Drug Delivery Approaches for Managing Overactive Bladder (OAB): A Systematic Review
Abstract
:1. Introduction
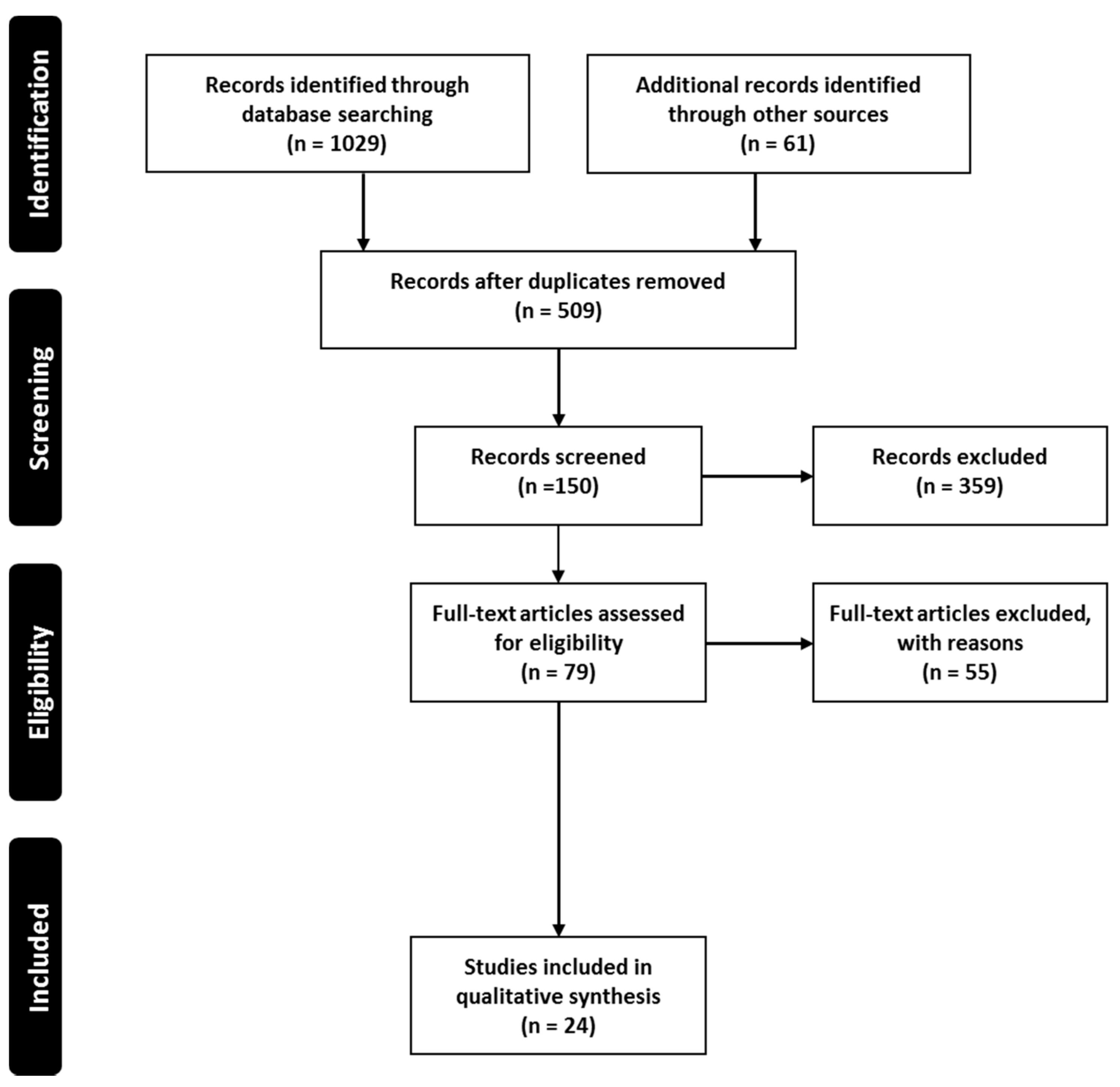
2. Methodology
2.1. Search Plot, Information Sources and Screening Process
2.2. Study Selection
2.3. Data Extraction and Collection
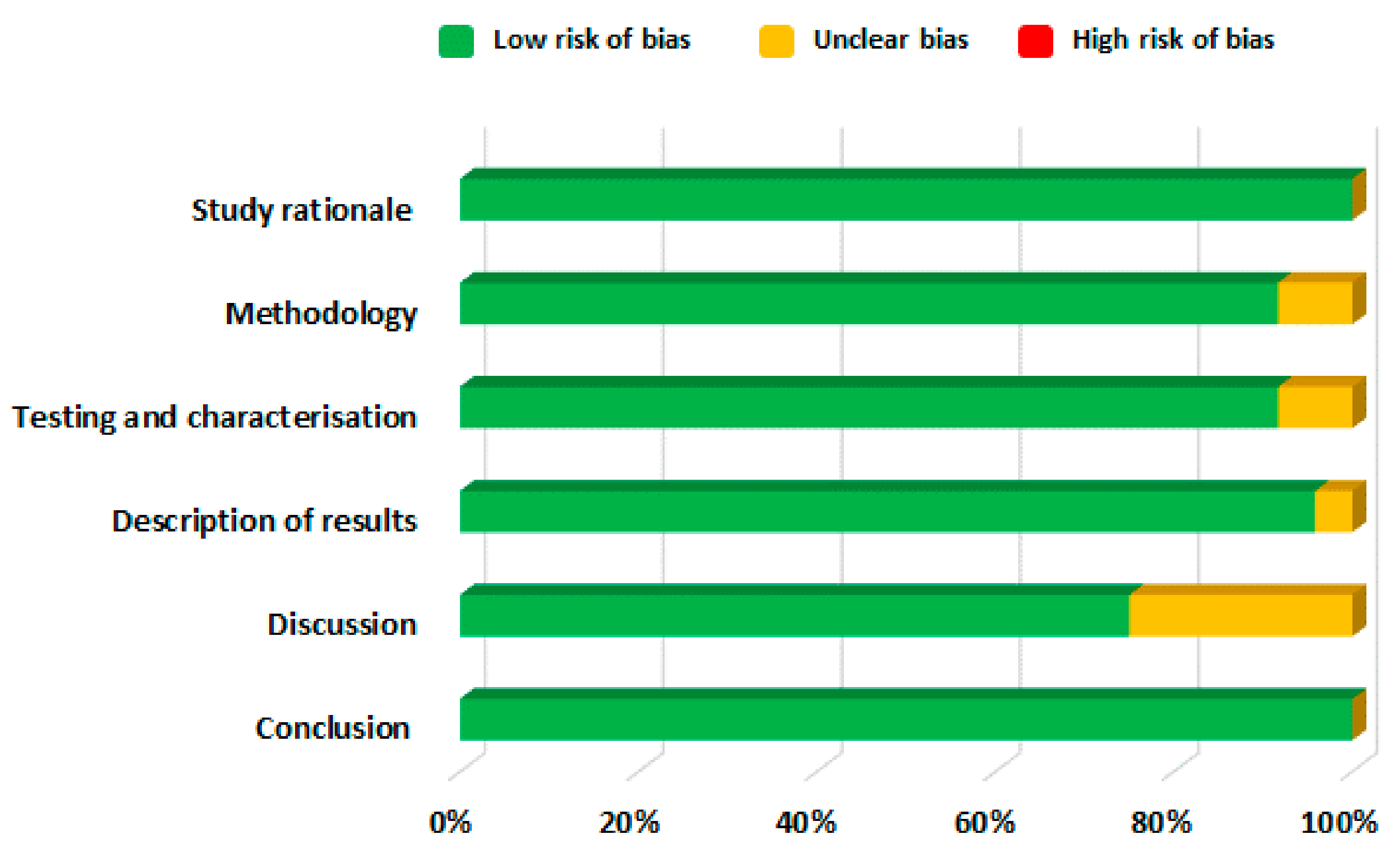
2.4. Risk of Bias Assessment
3. Results and Discussion
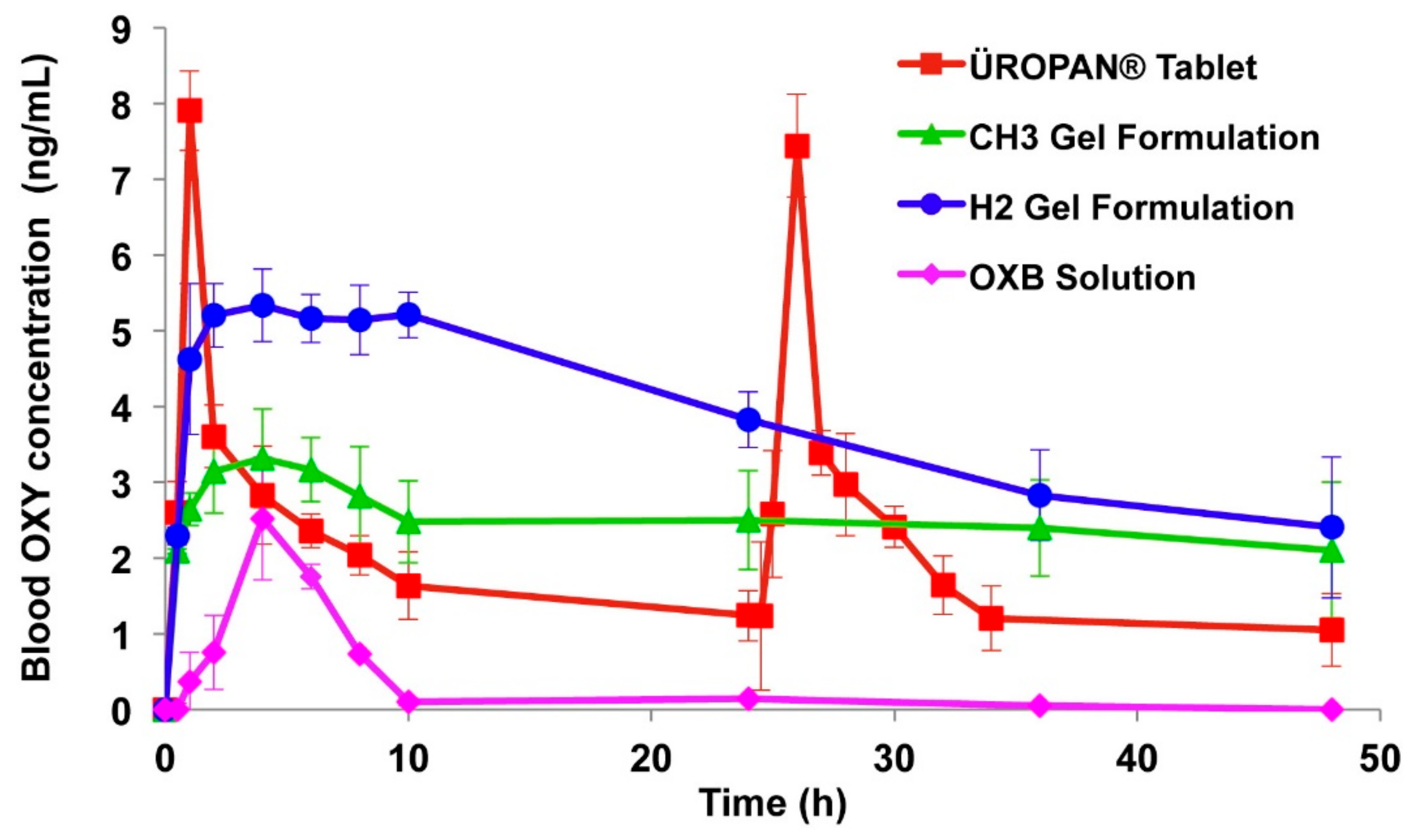
3.1. Transdermal Route
3.2. Intravesical Route
3.3. Vaginal Route
3.4. Intramuscular Route
3.5. Oral Route
4. Conclusions
5. Limitations of Current Systematic Review
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, D.; Cardozo, L. Managing overactive bladder. Climacteric 2019, 22, 250–256. [Google Scholar] [CrossRef]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Benner, J.S.; Becker, R.; Fanning, K.; Jumadilova, Z.; Bavendam, T.; Brubaker, L. OAB Medication Use Study Steering Committee Bother Related to Bladder Control and Health Care Seeking Behavior in Adults in the United States. J. Urol. 2009, 181, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Kelleher, C.J.; Kerr, L.A.; Rogers, R.G. Overactive bladder significantly affects quality of life. Am. J. Manag. Care 2000, 6, S580–S590. [Google Scholar]
- Filipetto, F.A.; Fulda, K.G.; Holthusen, A.E.; McKeithen, T.M.; McFadden, P. The patient perspective on overactive bladder: A mixed-methods needs assessment. BMC Fam. Pr. 2014, 15, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, F.M.; Dmochowski, R. Pathophysiology of Overactive Bladder. Am. J. Med. 2006, 119, 3–8. [Google Scholar] [CrossRef]
- Meng, E.; Lin, W.; Lee, W.; Chuang, Y. Pathophysiology of Overactive Bladder. LUTS Low. Urin. Tract Symptoms 2012, 4, 48–55. [Google Scholar] [CrossRef]
- O’Reilly, B.A.; Kosaka, A.H.; Knight, G.F.; Chang, T.K.; Ford, A.P.D.W.; Rymer, J.M.; Popert, R.; Burnstock, G.; McMahon, S.B. P2X receptors and their role in female idiopathic detrusor instability. J. Urol. 2002, 167, 157–164. [Google Scholar] [CrossRef]
- Brading, A.F. A myogenic basis for the overactive bladder. Urology 1997, 50, 57–67. [Google Scholar] [CrossRef]
- Sibley, G.N. Developments in our understanding of detrusor instability. Br. J. Urol. 1997, 80, 54–61. [Google Scholar]
- Maggi, C.A.; Santicioli, P.; Parlani, M.; Astolfi, M.; Patacchini, R.; Meli, A. The presence of mucosa reduces the contractile response of the guinea-pig urinary bladder to substance P. J. Pharm. Pharmacol. 1987, 39, 653–655. [Google Scholar] [CrossRef]
- De Nunzio, C.; Franco, G.; Rocchegiani, A.; Iori, F.; Leonardo, C.; Laurenti, C. The evolution of detrusor overactivity after watchful waiting, medical therapy and surgery in patients with bladder outlet obstruction. J. Urol. 2003, 169, 535–539. [Google Scholar] [CrossRef]
- Yu, H.-J.; Liu, C.-Y.; Lee, K.-L.; Lee, W.-C.; Chen, T.H.-H. Overactive Bladder Syndrome among Community-Dwelling Adults in Taiwan: Prevalence, Correlates, Perception, and Treatment Seeking. Urol. Int. 2006, 77, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-T.; Chung, M.-S.; Lee, W.-C.; Chang, S.-W.; Huang, S.-T.; Yang, K.D.; Chancellor, M.B.; Chuang, Y.-C. Prevalence of Overactive Bladder and Associated Risk Factors in 1359 Patients with Type 2 Diabetes. Urology 2011, 78, 1040–1045. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Tyagi, V.; Liu, R.-T.; Chancellor, M.B.; Tyagi, P. Urine and Serum C-Reactive Protein Levels as Potential Biomarkers of Lower Urinary Tract Symptoms. Urol. Sci. 2010, 21, 132–136. [Google Scholar] [CrossRef] [Green Version]
- National Collaborating Centre for Women’s and Children’s Health (UK). Urinary Incontinence: The Management of Urinary Incontinence in Women; RCOG Press: London, UK, 2006; pp. 48–83. [Google Scholar]
- Chapple, C.R.; Gormley, E.A. Developments in pharmacological therapy for the overactive bladder. BJU Int. 2006, 98, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, H.; Shariat, S.F.; Salehi-Pourmehr, H.; Janisch, F.; Mori, K.; Quhal, F.; Hajebrahimi, S. The clinical pharmacology of the medical treatment for overactive bladder in adults. Expert Rev. Clin. Pharmacol. 2020, 13, 707–720. [Google Scholar] [CrossRef]
- Painter, C.E.; Suskind, A.M. Advances in Pharmacotherapy for the Treatment of Overactive Bladder. Curr. Bl. Dysfunct. Rep. 2019, 14, 377–384. [Google Scholar] [CrossRef]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Benner, J.S.; Nichol, M.B.; Rovner, E.S.; Jumadilova, Z.; Alvir, J.; Hussein, M.; Fanning, K.; Trocio, J.N.; Brubaker, L. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010, 105, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Nazir, J.; Hakimi, Z.; Bowditch, S.; Fatoye, F.; Guelfucci, F.; Khemiri, A.; Siddiqui, E.; Wagg, A. Persistence and Adherence with Mirabegron versus Antimuscarinic Agents in Patients with Overactive Bladder: A Retrospective Observational Study in UK Clinical Practice. Eur. Urol. 2017, 72, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, C.C.; Notte, S.M.; Maroulis, C.; Dmochowski, R.R.; Cardozo, L.; Subramanian, D.; Coyne, K.S. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int. J. Clin. Pr. 2011, 65, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Veenboer, P.W.; Bosch, J.R. Long-Term Adherence to Antimuscarinic Therapy in Everyday Practice: A Systematic Review. J. Urol. 2014, 191, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Ita, K. Transdermal drug delivery: Progress and challenges. J. Drug Deliv. Sci. Technol. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Tyagi, P.; Kashyap, M.; Hensley, H.; Yoshimura, N. Advances in intravesical therapy for urinary tract disorders. Expert Opin. Drug Deliv. 2015, 13, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Acarturk, F. Mucoadhesive Vaginal Drug Delivery Systems. Recent Pat. Drug Deliv. Formul. 2009, 3, 193–205. [Google Scholar] [CrossRef]
- Raju, R.; Linder, B.J. Evaluation and Treatment of Overactive Bladder in Women. Mayo Clin. Proc. 2020, 95, 370–377. [Google Scholar] [CrossRef]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecology J. 2012, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousaf, M.; Nirwan, J.S.; Smith, A.M.; Timmins, P.; Conway, B.R.; Ghori, M.U. Raft-forming polysaccharides for the treatment of gastroesophageal reflux disease (GORD): Systematic review. J. Appl. Polym. Sci. 2019, 136, 48012. [Google Scholar] [CrossRef] [Green Version]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.-U.-D.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef]
- Hasan, S.S.; Zaidi, S.T.R.; Nirwan, J.S.; Ghori, M.U.; Javid, F.; Ahmadi, K.; Babar, Z.-U.-D. Use of Central Nervous System (CNS) Medicines in Aged Care Homes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1292. [Google Scholar] [CrossRef] [Green Version]
- Nirwan, J.S.; Hasan, S.S.; Conway, B.R.; Ghori, M.U. Investigating the association between education level and gastroesophageal reflux disease (GERD): A systematic review and meta-analysis. Turk. J. Gastroenterol. 2019, 30 (Suppl. 3), S892–S893. [Google Scholar]
- Electronic Medicines Compendium, Database on the Internet. Datapharm Communications Ltd. 2021. Available online: http://emc.medicines.org.uk (accessed on 7 April 2021).
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Faculty of Medicine, University of Calgary: Calgary, AB, Canada, 2004; pp. 1–4. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- O’Neil, M.E.; Peterson, K.; Low, A.; Carson, S.; Denneson, L.M.; Haney, E.; Shiroma, P.; Kansagara, D. Suicide Prevention Interventions and Referral/Follow-Up Services: A Systematic Review; Department of Veterans Affairs: Washington, DC, USA, 2012. [Google Scholar]
- Walsh, D.; Downe, S. Appraising the quality of qualitative research. Midwifery 2006, 22, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Korakakis, V.; Whiteley, R.; Tzavara, A.; Malliaropoulos, N. The effectiveness of extracorporeal shockwave therapy in common lower limb conditions: A systematic review including quantification of patient-rated pain reduction. Br. J. Sports. Med. 2018, 52, 387–407. [Google Scholar] [CrossRef] [Green Version]
- Nicoli, S.; Penna, E.; Padula, C.; Colombo, P.; Santi, P. New transdermal bioadhesive film containing oxybutynin: In vitro permeation across rabbit ear skin. Int. J. Pharm. 2006, 325, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Banu, T.S.; Som, S.; Havannavar, N.T. An approach to the formulation of transdermal film of oxybutynin. Res. J. Pharm. Bio. Chem. Sci. 2010, 1, 412–421. [Google Scholar]
- Bakshi, A.; Bajaj, A.; Malhotra, G.; Madan, M.; Amrutiya, N.; Bakshi, A. A novel metered dose transdermal spray formulation for oxybutynin. Indian J. Pharm. Sci. 2008, 70, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Rajabalaya, R.; David, S.R.; Chellian, J.; Xin Yun, G.; Chakravarthi, S. Transdermal delivery of oxybutynin chloride proniosomal gels for the treatment of overactive bladder. Drug Deliv. 2016, 23, 578–1587. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, C.; Luo, Z.; Wan, X.; Fang, L. Investigation of molecular mobility of pressure-sensitive-adhesive in oxybutynin patch in vitro and in vivo Effect of sorbitan monooleate on drug release and patch mechanical property. Eur. J. Pharm. Sci. 2018, 122, 116–124. [Google Scholar] [CrossRef]
- Pandit, V.; Khanum, A.; Bhaskaran, S.; Banu, V. Formulation and evaluation of transdermal films for the treatment of overactive bladder. Int. J. Pharm. Tech. Res. 2009, 1, 799–804. [Google Scholar]
- Sun, F.; Sui, C.; Zhou, Y.; Liu, X.; Shi, Y.; Wu, Y. Preparation, characterization and pharmacological evaluation of tolterodine hydrogels for the treatment of overactive bladder. Int. J. Pharm. 2013, 454, 532–538. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Yu, K.; Sun, X.; Fan, C.; Long, C.; Liu, N.; Li, S.; Wu, B.; Xu, Q.; et al. Design of hydrogels of 5-hydroxymethyl tolterodine and their studies on pharmacokinetics, pharmacodynamics and transdermal mechanism. Eur. J. Pharm. Sci. 2017, 96, 530–541. [Google Scholar] [CrossRef]
- Liu, X.; Fu, L.; Dai, W.; Liu, W.; Zhao, J.; Wu, Y.; Teng, L.; Sun, F.; Li, Y. Design of transparent film-forming hydrogels of tolterodine and their effects on stratum corneum. Int. J. Pharm. 2014, 471, 322–331. [Google Scholar] [CrossRef]
- Rajabalaya, R.; Mun, C.Y.; Chellian, J.; Chakravarthi, S.; David, S.R. Transdermal delivery of tolterodine tartrate for overactive bladder treatment: In vitro and in vivo evaluation. Acta Pharm. 2017, 67, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Chancellor, M.B.; Li, Z.; De Groat, W.C.; Yoshimura, N.; Fraser, M.O.; Huang, L. Urodynamic and Immunohistochemical Evaluation of Intravesical Capsaicin Delivery Using Thermosensitive Hydrogel and Liposomes. J. Urol. 2004, 171, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Tyagi, P.; Huang, C.-C.; Yoshimura, N.; Wu, M.; Kaufman, J.; Chancellor, M.B. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J. Urol. 2009, 182, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Hopmann, C.; Kaltbeitzel, D.; Kauth, T.; Dittrich, B.; Grosse, J.; Huppertz, N.; Schwantes, U.; Neumeister, C.; von Walter, M.; Hemateq, A.G. Degradation of Microcellular PLGA-PEG Copolymer for Use in a Drug Delivery System for the Urinary Bladder. Plast Eng. 2015, 71, 60–64. [Google Scholar] [CrossRef]
- Haupt, M.; Thommes, M.; Heidenreich, A.; Breitkreutz, J. Lipid-based intravesical drug delivery systems with controlled release of trospium chloride for the urinary bladder. J. Control. Release 2013, 170, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tuğcu-Demiröz, F.; Acartürk, F.; Erdoğan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sui, C.; Teng, L.; Liu, X.; Teng, L.; Meng, Q.; Li, Y. Studies on the preparation, characterization and pharmacological evaluation of tolterodine PLGA microspheres. Int. J. Pharm. 2010, 397, 44–49. [Google Scholar] [CrossRef]
- Ploen, J.; Andersch, J.; Heschel, M.; Leopold, C.S. Citric acid as a pH-modifying additive in an extended release pellet formulation containing a weakly basic drug. Drug Dev. Ind. Pharm. 2009, 35, 1210–1218. [Google Scholar] [CrossRef]
- Pradhan, R.; Kim, Y.-I.; Chang, S.W.; Kim, J.O. Preparation and evaluation of once-daily sustained-release coated tablets of toltero-dine-L-tartrate. Int. J. Pharm. 2014, 460, 205–211. [Google Scholar] [CrossRef]
- Patil, V.; Belsare, D. Development and evaluation of novel drug delivery system of tolterodine tartrate. Int. J. Appl. Pharm. 2017, 9, 29. [Google Scholar] [CrossRef]
- Naik, S.; Venkateswarlu, K.; Chandrasekhar, K. Formulation and evaluation of oxybutynin chloride extended release matrix tablets. Indo. Am. J. Pharm. Res. 2016, 6, 4179–4184. [Google Scholar]
- Sudarsan, G.V.; Reddy, T.V.B. Formulation and evaluation of darifenacin hydrobromide extended release formulation using res-ervoir drug delivery system. J. Glob. Trends Pharm. 2014, 5, 1699–1705. [Google Scholar]
- Sreeharsha, N.; Shariff, A.; Shendkar, Y.A.; Al-Dhubiab, B.E.; Meravanige, G. Development and Evaluation of a (SEDDS) Self—Emulsifying Drug Delivery System for Darifenacin Hydrobromide. Indian J. Pharm. Educ. Res. 2019, 53, s204–s212. [Google Scholar] [CrossRef] [Green Version]
- Sonvico, F.; Conti, C.; Colombo, G.; Buttini, F.; Colombo, P.; Bettini, R.; Barchielli, M.; Leoni, B.; Loprete, L.; Rossi, A. Multi-kinetics and site-specific release of gabapentin and flurbiprofen from oral fixed-dose combination: In vitro release and in vivo food effect. J. Control. Release 2017, 262, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.K.; Rajab, N.A.; Hussein, A.A. Formulation and in-vitro evaluation of darifenacin hydrobromide as buccal films. Iraqi J. Pharm. Sci. 2019, 28, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Waller, D.G.; Sampson, A.P. 15—Disorders of micturition. In Medical Pharmacology and Therapeutics, 5th ed.; Waller, D.G., Sampson, A.P., Eds.; Elsevier: Oxford, UK, 2018; pp. 231–237. [Google Scholar]
- Yarker, Y.E.; Goa, K.L.; Fitton, A. Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging 1995, 6, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Davila, G.W.; Starkman, J.S.; Dmochowski, R.R. Transdermal Oxybutynin for Overactive Bladder. Urol. Clin. N. Am. 2006, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Chapple, C.R. Oxybutynin and the overactive bladder. World J. Urol. 2001, 19, 319–323. [Google Scholar] [CrossRef]
- Salinas-Casado, J.; Esteban-Fuertes, M.; Serrano, O.; Galván, J. The value of oxybutynin in transdermal patches for treating overactive bladder. Actas Urológicas Españolas 2015, 39, 599–604. [Google Scholar] [CrossRef]
- Dmochowski, R. Improving the Tolerability of Anticholinergic Agents in the Treatment of Overactive Bladder. Drug Saf. 2005, 28, 583–600. [Google Scholar] [CrossRef]
- Dmochowski, R.R.; Sand, P.K.; Zinner, N.R.; Gittelman, M.C.; Davila, G.W.; Sanders, S.W. Transdermal Oxybutynin Study Groupet. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology 2003, 62, 237–242. [Google Scholar] [CrossRef]
- Kennelly, M.J. A comparative review of oxybutynin chloride formulations: Pharmacokinetics and therapeutic efficacy in overactive bladder. Rev. Urol. 2010, 12, 12. [Google Scholar]
- Slotoroff, C.B.; Shupp-Byrne, D.; Shenot, P.J. Intravesical Treatments for Overactive Bladder. In Female Urology; Humana Press: Totowa, NJ, USA, 2007; pp. 201–212. [Google Scholar]
- Zargar, H.; Aning, J.; Ischia, J.; So, A.; Black, P. Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat. Rev. Urol. 2014, 11, 220–230. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Liu, H.-T.; Chuang, Y.-C.; Birder, L.A.; Chancellor, M.B. Pilot Study of Liposome-encapsulated OnabotulinumtoxinA for Patients with Overactive Bladder: A Single-center Study. Eur. Urol. 2014, 65, 1117–1124. [Google Scholar] [CrossRef]
- Parkin, J.; Shea, C.; Sant, G.R. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis—A practical approach. Urology 1997, 49 (Suppl. 5A), 105–107. [Google Scholar] [CrossRef]
- Eldrup, J.; Thorup, J.; Nielsen, S.L.; Hald, T.; Hainau, B. Permeability and Ultrastructure of Human Bladder Epithelium. BJU Int. 1983, 55, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Hensley, H.; Jacobs, J.; Anthony, M.; Tyagi, V.; Tyagi, P. 1626 non-invasive imaging of near infrafred dye labeled liposomes facilitates evaluation of bioresidence time. J. Urol. 2010, 183, e628. [Google Scholar] [CrossRef]
- Chancellor, M.B.; De Groat, W.C. Intravesical Capsaicin and Resiniferatoxin Therapy: Spicing Up the Ways to Treat The overactive Bladder. J. Urol. 1999, 162, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Giannantoni, A.; Di Stasi, S.M.; Stephen, R.L.; Navarra, P.; Scivoletto, G.; Mearini, E.; Porena, M. Intravesical capsaicin versus resiniferatoxin in patients with detrusor hyperreflexia: A prospective randomized study. J. Urol. 2002, 167, 1710–1714. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.S.; Das, A.; Sedor, J.; Huang, B.; Rivas, D.A.; Flood, H.J. Effect of intravesical capsaicin and vehicle on bladder integrity in control and spinal cord injured rats. J. Urol. 1998, 159, 1074–1078. [Google Scholar] [CrossRef]
- Levin, R.M.; Whitbeck, C.; Borow, A.; Burden, O.; Leggett, R.E. Effectiveness of vaginally administered oxybutynin on rabbit bladder function. Urology 2003, 61, 1273–1277. [Google Scholar] [CrossRef]
- Malcolm, K.; Woolfson, D.; Russell, J.; Tallon, P.; McAuley, L.; Craig, D. Influence of silicone elastomer solubility and diffusivity on the in vitro release of drugs from intravaginal rings. J. Control. Release 2003, 90, 217–225. [Google Scholar] [CrossRef]
- Schröder, A.; Levin, R.M.; Kogan, B.A.; Das, A.K.; Kay, F.; Mahashabde, A. Absorption of oxybutynin from vaginal inserts: Drug blood levels and the response of the rabbit bladder. Urology 2000, 56, 1063–1067. [Google Scholar] [CrossRef]
- Woolfson, A.; Malcolm, R.; Gallagher, R. Design of a silicone reservoir intravaginal ring for the delivery of oxybutynin. J. Control Release 2003, 91, 465–476. [Google Scholar] [CrossRef]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vermani, K.; Garg, S. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 2000, 3, 359–364. [Google Scholar] [CrossRef]
- Kast, C.E.; Valenta, C.; Leopold, M.; Bernkop-Schnürch, A. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J. Control. Release 2002, 81, 347–354. [Google Scholar] [CrossRef]
- Greenbatt, D. Intramuscular injection of drugs. N. Eng. J. Med. 1976, 295, 542–546. [Google Scholar]
- Edlund, U.; Albertsson, A.C. Degradable polymer microspheres for controlled drug delivery. In Degradable Aliphatic Polyesters; Springer: New York, NY, USA, 2002; pp. 67–112. [Google Scholar]
- Zolnik, B.S.; Burgess, D.J. Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres. J. Control. Release 2008, 127, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Sakamura, Y.; Horikiri, Y.; Suzuki, T.; Yoshino, H. Evaluation of in vivo release characteristics of protein-loaded bio-degradable microspheres in rats and severe combined immunodeficiency disease mice. J. Control Release 2001, 73, 213–221. [Google Scholar] [CrossRef]
- Leonardi, A.; Guarneri, L.; Angelico, P. Alpha-2-Delta Ligand/Nsaid Therapeutic Treatment of Lower Urinary Tract Disorders. US Patent Application No. 11/962,552, 3 July 2008. [Google Scholar]




| Study ID | Drug | Drug Delivery Route | Type of Investigation | Excipients | Study Characteristics | Reference |
|---|---|---|---|---|---|---|
| Nicoli et al., 2006 | Oxybutynin | Transdermal | In vitro | PVA, sorbitol | The developed oxybutynin films showed good permeation characteristics across rabbit skin, oxybutynin permeation increased in a linear way for up to 7 h and 50% of the drug permeation was achieved after 24 h. | [44] |
| Banu et al., 2010 | Oxybutynin | Transdermal | In vitro | Ethyl cellulose, Carbopol-934P and PG | Films containing 2% Carbopol-934P and 30% PG showed 87.28% drug release across rat abdominal skin whereas drug release from formulation containing 2% of ethyl cellulose: Carbopol-934P (1:3) and 30% PG was 88.32%. | [45] |
| Bakshi et al., 2008 | Oxybutynin | Transdermal | In vitro | Lutrol F-127, Carbopol-940, myristyl lactate and glyceryl monooleate | The droplet size delivered from the formulations was within the range of 5–50 µm. The drug dose delivered per actuation of the pump was within the range of 101–106% by each formulation. Permeation studies were carried out on rabbit ear skin and showed that drug release was within the range of 45–50% during the period of 24 h. | [46] |
| Rajabalaya et al., 2016 | Oxybutynin chloride | Transdermal | In vitro and in vivo (rat model) | Span (S20, S40, and S60) and Tween (T20 and T80), cholesterol and glycerol distearate, isopropyl alcohol | All the formulations showed more than 87% entrapment efficiency which tended to increase with increase in surfactant. In vitro permeation studies determined that percent cumulative permeation after 8 h was higher for gels containing Span than gels containing Tween. | [47] |
| Wang et al., 2018 | Oxybutynin | Transdermal | In vitro and in vivo (rat model) | Acrylic adhesives, span 80 | The patch with AACONH2 functional group acrylic adhesive had the highest (763.5 ± 58.8 μg/cm2) oxybutynin cumulative levels during in vitro skin permeation study. The relative bioavailability of the developed patches was 97% in rats. | [48] |
| Pandit et al., 2009 | Tolterodine tartrate | Transdermal | In vitro | Ethyl cellulose, Carbopol-934P, Hypromellose and PG | Different concentrations of polymers and PG were investigated. Combination of cabopol-934P: hypromellose (1:3) with 30% PG was effective in producing films of high endurance flexibility and with uniform drug content. Permeation study showed that there was 68.72% drug release across rat abdominal skin and 81.12% release across cellophane membrane for 12 h. | [49] |
| Sun et al., 2013 | Tolterodine | Transdermal | In vitro and in vivo (rabbit model) | Carbopol 980, N-methyl pyrrolidone | The formulation showed permeation rate of 770.19 µg cm−2 h−1 during in vitro percutaneous permeation experiment. The absolute bioavailability was 11.47% during pharmacokinetic studies. | [50] |
| W. Liu et al., 2017 | 5-hydroxymethyl tolterodine | Transdermal | In vitro and in vivo (rat model) | Carbopol 934, 940 and 980, | The formulation showed 20.7% absolute bioavailability during in vivo studies and no skin irritation was observed during skin irritation study. | [51] |
| X. Liu et al., 2014 | Tolterodine | Transdermal | In vitro and in vivo (rat model) | Tween 80, Hypromellose, HPC, Carbopol 980 | The formulation showed 86.02% cumulative drug release rate in 24 h. The flux of tolterodine from the formulation was 81.82, 37.15, 18.55 and 15.83 µg cm−2 h−1, across subcutaneous tissue, dermis, epidermis and full rat skin, respectively. | [52] |
| Rajabalaya et al., 2017 | Tolterodine tartrate | Transdermal | In vitro and in vivo (rat model) | Eudragit (E 100, RSPO and RLPO), dibutyl sebacate (DBS), dibutyl phthalate (DBP) and triethyl citrate (TEC) and polyvinyl pyrrolidone (PVP) | Drug loaded formulations (i) E100, PVP, and DBS and (ii) RSPO, RLPO, PVP and DBS showed highest percent cumulative permeation and permeation rate during in vitro permeation studies | [53] |
| Tyagi et al., 2004 | Capsaicin | Intravesical | - | Phosphatidylcholine, Cholesterol, PEG-PLGA-PEG, Saline solution with 30% ethanol | Three types of formulations, liposomes, hydrogel and 30% ethanolic solution, were developed. The results showed that the liposomes and 30% ethanolic solution completely blocked the micturition reflexes after intravesical administration. However, hydrogel of capsaicin was not successful in blocking the micturition reflexes completely but there was significant decrease in bladder contractions | [54] |
| Chuang et al., 2009 | Botulinum toxin A | Intravesical | In vivo (rat model) | l-α-phosphatidylcholine and cholesterol | Intravesical delivery of lipotoxin (botulinum toxin A encapsulated in liposomes) was investigated to evaluate the effect of lipotoxin on bladder hyperactivity. The results showed that intercontraction interval was 21.1%. | [55] |
| Hopmann et al., 2015 | Barium sulphate | Intravesical | In vitro | PLGA-PEG, polydimethylsiloxane | Samples were incubated in artificial urine for 27 days and CESP parameters including temperature, pressure and pressure release gradient were measured. The spheres and pills at constant temperature of 65 °C and pressure of 50 bar were totally dissolved in 27 days with pressure release gradient of 5 and 20 bar/min, respectively. | [56] |
| Haupt et al., 2013 | Trospium chloride | Intravesical | In vitro | Glyceryl tristearate, magnesium stearate | Extrudates and mini-tablets released drug for more than 5 days. The drug release from mini-moulds was very low or negligible and it was concluded that lipids provides good matrix for highly soluble drugs and with drug loading of only 30% the drug was released from several days up to weeks. | [57] |
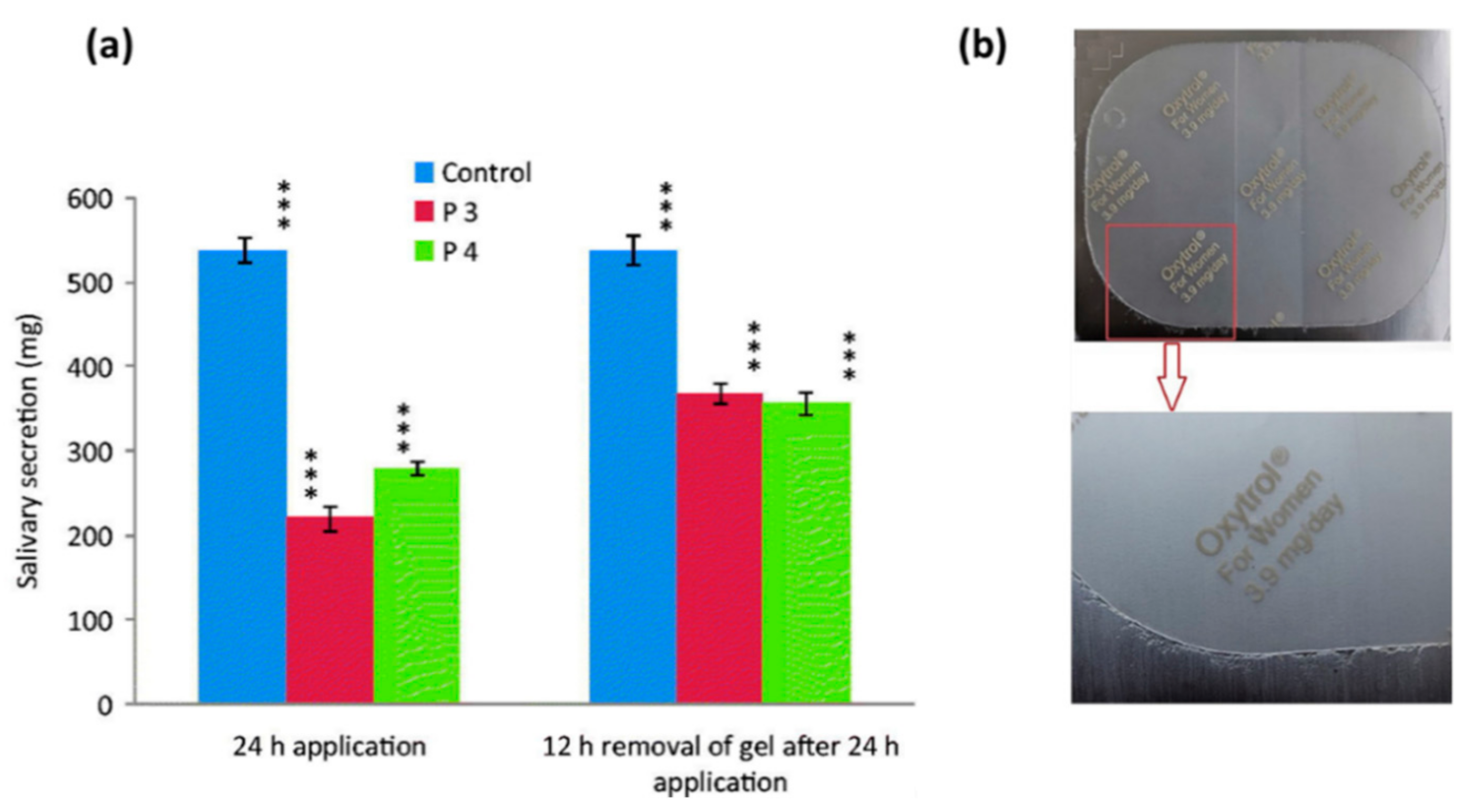
| Tuğcu-Demiröz et al., 2013 | Oxybutynin | Vaginal | In vitro and in vivo (rabbit model) | Chitosan, Hypromellose (K100M) and Poloxamer 407 (Pluronic F 127). | The hypromellose K100M formulation resulted in suitable permeation characteristics across the vaginal mucosa and also resulted in highest relative bioavailability and AUC during in vivo studies. | [58] |
| Sun et al., 2010 | Tolterodine | Intramuscular | In vivo (rat model) | PLGA, palmitic acid, stearic acid | Drug entrapment efficiency was increased upon adding palmitic or stearic acid. The formulation was administered intramuscularly to beagle s. A sustained release following an initial burst was observed for 18 days. | [59] |
| Ploen et al., 2009 | Propiverine hydrochloride | Oral | In vitro | Citric acid, Eudragit | At higher coating levels e drug release and citric acid release was reduced. The pellets extended the drug release for more than 16 h. | [60] |
| Pradhan et al., 2014 | Tolterodine-l-tartrate | Oral | In vitro and in vivo (human volunteers) | Hypromellose 2208 and hypromellose 2910 | The formulation showed sustained release profile up to 10 h during in vitro dissolution testing. Similar results were obtained from in vivo results. | [61] |
| Patil et al., 2017 | Tolterodine tartrate | Oral | In vitro | Mannitol, hypromellose | The in vitro dissolution profile of extended release capsule was similar to Detrol LA and resulted in more than 85% release during the period of 12 h. | [62] |
| Naik et al., 2016 | Oxybutynin chloride | Oral | In vitro | Hypromellose K4M, K100M, Carbopol, ethyl cellulose, PVP, sodium alginate | The formulation containing Hypromellose K4M along with ethyl cellulose was an optimised formulation that showed controlled drug release for period of 24 h and resulted in cumulative release of 95.59% of drug release. The formulation followed first order kinetics. | [63] |
| Sudarsan et al., 2014 | Darifenacin hydrobromide | Oral | In vitro | Ethyl cellulose, povidone, magnesium stearate | Formulation with highest level of ethyl cellulose coating was an optimised formulation as the results were satisfactory with regards to all parameters and drug release profile was similar to the marketed product. | [64] |
| SreeHarsha et al., 2019 | Darifenacin | Oral | In vitro | Surfactant (Labrafil 1944 CS) and co-surfactant (polyethylene glycol 400) and peanut oil | SEDDS were developed using surfactant, co-surfactant and peanut oil. The average globule size of SEDDS was less than 200 nm and depicted negative zeta potential. The rate of dissolution of the developed formulations was also increased upon comparison with pure darifenacin. | [65] |
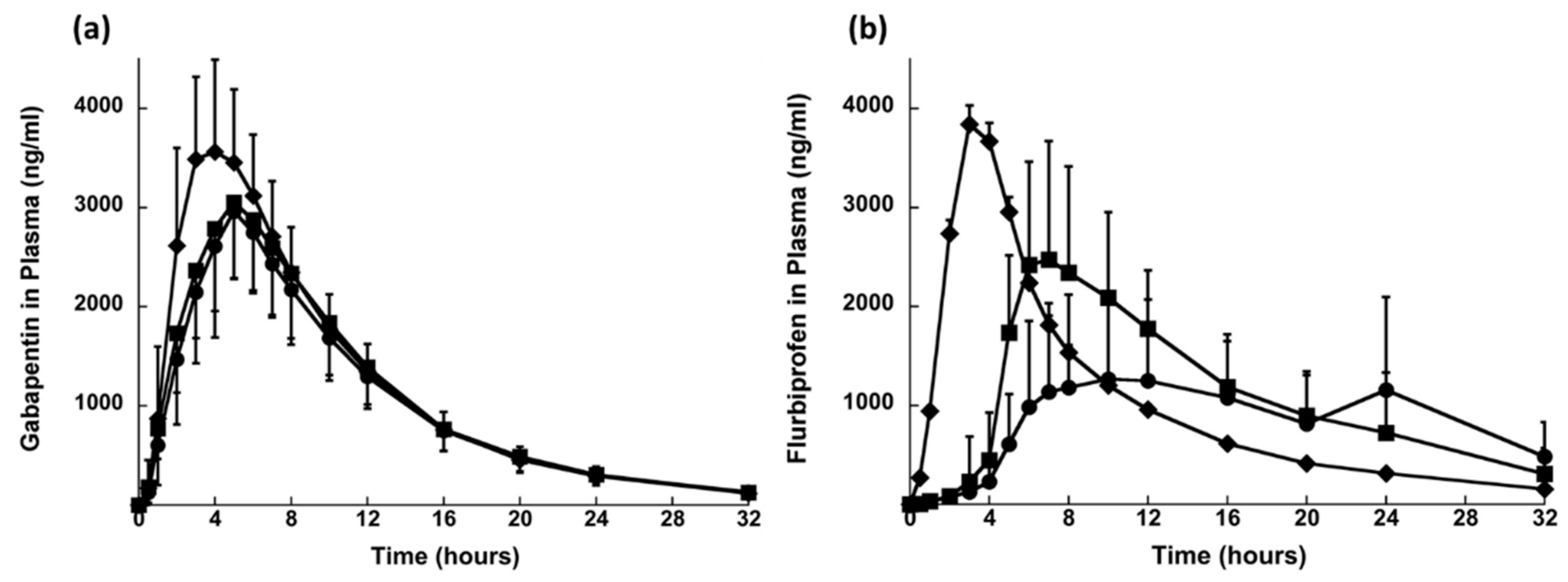
| Sonvico et al., 2017 | Gabapentin and flurbiprofen | Oral | In vitro and in vivo (human volunteers) | Hypromellose K15M, PEO, PVP K30, Mannitol, sodium croscarmellose, sodium alginate, B-cyclodextrin | This was a tri-layered formulation and, during in vitro dissolution testing, layer B disintegrated in few minutes splitting the layer A and C eventually. Layer A started to float and layer B sank down in the bottom. Layer A floated for about 7 h and for layer C there was no flurbiprofen release in the first 60 min. After transferring the layer C to pH 7.2 medium, accelerated dissolution was observed | [66] |
| Abbas et al., 2019 | Darifenacin hydrobromide | Buccal | In vitro | PVA, Tween 80, croscarmellose sodium, sodium starch glycolate, indion 414 | The formulation containing 4% w/w indion 414, 30% w/w glycerol, 2% w/v PVA, 0.5% w/v tween 80 and 7.5 mg of darifenacin hydrobromide was an optimum formulation by showing shortest disintegration time of 31.28 s. | [67] |
| Drug | Formulation Type | Company Name | Marketed Name |
|---|---|---|---|
| Oxybutynin hydrochloride | Tablet | Alliance Healthcare Ltd. | Oxybutynin 2.5, 3 and 5 mg tablets |
| Modified-release tablet | Janssen-Cilag Ltd. | Lyrinel XL | |
| Oral solution | Brillpharma Ltd. | Oxybutynin 2.5 mg/5 mL and 5 mg/5 mL oral solution | |
| Transdermal patch | Orion Pharma (UK) Ltd. | Kentera 3.9 mg/24 h patches | |
| Tolterodine tartrate | Tablet | Sandoz Ltd. | Tolterodine 1 mg tablets |
| Modified-release capsule | Aspire Pharma Ltd. | Neditol XL | |
| Capsaicin | Cream | Teva UK Ltd. | Zacin 0.025% cream |
| Axsain 0.075% cream | |||
| Cutaneous patch | Grunenthal Ltd. | Qutenza 179 mg | |
| Botulinum toxin type A | Powder for solution for injection | Allergan Ltd. | Botox 50, 100 and 200 unit powder for solution for injection vials |
| Galderma (UK) Ltd. | Azzalure 125 unit powder for solution for injection vials | ||
| Ipsen Ltd. | Dysport 300 and 500 unit powder for solution for injection vials | ||
| Trospium chloride | Tablet | Galen Ltd. | Flotros 20 mg tablets |
| Modified-release capsule | Contura Ltd. | Regurin XL 60 mg capsules | |
| Darifenacin hydrobromide | Modified-release tablet | Norgine Pharmaceuticals Ltd. | Emselex 7.5 and 15 mg modified-release tablets |
| Propiverine hydrochloride | Tablet | Advanz Pharma | Detrunorm 15 mg tablets |
| Modified-release capsule | Advanz Pharma | Detrunorm XL 30 and 45 mg capsules | |
| Gabapentin | Tablet | A A H Pharmaceuticals Ltd. | Gabapentin 600 mg tablets |
| Capsule | Accord Healthcare Ltd. | Gabapentin 100, 300 and 400 mg capsules | |
| Oral solution | A A H Pharmaceuticals Ltd. | Gabapentin 50 mg/mL oral solution sugar free | |
| Imported (United States) | Neurontin 250 mg/5 mL oral solution | ||
| Flurbiprofen | Tablet | Mylan | Flurbiprofen 50 and 100 mg tablets |
| Lozenge | Reckitt Benckiser Healthcare (UK) Ltd. | Strefen Honey and Lemon 8.75 mg lozenges |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khizer, Z.; Sadia, A.; Sharma, R.; Farhaj, S.; Nirwan, J.S.; Kakadia, P.G.; Hussain, T.; Yousaf, A.M.; Shahzad, Y.; Conway, B.R.; et al. Drug Delivery Approaches for Managing Overactive Bladder (OAB): A Systematic Review. Pharmaceuticals 2021, 14, 409. https://doi.org/10.3390/ph14050409
Khizer Z, Sadia A, Sharma R, Farhaj S, Nirwan JS, Kakadia PG, Hussain T, Yousaf AM, Shahzad Y, Conway BR, et al. Drug Delivery Approaches for Managing Overactive Bladder (OAB): A Systematic Review. Pharmaceuticals. 2021; 14(5):409. https://doi.org/10.3390/ph14050409
Chicago/Turabian StyleKhizer, Zara, Amina Sadia, Raman Sharma, Samia Farhaj, Jorabar Singh Nirwan, Pratibha G. Kakadia, Talib Hussain, Abid Mehmood Yousaf, Yasser Shahzad, Barbara R. Conway, and et al. 2021. "Drug Delivery Approaches for Managing Overactive Bladder (OAB): A Systematic Review" Pharmaceuticals 14, no. 5: 409. https://doi.org/10.3390/ph14050409







