In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry
Abstract
:1. Introduction
2. Results
2.1. BP Characterization Assay
2.1.1. BP Lytic Spectrum
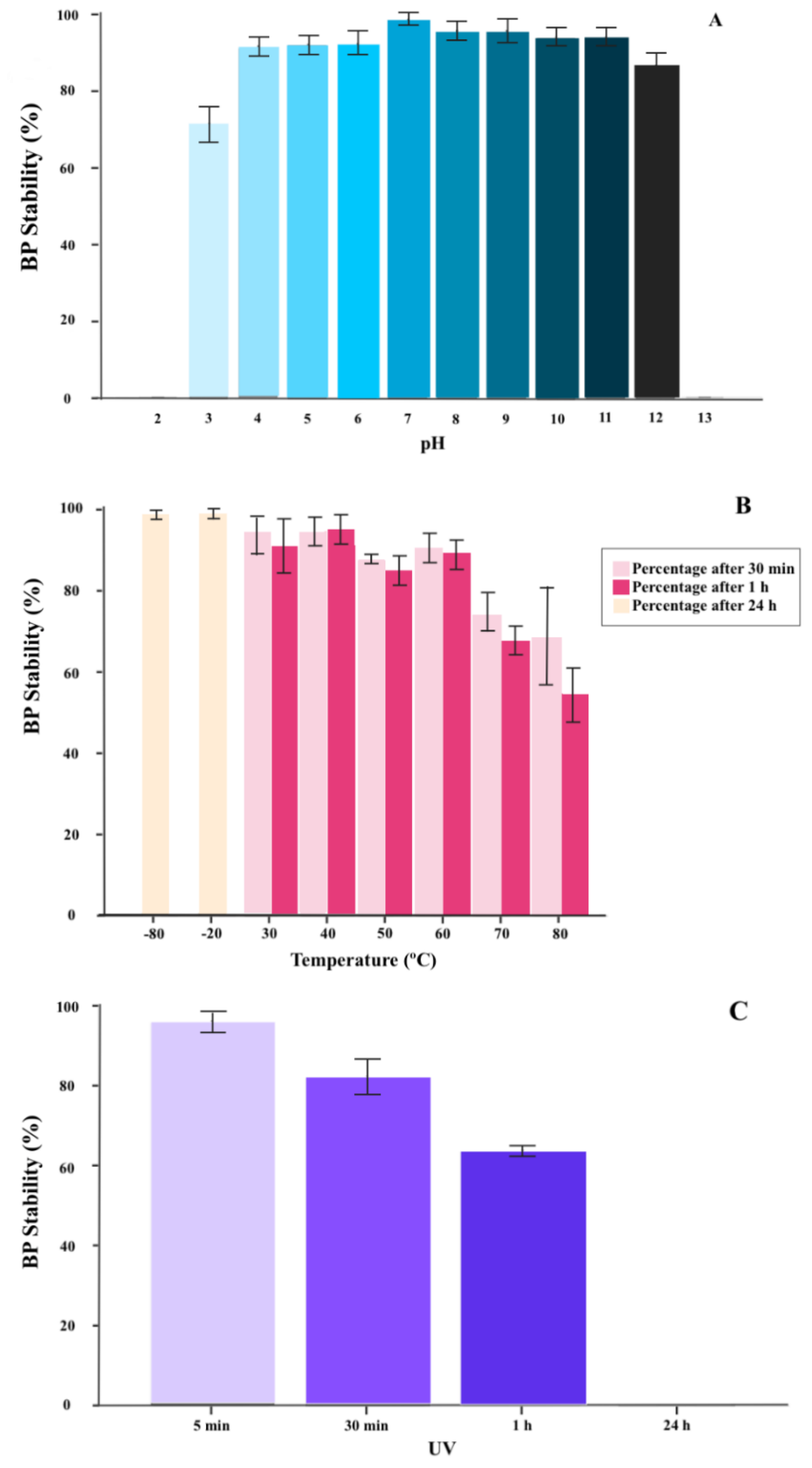
2.1.2. BP Thermal and pH Stability
2.1.3. BP Inactivation with UV Radiation
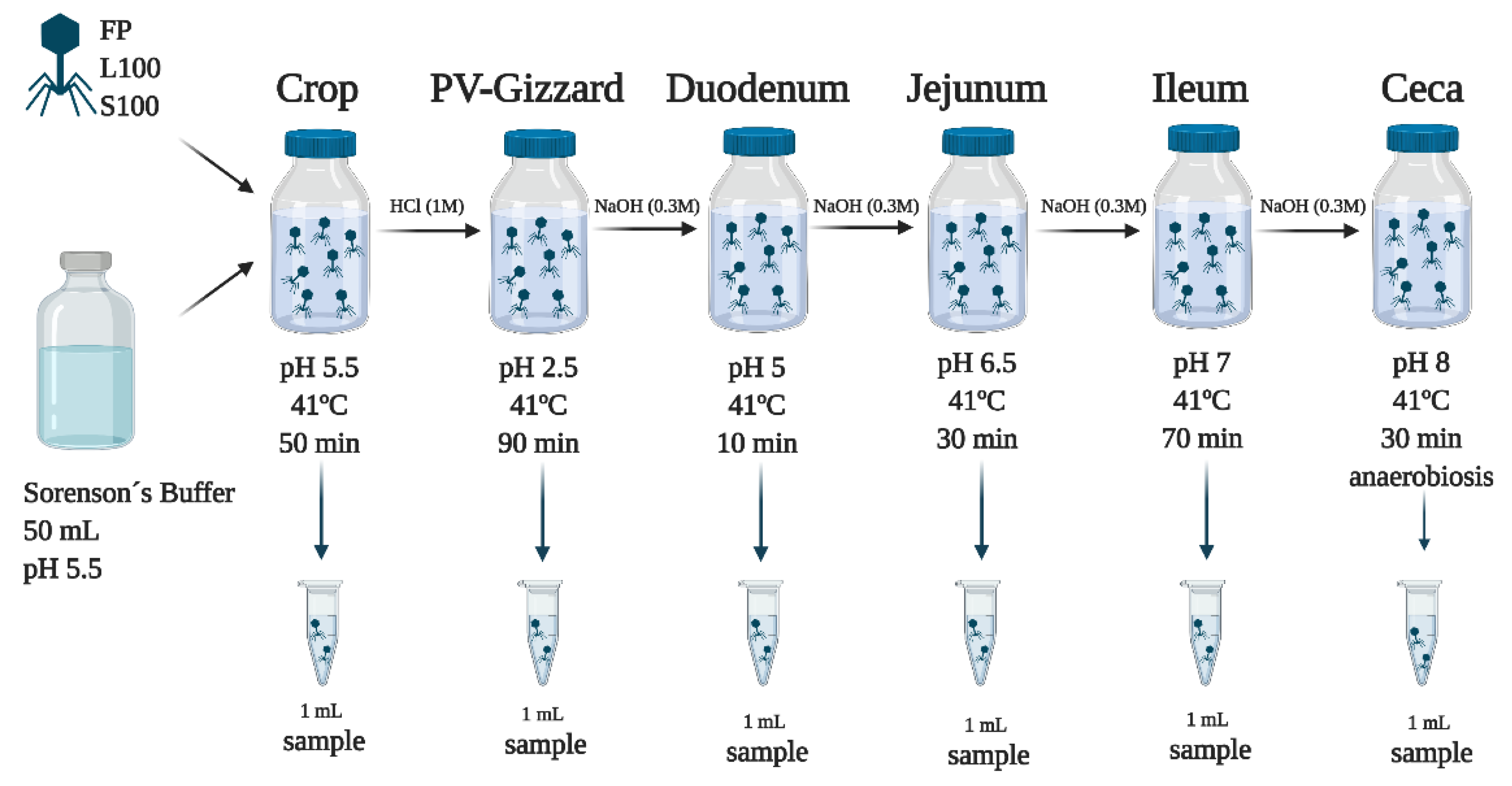
2.2. In Vitro Evaluation of the Release of BP under Different GIT Conditions (Experiment 1)
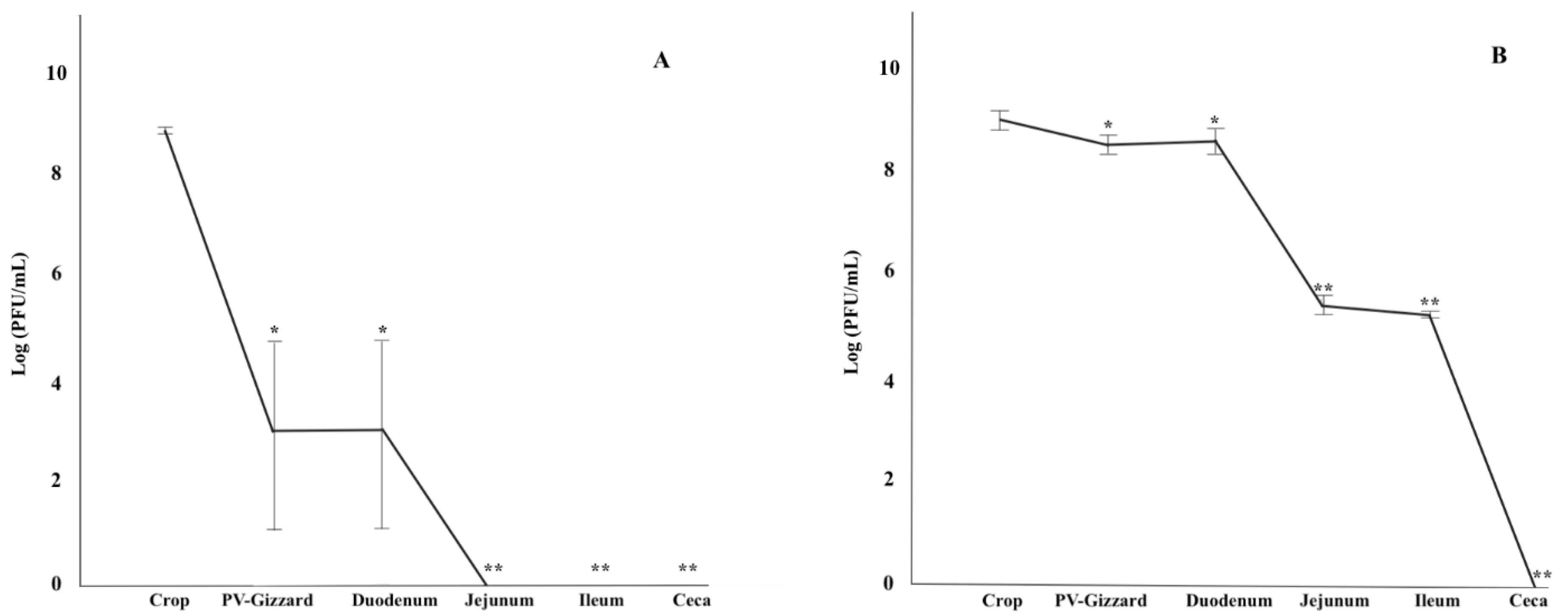
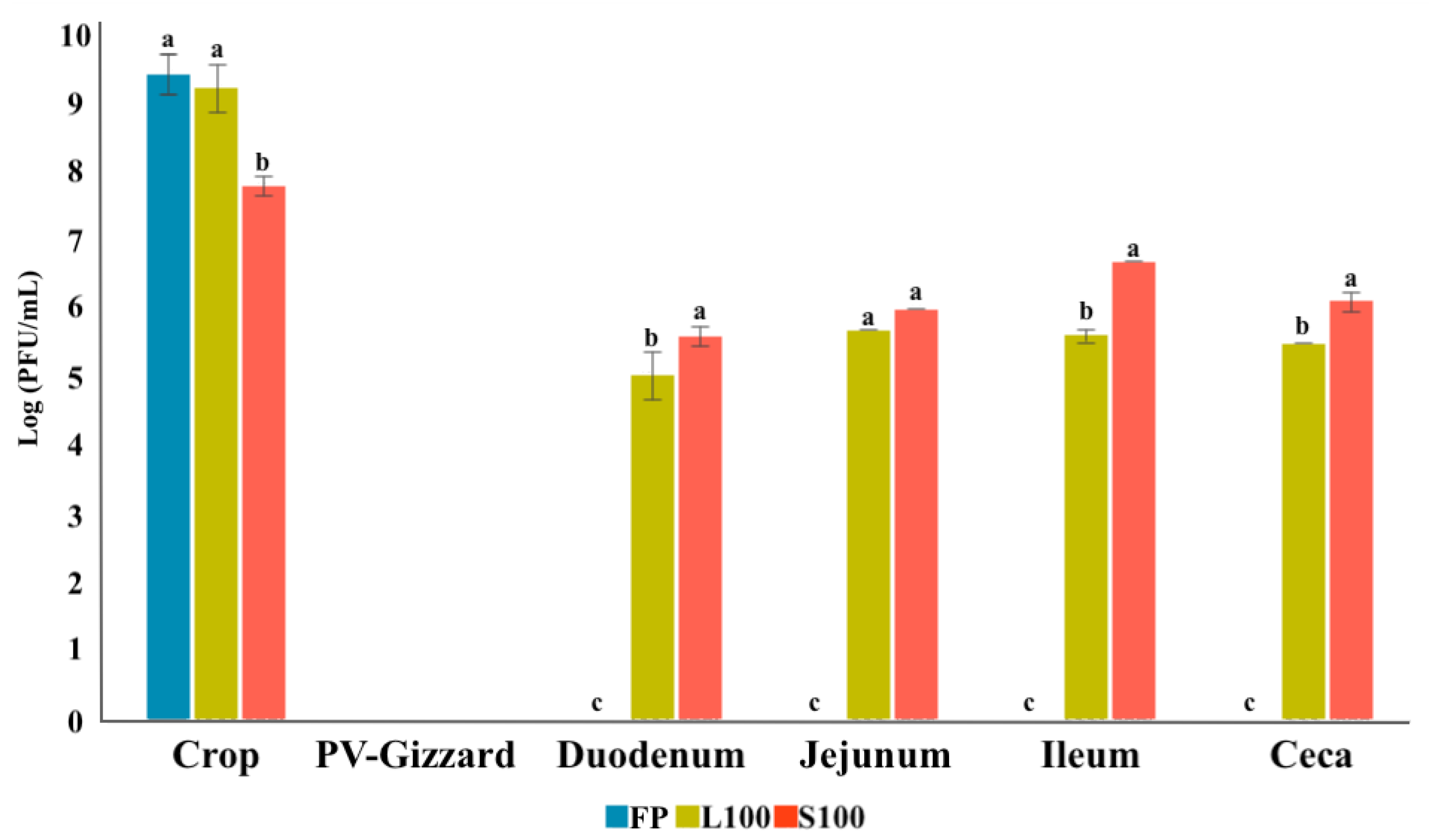
2.3. In Vitro Evaluation of BP Titres along the GIT (Experiment 2)
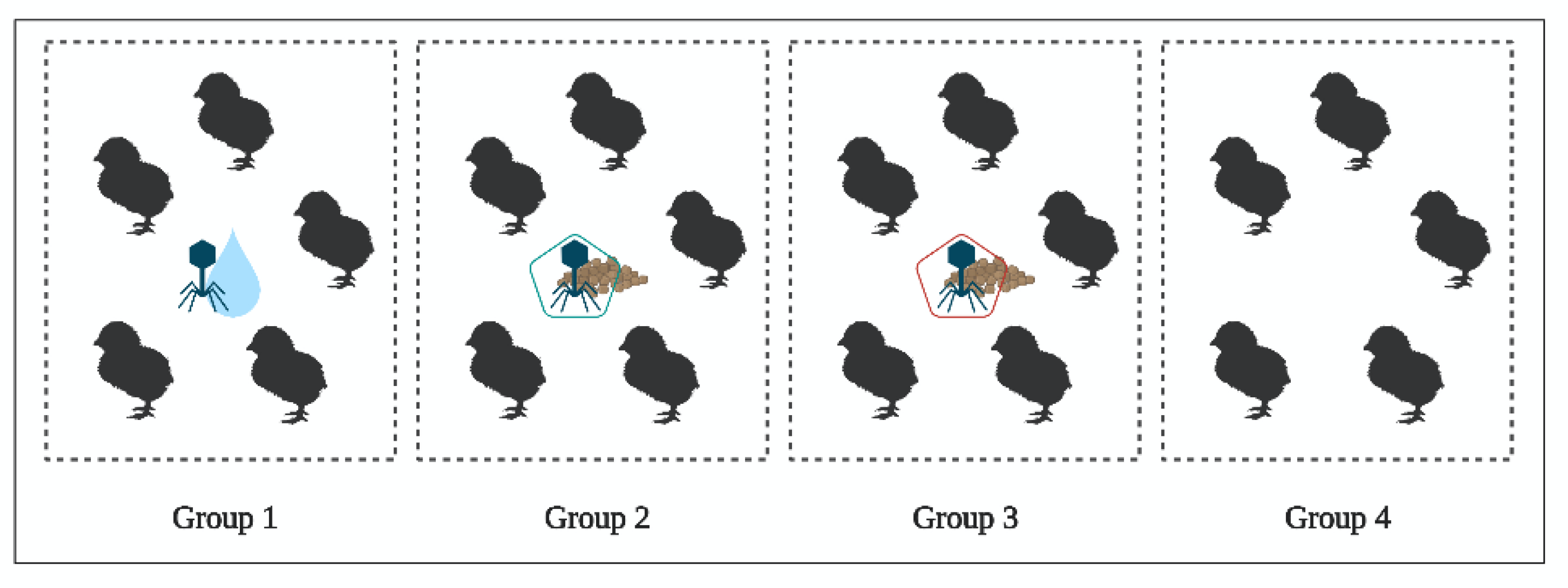
2.4. In Vivo Study of BP Survival along GIT (Experiment 3)
3. Discussion
4. Materials and Methods
4.1. BP Characterization Assay
4.1.1. BP Lytic Spectrum
4.1.2. BP Thermal and pH Stability
4.1.3. BP Inactivation with UV Radiation
4.1.4. BP Encapsulation
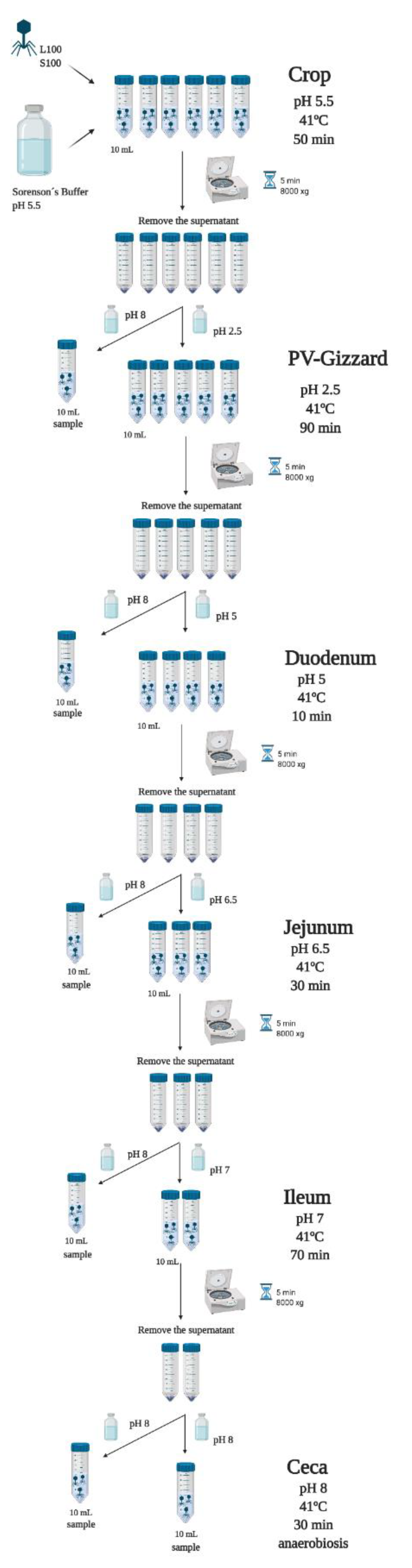
4.2. In Vitro Evaluation of the Release of BP under Different GIT Conditions (Experiment 1)
4.3. In Vitro Evaluation of BP Titres along the GIT (Experiment 2)
4.4. In Vivo Study of BP Survival along GIT (Experiment 3)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases; WHO: Geneva, Switzerland, 2015; pp. 1–16. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf;jsessionid=F5D0B289A1506C32255D8921C9119A5B?sequence=1 (accessed on 25 February 2021).
- WHO. 23 Million People Falling Ill from Unsafe Food Each Year in Europe is just the Tip of the Iceberg. Available online: https://www.who.int/news-room/detail/05-06-2019-23-million-people-falling-ill-from-unsafe-food-each-year-in-europe-is-just-the-tip-of-the-iceberg (accessed on 22 June 2020).
- EFSA. ECDC The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- EFSA; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Microbiology and food safety: Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef] [PubMed]
- EC. Regulation (EC) No 2160/2003 of the European Parlament and of the council of 17 November 2003 on the control of salmonella and other specified food-borne zoonotic agents. Off. J. 2003, L325, 1–15. [Google Scholar]
- Vandeplas, S.; Dubois Dauphin, R.; Beckers, Y.; Thonart, P.; Théwis, A. Salmonella in chicken: Current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010, 73, 774–785. [Google Scholar] [CrossRef]
- EFSA. ECDC The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA J. 2017, 15, 4694. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.; Wales, A. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef] [Green Version]
- Carrique-Mas, J.J.; Marín, C.; Breslin, M.; McLaren, I.; Davies, R. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian. Pathol. 2009, 38, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.K.; Jalukar, S.; Brake, J. The effect of refined functional carbohydrates from enzymatically hydrolyzed yeast on the transmission of environmental Salmonella Senftenberg among broilers and proliferation in broiler housing. Poult. Sci. 2018, 97, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Lainez, M. Salmonella detection in feces during broiler rearing and after live transport to the slaughterhouse. Poult. Sci. 2009, 88, 1999–2005. [Google Scholar] [CrossRef]
- Berry, E.; Wells, J. Reducing Foodborne pathogen persistence and transmission in animal production environments: Challenges and opportunities. Microbiol Spectr. 2016, 4, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Barrow, P.A.; Bumstead, N.; Marston, K.; Lovell, M.A.; Wigley, P. Faecal shedding and intestinal colonization of Salmonella enterica in in-bred chickens: The effect of host-genetic background. Epidemiol. Infect. 2004, 132, 117–126. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosby, D.E.; Cox, N.A.; Harrison, M.A.; Wilson, J.L.; Jeff Buhr, R.; Fedorka-Cray, P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015, 24, 408–426. [Google Scholar] [CrossRef]
- Shah, D.H.; Elder, J.R.; Chiok, K.L.; Paul, N.C. Genetic Basis of Salmonella Enteritidis Pathogenesis in Chickens. In Producing Safe Eggs: Microbial Ecology of Salmonella; Ricke, S.C., Gast, R.K., Eds.; Elsevier Inc.: London, UK, 2017; pp. 187–208. ISBN 9780128025826. [Google Scholar]
- Nair, D.V.T.; Johny, A.K. Salmonella in poultry meat production. In Food Safety in Poultry Meat Production; Venkitanarayanan, K., Thakur, S., Ricke, S.C., Eds.; Springer Nature: Gewerbestrasse, Switzerland, 2019; pp. 1–25. ISBN 978-3-030-05010-8. [Google Scholar]
- Ravindran, V. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 2013, 22, 628–636. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Navarro, S.; Marín, C.; Cortés, V.; García, C.; Vega, S.; Catalá-Gregori, P. Autophage as a control measure for Salmonella in laying hens. Poult. Sci. 2018, 97, 4367–4373. [Google Scholar] [CrossRef]
- Toro, H.; Price, S.B.; McKee, S.; Hoerr, F.J.; Krehling, J.; Perdue, M.; Bauermeister, L. Use of bacteriophages in combination with competitive exclusion to reduce salmonella from infected chickens. Avian Dis. 2005, 49, 118–124. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Van Bergen, M.A.P.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; De Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [Green Version]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017. [Google Scholar] [CrossRef]
- Sevilla-Navarro, S.; Catalá-Gregori, P.; García, C.; Cortés, V.; Marin, C. Salmonella infantis and salmonella enteritidis specific bacteriophages isolated form poultry faeces as a complementary tool for cleaning and disinfection against salmonella. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101405. [Google Scholar] [CrossRef] [PubMed]
- Zelasko, S.; Gorski, A.; Dabrowska, K. Delivering phage therapy per os: Benefits and barriers. Expert Rev. Anti. Infect. Ther. 2017, 15, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.; García-Rodríguez, A.; Cano-Sarabia, M.; Maspoch, D.; Marcos, R.; Cortés, P.; Llagostera, M. Biodistribution of liposome-encapsulated bacteriophages and their transcytosis during oral phage therapy. Front. Microbiol. 2019, 10, 689. [Google Scholar] [CrossRef] [Green Version]
- Romero-Calle, D.; Benevides, R.G.; Góes-Neto, A.; Billington, C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabouri, S.; Sepehrizadeh, Z.; Amirpour-Rostami, S.; Skurnik, M. A minireview on the in vitro and in vivo experiments with anti-Escherichia coli O157:H7 phages as potential biocontrol and phage therapy agents. Int. J. Food Microbiol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.; Marshall, D.L.; DePaola, A. Antacid increases survival of vibrio vulnificus and vibrio vulnificus phage in a gastrointestinal model. Appl. Environ. Microbiol. 2001, 67, 2895–2902. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.J. Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Pragya; Verma, N.; Mishra, A.K.; Nath, G.; Gaur, P.K.; Verma, A. An overview on bacteriophages: A natural nanostructured antibacterial agent. Curr. Drug Deliv. 2016, 15, 3–20. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.A.; Liu, H.; Wang, Q.; Zhong, F.; Guo, Q.; Balamurugan, S. Use of encapsulated bacteriophages to enhance farm to fork food safety. Crit. Rev. Food Sci. Nutr. 2015, 57, 2801–2810. [Google Scholar] [CrossRef]
- Ma, Y.H.; Islam, G.S.; Wu, Y.; Sabour, P.M.; Chambers, J.R.; Wang, Q.; Wu, S.X.Y.; Griffiths, M.W. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poult. Sci. 2016, 95, 2911–2920. [Google Scholar] [CrossRef]
- Vinner, G.K.; Richards, K.; Leppanen, M.; Sagona, A.P.; Malik, D.J. Microencapsulation of enteric bacteriophages in a pH-Responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 2019, 11, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowska, K. Interaction of bacteriophages with the immune system: Induction of bacteriophage-specific antibodies. In Methods in Molecular Biology; Humana Press Inc.: New York, NY, USA, 2018; Volume 1693, pp. 139–150. [Google Scholar]
- Sevilla-Navarro, S.; Catalá-Gregori, P.; Marin, C. Salmonella bacteriophage diversity according to most prevalent salmonella serovars in layer and broiler poultry farms from eastern spain. Animals 2020, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Amir Karimi Torshizi, M.; Rahimi, S.; Dennehy, J.J. Prophylactic bacteriophage administration more effective than post-infection administration in reducing Salmonella enterica serovar enteritidis shedding in Quail. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, P.A.; Cosby, D.E.; Cox, N.A.; Lee, J.H.; Kim, W.K. Effect of dietary bacteriophage supplementation on internal organs, fecal excretion, and ileal immune response in laying hens challenged by Salmonella Enteritidis. Poult. Sci. 2017, 96, 3264–3271. [Google Scholar] [CrossRef]
- Nabil, N.M.; Tawakol, M.M.; Hassan, H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018, 8. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [Green Version]
- Bull, J.J.; Gill, J.J. The habits of highly effective phages: Population dynamics as a framework for identifying therapeutic phages. Front. Microbiol. 2014, 5, 618. [Google Scholar] [CrossRef] [Green Version]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.W.; Shaw, N.M. Factors Influencing the Survival and Mu1 Tiplica tion of Bacteriophages in Calves and in Their Environment. Microbiology 1987, 133, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Chibani-Chennoufi, S.; Sidoti, J.; Bruttin, A.; Kutter, E.; Sarker, S.; Brüssow, H. In vitro and in vivo bacteriolytic activities of Escherichia coli phages: Implications for phage therapy. Antimicrob. Agents Chemother. 2004, 48, 2558–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of bacteriophage felix o1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; An, F.; Zou, M.-J.; Sun, J.; Hao, X.-H.; He, Y.-X. Time-and pH-dependent colon-specific drug delivery for orally administered diclofenac sodium and 5-aminosalicylic acid. World J. Gastroenterol. World J. Gastroenterol. 2004, 10, 1769–1774. [Google Scholar] [CrossRef]
- Asghar, L.F.A.; Chure, C.B.; Chandran, S. Colon specific delivery of indomethacin: Effect of incorporating pH sensitive polymers in xanthan gum matrix bases. AAPS Pharm. Sci. Tech. 2009, 10, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royal Degree 53/2013, 1st of Febrary, por el que se Establecen las Normas Básicas Aplicables Para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. Available online: https://www.boe.es/diario_boe/txt.php?id=BOE-A-2013-1337 (accessed on 1 May 2021).
- NSCP. Programa Nacional de Control de Determinados Serotipos de Salmonella en la Especie Gallus Gallus; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2020.
- Krasowska, A.; Biegalska, A.; Augustyniak, D.; Łoś, M.; Richert, M.; Łukaszewicz, M. Isolation and characterization of phages infecting bacillus subtilis. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Virk, S.M.; Shi, J.; Zhou, Y.; Willias, S.P.; Morsy, M.K.; Abdelnabby, H.E.; Liu, J.; Wang, X.; Li, J. Isolation, characterization, and application of Bacteriophage LPSE1 against Salmonella enterica in Ready to Eat (RTE) Foods. Front. Microbiol. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.H.; Rahman, M.; Kim, J. Characterization of a Salmonella Enteritidis bacteriophage showing broad lytic activity against Gram-negative enteric bacteria. J. Microbiol. 2018, 56, 917–925. [Google Scholar] [CrossRef]
- Kislalioglu, M.S.; Khan, M.A.; Blount, C.; Goettsch, R.W.; Bolton, S. Physical characterization and dissolution properties of ibuprofen: Eudragit coprecipitates. J. Pharm. Sci. 1991, 80, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Marcó, M.B.; Suárez, V.B.; Quiberoni, A.; Pujato, S.A. Inactivation of dairy bacteriophages by thermal and chemical treatments. Viruses 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strain | Source/Reference | Lysis by BP FGS011 |
|---|---|---|
| Salmonella Enteritidis | CCM160 | + |
| Salmonella Typhimurium | CCM157 | + |
| mST | CCM188 | + |
| Salmonella Virchow | CECAV | - |
| Salmonella Ohio | CECAV | - |
| Salmonella Kentucky | CECAV | - |
| Salmonella Infantis | CECAV | + |
| Salmonella Senftemberg | CECAV | + |
| Salmonella Indiana | CECAV | + |
| Salmonella Havana | CECAV | + |
| Escherichia coli | CCM099 | - |
| Citrobacter freundi | CCM091 | + |
| Pseudomonas aeruginosa | CCM054 | - |
| FP Log10 (PFU/g Content) | L100 Log10 (PFU/g Content) | S100 Log10 (PFU/g Content) | |
|---|---|---|---|
| Crop | 4.9 ± 0.4 | 6.3 ± 0.2 | 5.6 ± 0.3 |
| Proventriculus | 3.8 ± 0.4 | 5.7 ± 0.9 | 4.7 ± 0.7 |
| Gizzard | 4.5 ± 0.2 | 4.9 ± 0.5 | 4.6 ± 0.4 |
| Gut | 4.7 ± 0.2 b | 5.9 ± 0.5 a | 4.6 ± 0.5 b |
| Ceca | 5.2 ± 0.6 | 5.9 ± 0.2 | 4.7 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry. Pharmaceuticals 2021, 14, 434. https://doi.org/10.3390/ph14050434
Lorenzo-Rebenaque L, Malik DJ, Catalá-Gregori P, Marin C, Sevilla-Navarro S. In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry. Pharmaceuticals. 2021; 14(5):434. https://doi.org/10.3390/ph14050434
Chicago/Turabian StyleLorenzo-Rebenaque, Laura, Danish J. Malik, Pablo Catalá-Gregori, Clara Marin, and Sandra Sevilla-Navarro. 2021. "In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry" Pharmaceuticals 14, no. 5: 434. https://doi.org/10.3390/ph14050434
APA StyleLorenzo-Rebenaque, L., Malik, D. J., Catalá-Gregori, P., Marin, C., & Sevilla-Navarro, S. (2021). In Vitro and In Vivo Gastrointestinal Survival of Non-Encapsulated and Microencapsulated Salmonella Bacteriophages: Implications for Bacteriophage Therapy in Poultry. Pharmaceuticals, 14(5), 434. https://doi.org/10.3390/ph14050434







