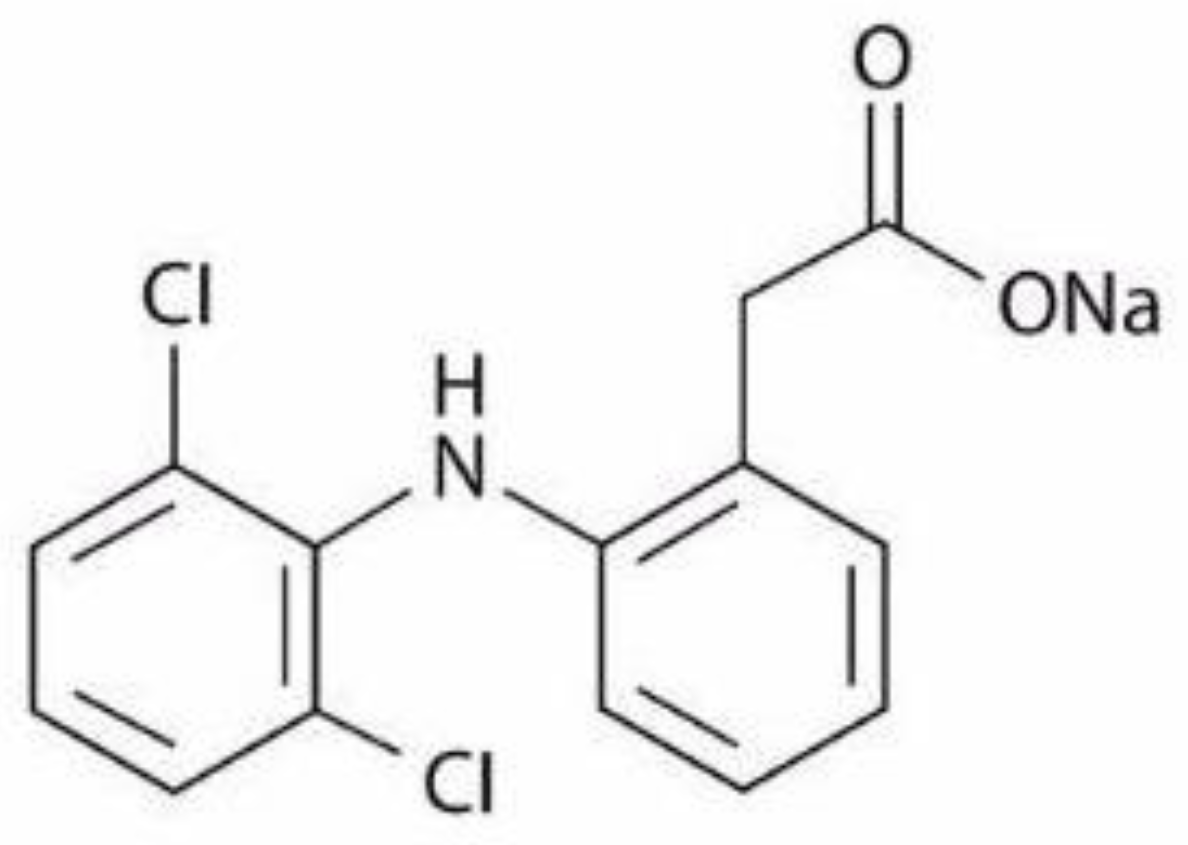
Quantification and Classification of Diclofenac Sodium Content in Dispersed Commercially Available Tablets by Attenuated Total Reflection Infrared Spectroscopy and Multivariate Data Analysis
Abstract
1. Introduction
2. Results and Discussion
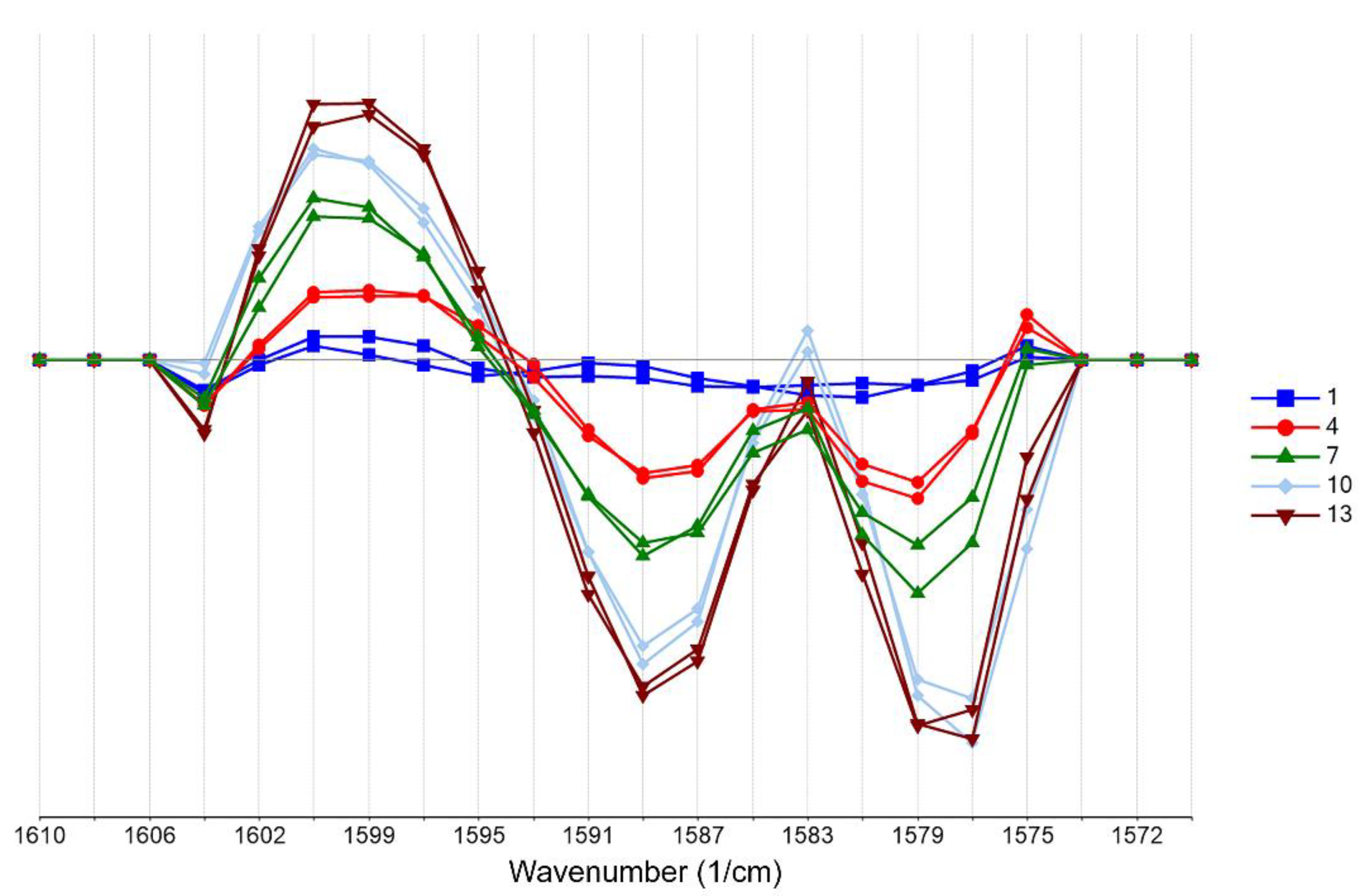
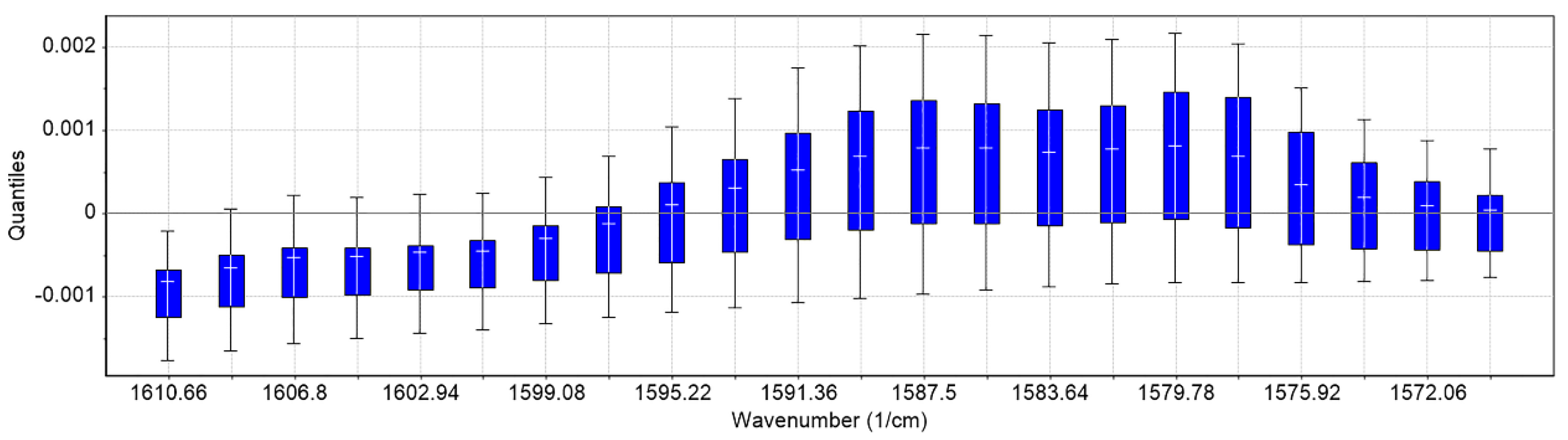
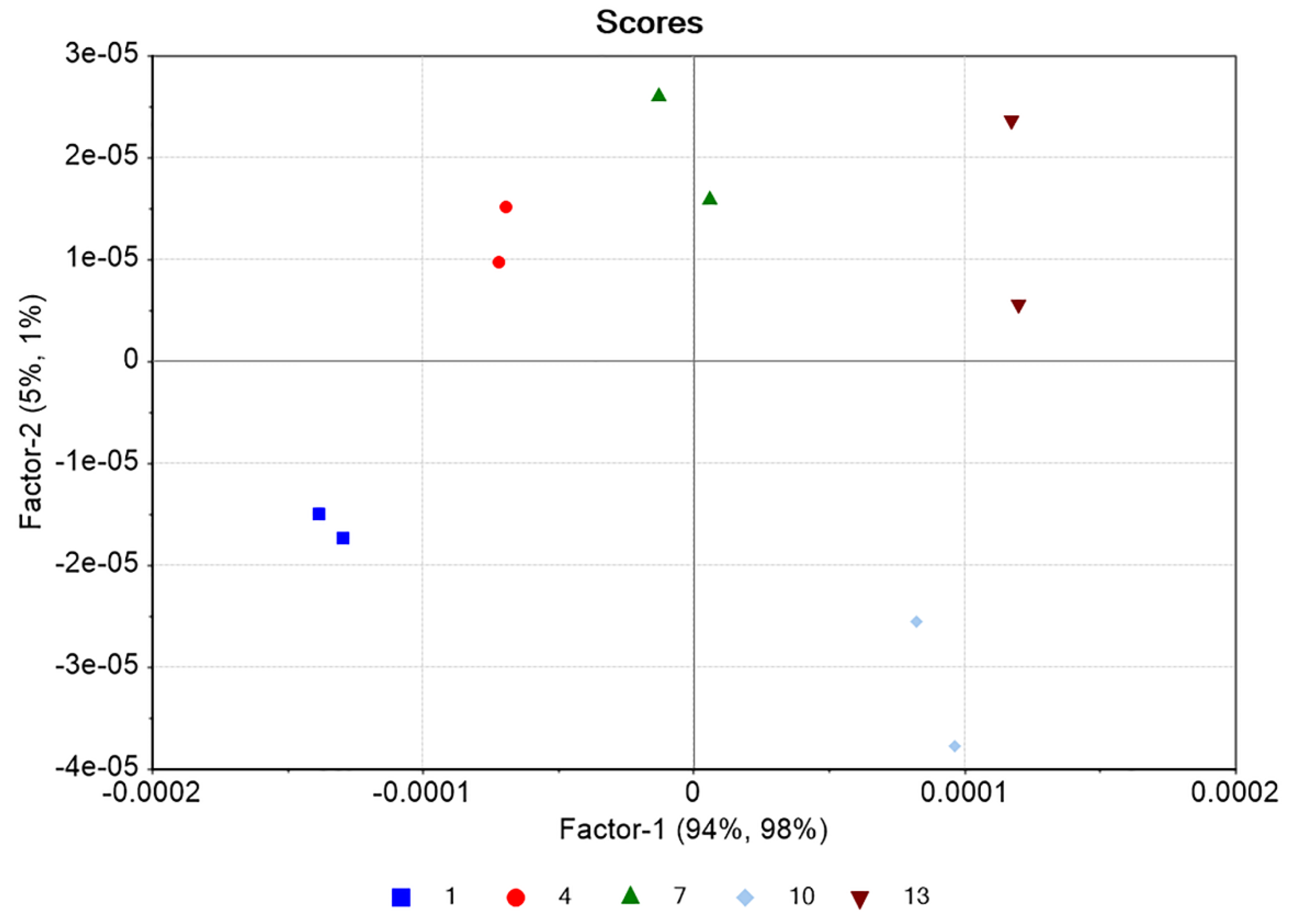
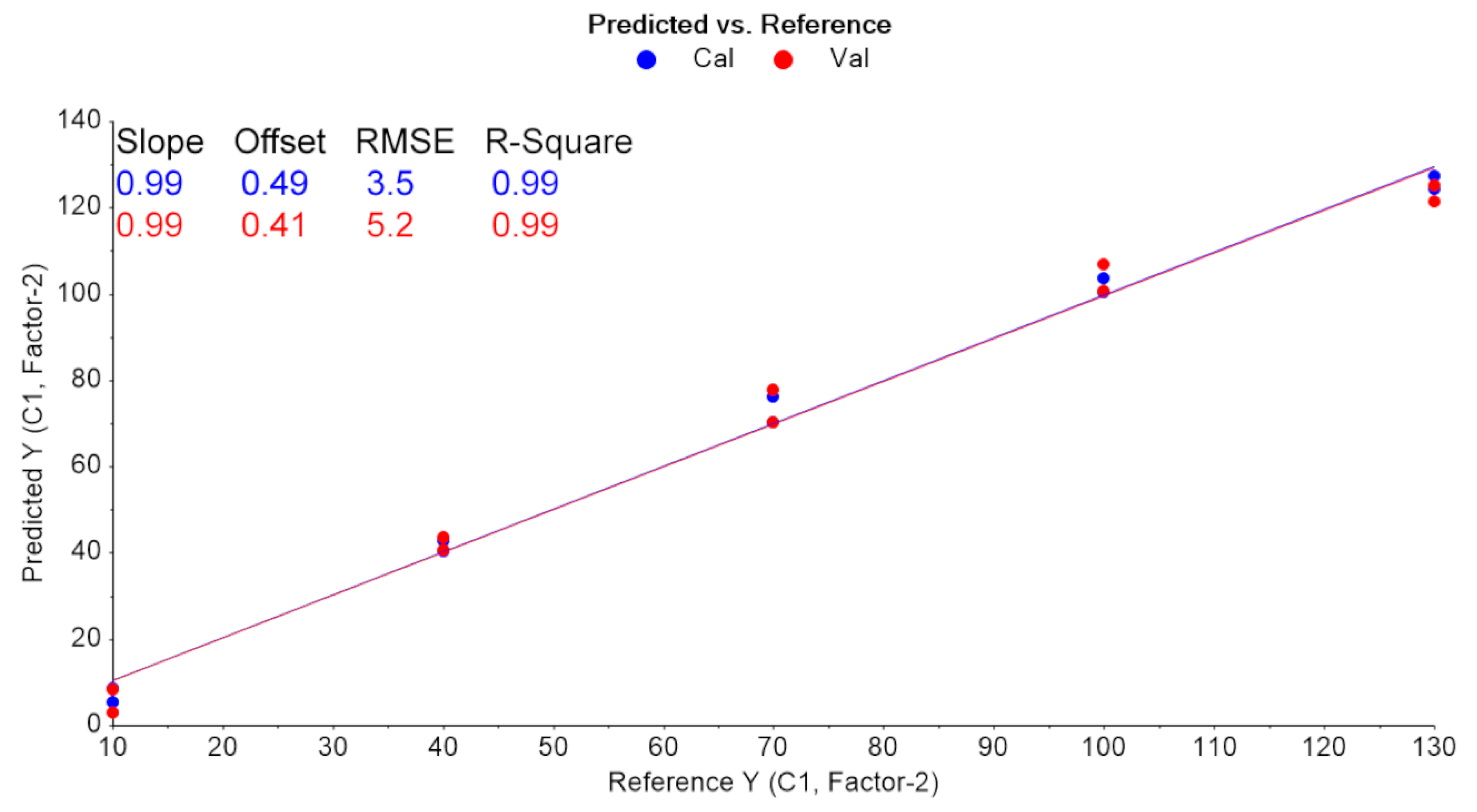
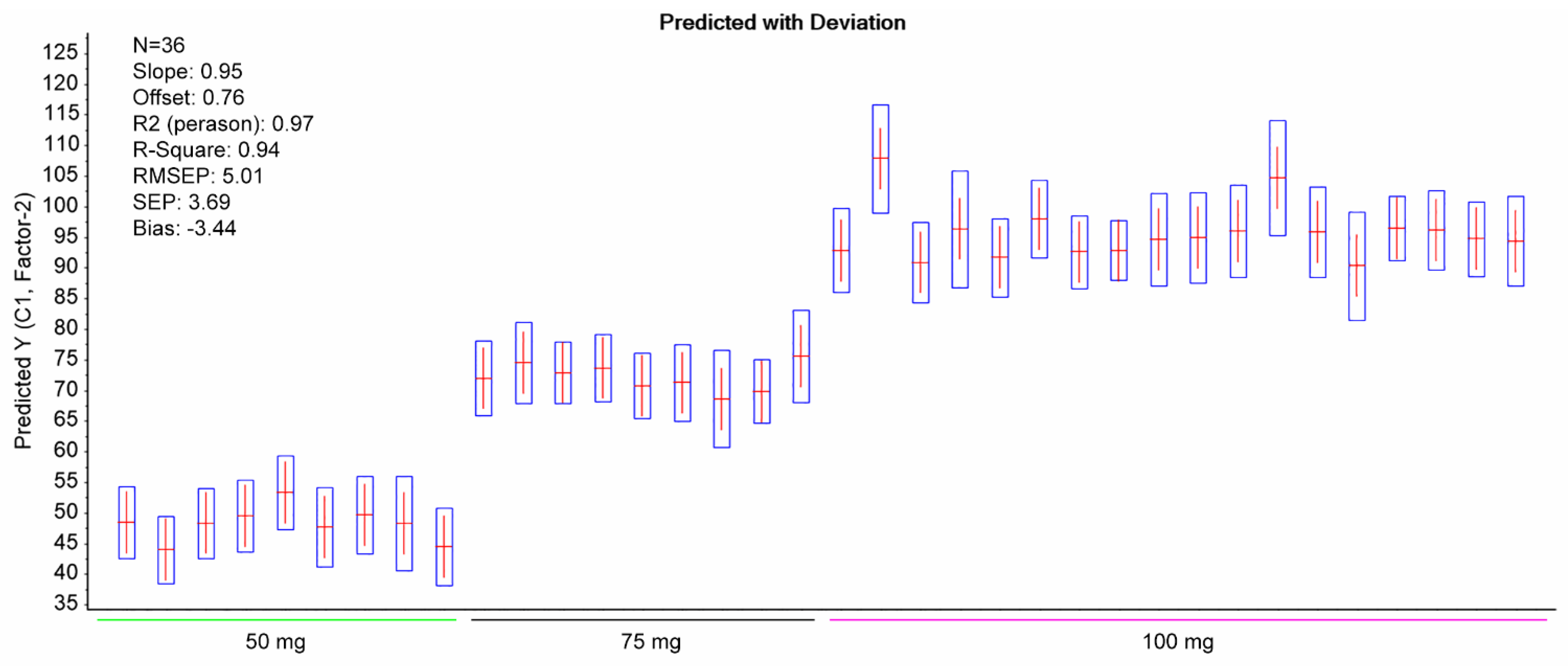
2.1. Development of the Chemometrics Model
2.2. Comparison with Reference Method
3. Materials and Methods
3.1. Samples and Reagents
3.2. Preparation of Training Set
3.3. Preparation of the Test Set
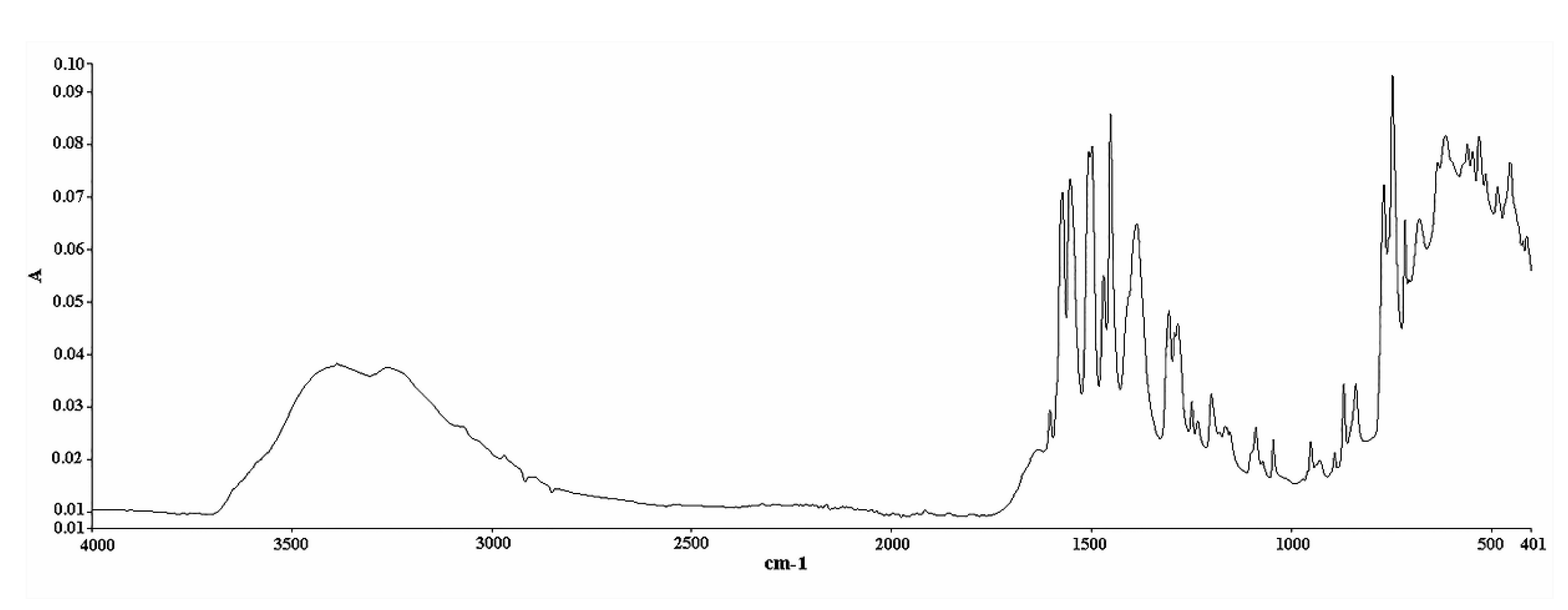
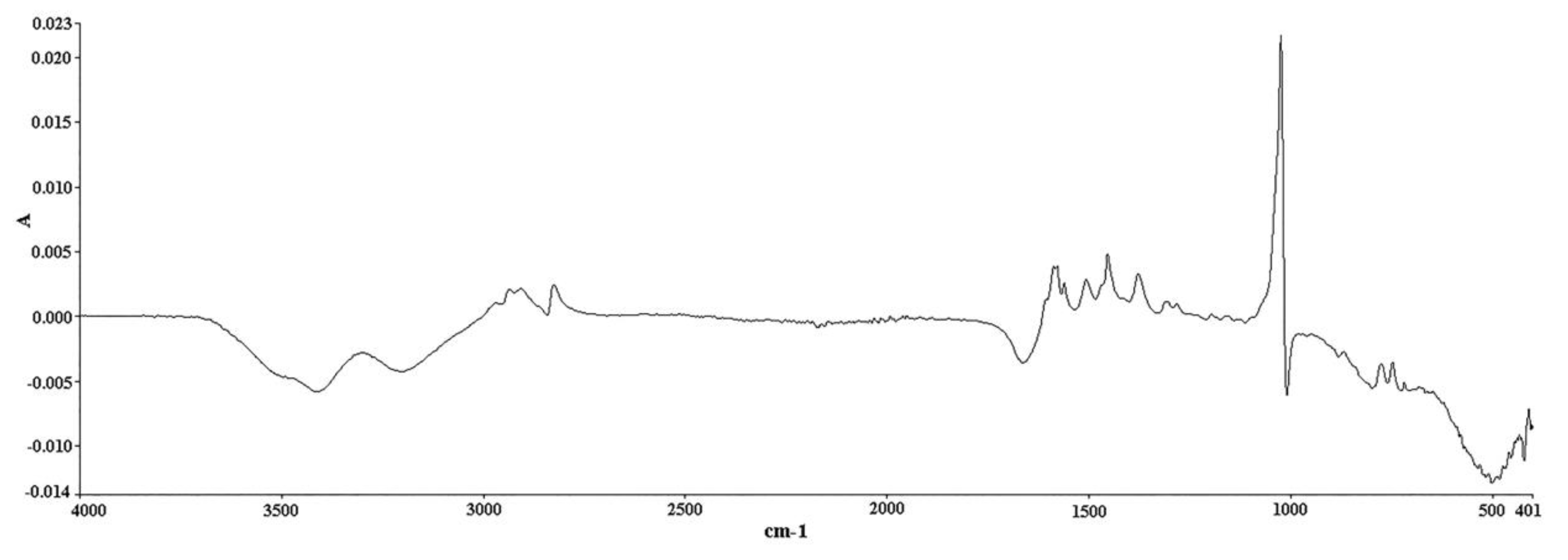
3.4. ATR FT-IR Analysis
3.5. Multivariate Analysis
3.6. Reference UV/Vis Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charles, R.E.S.; Craig, R. Modern Pharmacology with Clinical Applications, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Golan, D.E.; Tashjian, A.H.; Armstrong, E.J. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Lewis, A.J.; Furst, D.E. Nonsteroidal Anti-Inflammatory Drugs: Mechansms and Clinical Uses, 2nd ed.; Marcell Dekker Inc.: New York, NY, USA, 1994. [Google Scholar]
- Saeb-Parsy, K.; Assomull, R.G.; Khan, F.Z.; Saeb-Parsy, K.; Kelly, E. Instant Pharmacology; John Willey & Sons Ltd.: New York, NY, USA, 1999. [Google Scholar]
- Diclofenac. Available online: https://go.drugbank.com/drugs/DB00586 (accessed on 3 April 2021).
- Alexander, A.; Giri, T.K.; Parveen, N.; Thakur, D.; Badwaik, H.; Tripathi, D.K. In Vitro Evaluation of Commercially Available Enteric Coated Tablet Containing Diclofenac Sodium. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 875–881. [Google Scholar]
- US Pharmacopoeia—Diclofenac Sodium. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m24962.html (accessed on 3 April 2021).
- European Pharmacopoeia 7.0—Diclofenac Sodium. Available online: www.drugfuture.com/Pharmacopoeia/EP7/DATA/1002E.PDF (accessed on 3 April 2021).
- US Pharmacopoeia—Diclofenac Sodium Delayed-Release Tablets. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m24970.html (accessed on 3 April 2021).
- Agrawal, Y.; Shivramchandra, K. Spectrophotometric determination of diclofenac sodium in tablets. J. Pharm. Biomed. Anal. 1991, 9, 97–100. [Google Scholar] [CrossRef]
- Palomo, M.; Ballesteros, M.; Frutos, P. Analysis of diclofenac sodium and derivatives. J. Pharm. Biomed. Anal. 1999, 21, 83–94. [Google Scholar] [CrossRef]
- Matin, A.; Farajzadeh, M.; Jouyban, A. A simple spectrophotometric method for determination of sodium diclofenac in pharmaceutical formulations. II Farmaco 2005, 60, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Ansari, T.M.; Niazi, S.B.; Bukhari, S.I.H. A Simple Spectrophotometric Determination of Diclofenac Sodium in Commercial Dosage Forms Using 2,3-Dichloro-5,6-Dicyano-1,4-Benzoquinone (DDQ). Pak. J. Anal. Chem. 2005, 6, 5–9. [Google Scholar]
- Kormosh, Z.O.; Hunka, I.P.; Bazel, Y.R. Extraction and Spectrophotometric Determination of Diclofenac in Pharmaceuticals. J. Chin. Chem. Soc. 2008, 55, 356–361. [Google Scholar] [CrossRef]
- Rashid, Q.N.; Bakir, M.H.; Baban, S.O. Spectrophotometric determination of Diclofenac Sodium in pure form and in the pharmaceutical preparations. Tikrit J. Pure Sci. 2016, 21, 76–80. [Google Scholar]
- Mazurek, S.; Szostak, R. Quantitative determination of diclofenac sodium and aminophylline in injection solutions by FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 1235–1242. [Google Scholar] [CrossRef]
- Mazurek, S.; Szostak, R. Quantitative determination of diclofenac sodium in solid dosage forms by FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 48, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, G.; Dou, Y.; Liang, L.; Zhang, H.; Ren, Y. Quantitative analysis of diclofenac sodium powder via near-infrared spectroscopy combined with artificial neural network. J. Pharm. Biomed. Anal. 2009, 50, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Szostak, R. Comparison of infrared attenuated total reflection and Raman spectroscopy in the quantitative analysis of diclofenac sodium in tablets. Vib. Spectrosc. 2011, 57, 157–162. [Google Scholar] [CrossRef]
- Szostak, R.; Mazurek, S. The influence of sample area on diclofenac sodium quantification by diffuse reflectance IR spectroscopy. Talanta 2011, 84, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.; Araújo, A.; Montenegro, M. Simultaneous potentiometric and fluorimetric determination of diclofenac in a sequential injection analysis system. Anal. Chim. Acta 2002, 470, 185–194. [Google Scholar] [CrossRef]
- Hassan, S.S.; Mahmoud, W.H.; Elmosallamy, M.A.; Almarzooqi, M.H. Iron(II)-phthalocyanine as a novel recognition sensor for selective potentiometric determination of diclofenac and warfarin drugs. J. Pharm. Biomed. Anal. 2005, 39, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Pezza, H.; Pezza, L. Determination of diclofenac in pharmaceutical preparations using a potentiometric sensor immobilized in a graphite matrix. Talanta 2006, 68, 636–642. [Google Scholar] [CrossRef]
- Santos, E.M.; Araujo, A.N.; Couto, C.M.; Montenegro, M.C.B. Potentiometric behaviour of ion selective electrodes based on iron porphyrins: The influence of porphyrin substituents on the response properties and analytical determination of diclofenac in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2006, 42, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Kormosh, Z.; Hunka, I.; Bazel, Y. Potentiometric determination of diclofenac in pharmaceutical formulation by membrane electrode based on ion associate with base dye. Chin. Chem. Lett. 2007, 18, 1103–1106. [Google Scholar] [CrossRef]
- Lenik, J. A new potentiometric electrode incorporating functionalized β-cyclodextrins for diclofenac determination. Mater. Sci. Eng. C 2014, 45, 109–116. [Google Scholar] [CrossRef]
- Hájková, R.; Solich, P.; Pospíšilová, M.; Šícha, J. Simultaneous determination of methylparaben, propylparaben, sodium diclofenac and its degradation product in a topical emulgel by reversed-phase liquid chromatography. Anal. Chim. Acta 2002, 467, 91–96. [Google Scholar] [CrossRef]
- Elkady, E.F. Simultaneous determination of diclofenac potassium and methocarbamol in ternary mixture with guaifenesin by reversed phase liquid chromatography. Talanta 2010, 82, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.D.; Butle, S.; Patil, S.D.; Jagtap, P. Validated stability indicating RP-HPLC method for simultaneous determination and in vitro dissolution studies of thiocolchicoside and diclofenac potassium from tablet dosage form. Arab. J. Chem. 2015, 8, 118–128. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ciltas, U. Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods. J. Pharm. Anal. 2015, 5, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mubengayi, C.K.; Ramli, Y.; Routaboul, C.; Gilard, V.; El Karbane, M.; Cherrah, Y.; Essassi, M.M. Quality Evaluation of Diclofenac Formulations Manufactured in DR Congo. Pharm. Anal. Chem. Open Access 2016, 2, 112. [Google Scholar] [CrossRef]
- Elzayat, E.M.; Ibrahim, M.F.; Abdel-Rahman, A.A.; Ahmed, S.M.; Alanazi, F.K.; Habib, W.A. A validated stability-indicating UPLC method for determination of diclofenac sodium in its pure form and matrix formulations. Arab. J. Chem. 2017, 10, S3245. [Google Scholar] [CrossRef]
- Alquadeib, B.T. Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm. J. 2019, 27, 66–70. [Google Scholar] [CrossRef]
- Van Overbeke, A.; Baeyens, W.; Bossche, W.V.D. Quantitative Fourier transform infrared/attenuated total reflectance analysis of ketoprofen in some pharmaceutical formulations. Vib. Spectrosc. 1995, 9, 121–130. [Google Scholar] [CrossRef]
- Boyer, C.; Brégère, B.; Crouchet, S.; Gaudin, K.; Dubost, J. Direct determination of niflumic acid in a pharmaceutical gel by ATR/FTIR spectroscopy and PLS calibration. J. Pharm. Biomed. Anal. 2006, 40, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.; Ogwu, J.; Tanna, S. Counterfeit Tablet Investigations: Can ATR FT/IR Provide Rapid Targeted Quantitative Analyses? J. Anal. Bioanal. Tech. 2014, 5. [Google Scholar] [CrossRef]
- Hassib, S.T.; Hassan, G.S.; El-Zaher, A.A.; Fouad, M.A.; Taha, E.A. Quantitative analysis of anti-inflammatory drugs using FTIR-ATR spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.E.; Ferrão, M.F.; Parisotto, G.; Muller, E.I.; Flores, E.M. Simultaneous determination of sulphamethoxazole and trimethoprim in powder mixtures by attenuated total reflection-Fourier transform infrared and multivariate calibration. J. Pharm. Biomed. Anal. 2009, 49, 800–805. [Google Scholar] [CrossRef]
- Kandhro, A.A.; Laghari, A.H.; Mahesar, S.A.; Saleem, R.; Nelofar, A.; Khan, S.T.; Sherazi, S. Application of attenuated total reflectance Fourier transform infrared spectroscopy for determination of cefixime in oral pharmaceutical formulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Salari, A.; Young, R.E. Application of attenuated total reflectance FTIR spectroscopy to the analysis of mixtures of pharmaceutical polymorphs. Int. J. Pharm. 1998, 163, 157–166. [Google Scholar] [CrossRef]
- Helmy, R.; Zhou, G.X.; Chen, Y.W.; Crocker, L.; Wang, T.; Wenslow, R.M.; Vailaya, A. Characterization and Quantitation of Aprepitant Drug Substance Polymorphs by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Anal. Chem. 2002, 75, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumelis, N.; Demertzis, M.A.; Kovala-Demertzi, D.; Koutsodimou, A.; Moukarika, A. Preparations and spectroscopic studies of organotin complexes of diclofenac. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Sherazi, S.T.H.; Bhanger, M.I.; Mahesar, S.A.; Bajeer, M.A. A rapid Fourier-transform infrared (FTIR) spectroscopic method for direct quantification of paracetamol content in solid pharmaceutical formulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Li, L.; Zeng, Z.; Zhao, W.; Wang, G.; Guan, X. Simple and Green Fabrication of a Superhydrophobic Surface by One-Step Immersion for Continuous Oil/Water Separation. J. Phys. Chem. A 2016, 120, 5617–5623. [Google Scholar] [CrossRef]
- Fabre, H.; Sun, S.W.; Mandrou, B.; Maillols, H. Assay validation for an active ingredient in a pharmaceutical formulation: Practical approach using ultraviolet spectrophotometry. Analyst 1993, 118, 1061–1064. [Google Scholar] [CrossRef]
| DS Content | Calculated (Mean ± SD) |
|---|---|
| 50 mg | 48.10 ± 2.12 |
| 75 mg | 72.03 ± 1.73 |
| 100 mg | 95.55 ± 2.20 |
| DS Content | Calculated (Mean ± SD) UV/Vis |
|---|---|
| 50 mg | 49.31 ± 0.48 |
| 75 mg | 74.29 ± 0.64 |
| 100 mg | 98.79 ± 1.06 |
| Paired t-Test | ||
|---|---|---|
| Variable 1 (ATR FT-IR) | Variable 2 (UV) | |
| Mean | 71.89 | 74.13 |
| Deviation | 562.89 | 612.17 |
| Sample size | 3 | 3 |
| Pearson correlation | 0.99 | |
| Hypothetical mean difference | 0 | |
| Degrees of freedom | 2 | |
| t | 3.81 | |
| P(T ≤ t) one-tailed | 0.03 | |
| t critical, one-tailed | 2.92 | |
| P(T ≤ t) two-tailed | 0.06 | |
| t critical, two-tailed | 4.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siozou, E.; Sakkas, V.; Kourkoumelis, N. Quantification and Classification of Diclofenac Sodium Content in Dispersed Commercially Available Tablets by Attenuated Total Reflection Infrared Spectroscopy and Multivariate Data Analysis. Pharmaceuticals 2021, 14, 440. https://doi.org/10.3390/ph14050440
Siozou E, Sakkas V, Kourkoumelis N. Quantification and Classification of Diclofenac Sodium Content in Dispersed Commercially Available Tablets by Attenuated Total Reflection Infrared Spectroscopy and Multivariate Data Analysis. Pharmaceuticals. 2021; 14(5):440. https://doi.org/10.3390/ph14050440
Chicago/Turabian StyleSiozou, Eirini, Vasilios Sakkas, and Nikolaos Kourkoumelis. 2021. "Quantification and Classification of Diclofenac Sodium Content in Dispersed Commercially Available Tablets by Attenuated Total Reflection Infrared Spectroscopy and Multivariate Data Analysis" Pharmaceuticals 14, no. 5: 440. https://doi.org/10.3390/ph14050440
APA StyleSiozou, E., Sakkas, V., & Kourkoumelis, N. (2021). Quantification and Classification of Diclofenac Sodium Content in Dispersed Commercially Available Tablets by Attenuated Total Reflection Infrared Spectroscopy and Multivariate Data Analysis. Pharmaceuticals, 14(5), 440. https://doi.org/10.3390/ph14050440








