhuin, H.; Raes, G.; Everaert, H.; Movahedi, K.; et al. Clinical translation of 68Ga-NOTA-anti-MMR-sdAb for PET/CT imaging of protumorigenic macrophages. Mol. Imaging Biol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Mueller, B.; Wrasidlo, W.; Reisfeld, R. Determination of the number of e-amino groups available for conjugation of effector molecules to monoclonal antibodies. Hybridoma 1988, 7, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Bertozzi, C.R. Site-specific antibody-drug conjugates: The nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 2015, 26, 176–192. [Google Scholar] [CrossRef] [Green Version]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. MAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Buecheler, J.W.; Winzer, M.; Tonillo, J.; Weber, C.; Gieseler, H. Impact of Payload hydrophobicity on the stability of antibody-drug conjugates. Mol. Pharm. 2018, 15, 2656–2664. [Google Scholar] [CrossRef]
- Pham, D.T.; Kaspersen, F.M.; Bos, E.S. Electrophoretic method for the quantitative determination of a benzyl-DTPA ligand in DTPA monoclonal antibody conjugates. Bioconjugate Chem. 1995, 6, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, A.; Petrick, A.T.; Thomas, P. Modification of antibody isoelectric point affects biodistribution of 111-indium-labeled antibody. Nucl. Med. Biol. 1996, 23, 257–261. [Google Scholar] [CrossRef]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.P.; Fielder, P.J.; Khawli, L.A. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjugate Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef]
- Dennler, P.; Fischer, E.; Schibli, R. Antibody conjugates: From heterogeneous populations to defined reagents. Antibodies 2015, 4, 197–224. [Google Scholar] [CrossRef]
- Shin, I.S.; Lee, S.-M.; Kim, H.S.; Yao, Z.; Regino, C.; Sato, N.; Cheng, K.T.; Hassan, R.; Campo, M.F.; Albone, E.F.; et al. Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody. Nucl. Med. Biol. 2011, 38, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Grünberg, J.; Novak-Hofer, I.; Honer, M.; Zimmermann, K.; Knogler, K.; Bläuenstein, P.; Ametamey, S.; Maecke, H.R.; Schubiger, P.A. In vivo evaluation of 177Lu- and 67/64Cu-labeled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM-positive tumors. Clin. Cancer Res. 2005, 11, 5112–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knogler, K.; Grünberg, J.; Novak-Hofer, I.; Zimmermann, K.; Schubiger, P.A. Evaluation of 177Lu-DOTA-labeled aglycosylated monoclonal anti-L1-CAM antibody chCE7: Influence of the number of chelators on the in vitro and in vivo properties. Nucl. Med. Biol. 2006, 33, 883–889. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Zhou, Z.; McDougald, D.; Meshaw, R.L.; Vaidyanathan, G.; Zalutsky, M.R. Site-specific radioiodination of an anti-HER2 single domain antibody fragment with a residualizing prosthetic agent. Nucl. Med. Biol. 2021, 92, 171–183. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; De Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjugate Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; Van Leeuwen, F.W.B.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, E.I.; Gburek, J. Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiolog. Pediatr. Nephrol. 2004, 19, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Dagil, R.; O’Shea, C.; Nykjær, A.; Bonvin, A.M.; Kragelund, B.B. Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: Insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin. J. Biol. Chem. 2013, 288, 4424–4435. [Google Scholar] [CrossRef] [Green Version]
- Giebisch, G.; Windhager, E. Transport of sodium and chloride. In Medical Physiology: A cellular and Molecular Approach; Saunders Elsevier: Amsterdam, The Netherlands, 2009; Chapter 35; pp. 782–796. [Google Scholar]
- Linehan, S.A.; Martínez-Pomares, L.; Stahl, P.D.; Gordon, S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J. Exp. Med. 1999, 189, 1961–1972. [Google Scholar] [CrossRef]
- Linehan, S.A. The mannose receptor is expressed by subsets of APC in non-lymphoid organs. BMC Immunol. 2005, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.E. Structure and function of the macrophage mannose receptor. Mamm. Carbohydr. Recognit. Syst. 2001, 33, 105–121. [Google Scholar]
- Dekempeneer, Y.; Bäck, T.; Aneheim, E.; Jensen, H.; Puttemans, J.; Xavier, C.; Keyaerts, M.; Palm, S.; Albertsson, P.; Lahoutte, T.; et al. Labeling of Anti-HER2 nanobodies with astatine-211: Optimization and the effect of different coupling reagents on their in vivo behavior. Mol. Pharm. 2019, 16, 3524–3533. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.; Caveliers, V.; Ooms, M.; Maertens, D.; Gysemans, M.; Lahoutte, T.; Xavier, C.; Lecocq, Q.; Maes, K.; Covens, P.; et al. Therapeutic efficacy of (213)Bi-labeled sdAbs in a preclinical model of ovarian cancer. Mol. Pharm. 2020, 17, 3553–3566. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Remels, L.M.; de Baetselier, P.C. Characterization of 3LL-tumor variants generated by in vitro macrophage-mediated selection. Int. J. Cancer 1987, 39, 343–352. [Google Scholar] [CrossRef] [PubMed]







| Compound | Affinity—KD (nM) | ||||
|---|---|---|---|---|---|
| Reference * | Mixture ** | Fraction 1 | Fraction 2 | Fraction 3 | |
| NOTA-anti-HER2 | 3.7, σ = 0.7 | 4.8, σ = 1.6 | 4.1 | 5.0 | 7.4 |
| DTPA-anti-HER2 | 3.7, σ = 0.7 | NA *** | 3.3 | 6.2 | 10.0 |
| NOTA-anti-MMR | 1.2, σ = 0.3 | 1.2, σ = 0.2 | 1.3 | 1.7 | 2.9 |
| DTPA-anti-MMR | 1.2, σ = 0.3 | NA *** | 1.6 | 2.4 | 4.8 |
| Compound | pI | |||
|---|---|---|---|---|
| Reference * | Fraction 1 | Fraction 2 | Fraction 3 | |
| NOTA-anti-HER2 | >8.3 | 5.2, 5.0 | 5.0, 4.1, 3.5 | 3.5, 3.4 |
| DTPA-anti-HER2 | >8.3 | 5.0, 4.5 | 3.5, 3.3 | 3.1, 2.9 |
| NOTA-anti-MMR | >8.3 | 5.4, 5.2, 4.9 | 4.7, 3.7, 3.5 | 3.5, 3.2 |
| DTPA-anti-MMR | >8.3 | 5.0, 4.8, 3.8 | 3.4, 3.1 | 3.1, 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudhuin, H.; Puttemans, J.; Hanssens, H.; Vanwolleghem, P.; Hernot, S.; Raes, G.; Xavier, C.; Lahoutte, T.; Debie, P. Decorating sdAbs with Chelators: Effect of Conjugation on Biodistribution and Functionality. Pharmaceuticals 2021, 14, 407. https://doi.org/10.3390/ph14050407
Baudhuin H, Puttemans J, Hanssens H, Vanwolleghem P, Hernot S, Raes G, Xavier C, Lahoutte T, Debie P. Decorating sdAbs with Chelators: Effect of Conjugation on Biodistribution and Functionality. Pharmaceuticals. 2021; 14(5):407. https://doi.org/10.3390/ph14050407
Chicago/Turabian StyleBaudhuin, Henri, Janik Puttemans, Heleen Hanssens, Philippe Vanwolleghem, Sophie Hernot, Geert Raes, Catarina Xavier, Tony Lahoutte, and Pieterjan Debie. 2021. "Decorating sdAbs with Chelators: Effect of Conjugation on Biodistribution and Functionality" Pharmaceuticals 14, no. 5: 407. https://doi.org/10.3390/ph14050407
APA StyleBaudhuin, H., Puttemans, J., Hanssens, H., Vanwolleghem, P., Hernot, S., Raes, G., Xavier, C., Lahoutte, T., & Debie, P. (2021). Decorating sdAbs with Chelators: Effect of Conjugation on Biodistribution and Functionality. Pharmaceuticals, 14(5), 407. https://doi.org/10.3390/ph14050407







