Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review)
Abstract
:1. Introduction
2. Immune Checkpoint Inhibition Therapy
3. Photodynamic Therapy (PDT)
3.1. PDT-Induced Immunogenic Cell Death (ICD) and Activation of Damage-Associated Molecular Patterns (DAMPs)
3.2. PDT-Induced Inflammation and Activation of Innate Immunity
3.3. Activation of the Adaptive Immune System by PDT
3.4. Combination of Immune Checkpoint Inhibition with PDT
4. Photothermal Therapy (PTT)
4.1. PTT-Induced Immunogenic Cell Death (ICD), Activation of Damage-Associated Molecular Patterns (DAMPs) and Activation of Anti-Tumor Immunity
4.2. Nanoparticle-Based Photothermal Immunotherapy
4.3. Combination of Immune Checkpoint Inhibition (ICI) with Photothermal Therapy (PTT)
5. Radiation Therapy (RT)
5.1. Radiation Therapy-Induced Cell Death, Immunogenic Cell Death (ICD), and Activation of Anti-Tumor Responses
5.2. Combination of Immune Checkpoint Inhibition (ICI) with Radiation Therapy (RT)
5.3. Preclinical Scenario
5.4. Clinical Trials with Combination of Immune Checkpoint Inhibition with Radiation, Photodynamic, or Photothermal Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aminolevulinic acid-photodynamic therapy | ALA-PDT |
| Antigen-presenting cells | APC |
| Ataxia-telangiectasia mutated | ATM |
| Adenosine triphosphate | ATP |
| B7 protein family dendritic cell molecule | B7-DC |
| Bristol Mayers Squibb 202 | BMS-202 |
| Bursa of Fabricius cells | B cell |
| Calreticulin | CRT |
| Chemokine (C-X-C motif) ligand 2 | CXCL2 |
| Cluster of differentiation 8 | CD8 |
| Cytotoxic T lymphocyte | CTL |
| Cytotoxic T lymphocyte antigen 4 | CTLA4 |
| Damage-associated molecular patterns | DAMP |
| Damage-regulated autophagy modulator | DRAM |
| Dendritic cells | DC |
| Draining lymph nodes | DLN |
| External beam radiation therapy | EBRT |
| Forkhead box P3 | FoxP3 |
| Genetically engineered mouse model | GEMM |
| Glysated chitosan | GC |
| Gold nanorod | GNR |
| Gold nanostar | GNS |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF)-transfected tumor cell vaccine | GVAX |
| Head and neck squamous cell carcinoma | HNSCC |
| Heat shock proteins | HSP |
| High mobility group box 1 | HMGB1 |
| Immune checkpoint blockade | ICB |
| Immune checkpoint inhibition | ICI |
| Immunogenic cell death | ICD |
| Indoleamine-pyrrole 2,3-dioxygenase | IDO |
| Interferon | IFN |
| Interferon gamma | IFNγ |
| Interleukin 1 beta | IL-1β |
| Interleukin 6 | IL-6 |
| Interleukin 12 | IL-12 |
| Interleukin 17 | IL-17 |
| Ionizing radiation | IR |
| Lymphocyte-activation gene 3 | LAG3 |
| Lipopolysaccharide | LPS |
| Monoclonal antibodies | mAbs |
| Macrophage inflammatory protein 2 | MIP2 |
| Magnetic Fe3O4 photothermal nanoparticle | MNP |
| Major histocompatibility complex I and II | MHC I and II |
| MHC class I polypeptide-related sequence A | MICA |
| Myeloid-derived suppressor cells | MDSC |
| Natural killer | NK |
| Natural killer group 2D | NKG2D |
| Near-infrared radiation | NIR |
| Non-small-cell lung carcinoma | NSCLC |
| Organic semiconducting pro-nano stimulant | OSPS |
| Pancreatic ductal adenocarcinoma | PDAC |
| Pattern recognition receptors | PRR |
| Photodynamic therapy | PDT |
| Photosensitizer | PS |
| Photothermal agents | PTA |
| Photothermal therapy | PTT |
| Poly(lactic-co-glycolic) acid-indocyanine green-R837 | PLGA-ICG-R837 |
| Programmed cell death protein 1 | PD1/PDCD1 |
| Programmed cell death protein 1 ligand 1 | PDL1 |
| Prussian blue nanoparticle | PBNP |
| Radiation therapy | RT |
| Reactive oxygen species | ROS |
| Regulatory T cells | Treg |
| Renal cell carcinoma | RCC |
| Stereotactic body radiation therapy | SBRT |
| Small-cell lung cancer | SCLC |
| Single-walled carbon nanotubes | SWCNT |
| Silica–gold nanoshell | AuNS |
| T cell receptor | TCR |
| T helper 17 | Th17 |
| T lymphocytes | T cell |
| Toll-like receptors | TLR |
| Transforming growth factor beta | TGFβ |
| Tumor-associated macrophages | TAM |
| Tumor microenvironment | TME |
| Tumor necrosis factor alpha | TNFα |
| Tumor neoantigens | TNA |
| Tumor-specific antigens | TSA |
| Urothelial carcinoma | UC |
| Vascular endothelial growth factor | VEGF |
References
- Mukerjee, S. The Emperor of All Maladies: A Biography of Cancer; Scribner: New York, NY, USA, 2010. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rosenberg, S.A. Progress in the development of immunotherapy for the treatment of patients with cancer. J. Intern. Med. 2001, 250, 462–475. [Google Scholar] [CrossRef]
- Rosenberg, S.A. Progress in human tumour immunology and immunotherapy. Nature 2001, 411, 380–384. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.Y.; Huang, L. Cancer immunotherapy and nanomedicine. Pharm. Res. 2011, 28, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Takeyama, H.; Guha, S. Cytokine network: New targeted therapy for pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2416–2419. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Cramer, G.M.; Moon, E.K.; Cengel, K.A.; Busch, T.M. Photodynamic Therapy and Immune Checkpoint Blockade. Photochem. Photobiol. 2020, 96, 954–961. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochem. ically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Liang, F. Nanomaterial-Based Tumor Photothermal Immunotherapy. Int. J. Nanomed. 2020, 15, 9159–9180. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.D.; Gollnick, S.O. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran Hernandez, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Govande, M.; Yasinchak, A.; Heusinkveld, L.; Shakya, S.; Fairchild, R.L.; Maytin, E.V. Painless Photodynamic Therapy Triggers Innate and Adaptive Immune Responses in a Murine Model of UV-induced Squamous Skin Pre-cancer. Photochem. Photobiol. 2020. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996, 183, 2533–2540. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Allison, J.P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 2020, 20, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.W.; Oh, G.; Ahn, J.C.; Chung, E. Non-Oncologic Applications of Nanomedicine-Based Phototherapy. Biomedicines 2021, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Pogue, B.W.; O’Hara, J.A.; Demidenko, E.; Wilmot, C.M.; Goodwin, I.A.; Chen, B.; Swartz, H.M.; Hasan, T. Photodynamic therapy with verteporfin in the radiation-induced fibrosarcoma-1 tumor causes enhanced radiation sensitivity. Cancer Res. 2003, 63, 1025–1033. [Google Scholar]
- Korbelik, M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006, 38, 500–508. [Google Scholar] [CrossRef]
- Preise, D.; Scherz, A.; Salomon, Y. Antitumor immunity promoted by vascular occluding therapy: Lessons from vascular-targeted photodynamic therapy (VTP). Photochem. Photobiol. Sci. 2011, 10, 681–688. [Google Scholar] [CrossRef]
- Maas, A.L.; Carter, S.L.; Wileyto, E.P.; Miller, J.; Yuan, M.; Yu, G.; Durham, A.C.; Busch, T.M. Tumor vascular microenvironment determines responsiveness to photodynamic therapy. Cancer Res. 2012, 72, 2079–2088. [Google Scholar] [CrossRef] [Green Version]
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar]
- Korbelik, M.; Sun, J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006, 55, 900–909. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Korbelik, M. Cancer vaccines generated by photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 664–669. [Google Scholar] [CrossRef]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.J.; Konstantinou, M.; Goode, E.F.; Meier, P. The Diversification of Cell Death and Immunity: Memento Mori. Mol. Cell 2019, 76, 232–242. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Yatim, N.; Cullen, S.; Albert, M.L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 2017, 17, 262–275. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. DAMPs and PDT-mediated photo-oxidative stress: Exploring the unknown. Photochem. Photobiol. Sci. 2011, 10, 670–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Wang, H. Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxid Med. Cell Longev. 2016, 2016, 5274084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Cecic, I.; Parkins, C.S.; Korbelik, M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002, 1, 690–695. [Google Scholar] [CrossRef]
- Krosl, G.; Korbelik, M.; Dougherty, G.J. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer 1995, 71, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowis, D.; Stoklosa, T.; Legat, M.; Issat, T.; Jakobisiak, M.; Golab, J. The influence of photodynamic therapy on the immune response. Photodiagnosis Photodyn. Ther. 2005, 2, 283–298. [Google Scholar] [CrossRef]
- Haanen, J. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell 2017, 170, 1055–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kousis, P.C.; Henderson, B.W.; Maier, P.G.; Gollnick, S.O. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007, 67, 10501–10510. [Google Scholar] [CrossRef] [Green Version]
- Brackett, C.M.; Muhitch, J.B.; Evans, S.S.; Gollnick, S.O. IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation. J. Immunol. 2013, 191, 4348–4357. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; de la Rosa, G.; Tewary, P.; Oppenheim, J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009, 30, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Jalili, A.; Makowski, M.; Switaj, T.; Nowis, D.; Wilczynski, G.M.; Wilczek, E.; Chorazy-Massalska, M.; Radzikowska, A.; Maslinski, W.; Bialy, L.; et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004, 10, 4498–4508. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, J.; Lv, T.; Tu, Q.; Huang, Z.; Wang, X. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Exp. Dermatol. 2013, 22, 362–363. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Mentucci, F.M.; Roselli, E.; Araya, P.; Rivarola, V.A.; Rumie Vittar, N.B.; Maccioni, M. Photodynamic Modulation of Type 1 Interferon Pathway on Melanoma Cells Promotes Dendritic Cell Activation. Front. Immunol. 2019, 10, 2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbelik, M.; Krosl, G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem. Photobiol. 1994, 60, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Cecic, I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999, 137, 91–98. [Google Scholar] [CrossRef]
- Korbelik, M.; Hamblin, M.R. The impact of macrophage-cancer cell interaction on the efficacy of photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Hao, N.B.; Lu, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belicha-Villanueva, A.; Riddell, J.; Bangia, N.; Gollnick, S.O. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers Surg. Med. 2012, 44, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Fan, Z.; Zhou, F.; Wang, X.; Shi, L.; Zhang, H.; Wang, P.; Yang, D.; Zhang, L.; Chen, W.R.; et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget 2015, 6, 17135–17146. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, M.J.; Nigro, A.; Mentucci, F.M.; Rumie Vittar, N.B.; Casolaro, V.; Dal Col, J. Dendritic Cells and Immunogenic Cancer Cell Death: A Combination for Improving Antitumor Immunity. Pharmaceutics 2020, 12, 256. [Google Scholar] [CrossRef] [Green Version]
- Korbelik, M.; Krosl, G.; Krosl, J.; Dougherty, G.J. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996, 56, 5647–5652. [Google Scholar] [PubMed]
- Preise, D.; Oren, R.; Glinert, I.; Kalchenko, V.; Jung, S.; Scherz, A.; Salomon, Y. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol. Immunother. 2009, 58, 71–84. [Google Scholar] [CrossRef]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting Tumor-Specific Immunity Using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sompayrac, L. How the Immune System Works, 6th ed.; John Wiley & Sons: Oxford, UK, 2019; p. 156. [Google Scholar]
- Huang, Y.Y.; Tanaka, M.; Vecchio, D.; Garcia-Diaz, M.; Chang, J.; Morimoto, Y.; Hamblin, M.R. Photodynamic therapy induces an immune response against a bacterial pathogen. Expert Rev. Clin. Immunol. 2012, 8, 479–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune response after photodynamic therapy increases anti-cancer and anti-bacterial effects. World J. Immunol. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Anzengruber, F.; Avci, P.; de Freitas, L.F.; Hamblin, M.R. T-cell mediated anti-tumor immunity after photodynamic therapy: Why does it not always work and how can we improve it? Photochem. Photobiol. Sci. 2015, 14, 1492–1509. [Google Scholar] [CrossRef] [Green Version]
- Wachowska, M.; Gabrysiak, M.; Muchowicz, A.; Bednarek, W.; Barankiewicz, J.; Rygiel, T.; Boon, L.; Mroz, P.; Hamblin, M.R.; Golab, J. 5-Aza-2’-deoxycytidine potentiates antitumour immune response induced by photodynamic therapy. Eur. J. Cancer 2014, 50, 1370–1381. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hady, E.S.; Martin-Hirsch, P.; Duggan-Keen, M.; Stern, P.L.; Moore, J.V.; Corbitt, G.; Kitchener, H.C.; Hampson, I.N. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001, 61, 192–196. [Google Scholar]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Orlandi, A.; Costanza, G.; Di Stefani, A.; Piccioni, A.; Di Cesare, A.; Chiricozzi, A.; Ferlosio, A.; Peris, K.; Fargnoli, M.C. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS ONE 2017, 12, e0183415. [Google Scholar] [CrossRef]
- Ding, B.; Shao, S.; Yu, C.; Teng, B.; Wang, M.; Cheng, Z.; Wong, K.L.; Ma, P.; Lin, J. Large-Pore Mesoporous-Silica-Coated Upconversion Nanoparticles as Multifunctional Immunoadjuvants with Ultrahigh Photosensitizer and Antigen Loading Efficiency for Improved Cancer Photodynamic Immunotherapy. Adv. Mater. 2018, 30, e1802479. [Google Scholar] [CrossRef]
- Knutson, K.L.; Disis, M.L. Augmenting T helper cell immunity in cancer. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2005, 5, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.L.; Disis, M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005, 54, 721–728. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Zhou, L.; Lopes, J.E.; Chong, M.M.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Shevach, E.M. Regulatory T cells in autoimmmunity*. Annu Rev. Immunol. 2000, 18, 423–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Tagami, T.; Yamazaki, S.; Uede, T.; Shimizu, J.; Sakaguchi, N.; Mak, T.W.; Sakaguchi, S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000, 192, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Roychoudhuri, R.; Eil, R.L.; Restifo, N.P. The interplay of effector and regulatory T cells in cancer. Curr. Opin. Immunol. 2015, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reginato, E.; Mroz, P.; Chung, H.; Kawakubo, M.; Wolf, P.; Hamblin, M.R. Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. Br. J. Cancer 2013, 109, 2167–2174. [Google Scholar] [CrossRef]
- Oh, D.S.; Kim, H.; Oh, J.E.; Jung, H.E.; Lee, Y.S.; Park, J.H.; Lee, H.K. Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses. Oncotarget 2017, 8, 47440–47453. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, C.; Gao, D.; Liu, H.; Yu, X.; Lai, J.; Wang, F.; Lin, J.; Liu, Z. Enhanced Anti-Tumor Efficacy through a Combination of Integrin alphavbeta6-Targeted Photodynamic Therapy and Immune Checkpoint Inhibition. Theranostics 2016, 6, 627–637. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.J.; Murray, K.S.; La Rosa, S.P.; Budhu, S.; Merghoub, T.; Somma, A.; Monette, S.; Kim, K.; Corradi, R.B.; Scherz, A.; et al. Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clin. Cancer Res. 2018, 24, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, Y.; Liang, X.; Sun, T.; Xue, H.; Tian, J.; Jin, Z. EGFR-targeted liposomal nanohybrid cerasomes: Theranostic function and immune checkpoint inhibition in a mouse model of colorectal cancer. Nanoscale 2018, 10, 16738–16749. [Google Scholar] [CrossRef]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Trempolec, N.; Doix, B.; Degavre, C.; Brusa, D.; Bouzin, C.; Riant, O.; Feron, O. Photodynamic Therapy-Based Dendritic Cell Vaccination Suited to Treat Peritoneal Mesothelioma. Cancers 2020, 12, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C.S. Immune Checkpoint Blockade Mediated by a Small-Molecule Nanoinhibitor Targeting the PD-1/PD-L1 Pathway Synergizes with Photodynamic Therapy to Elicit Antitumor Immunity and Antimetastatic Effects on Breast Cancer. Small 2019, 15, e1903881. [Google Scholar] [CrossRef]
- Bao, R.; Wang, Y.; Lai, J.; Zhu, H.; Zhao, Y.; Li, S.; Li, N.; Huang, J.; Yang, Z.; Wang, F.; et al. Enhancing Anti-PD-1/PD-L1 Immune Checkpoint Inhibitory Cancer Therapy by CD276-Targeted Photodynamic Ablation of Tumor Cells and Tumor Vasculature. Mol. Pharm. 2019, 16, 339–348. [Google Scholar] [CrossRef]
- Muchowicz, A.; Wachowska, M.; Stachura, J.; Tonecka, K.; Gabrysiak, M.; Wolosz, D.; Pilch, Z.; Kilarski, W.W.; Boon, L.; Klaus, T.J.; et al. Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur. J. Cancer 2017, 83, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kleinovink, J.W.; Fransen, M.F.; Lowik, C.W.; Ossendorp, F. Photodynamic-Immune Checkpoint Therapy Eradicates Local and Distant Tumors by CD8(+) T Cells. Cancer Immunol. Res. 2017, 5, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum, R.R.; Lin, W. Chlorin-Based Nanoscale Metal-Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef] [Green Version]
- Wachowska, M.; Stachura, J.; Tonecka, K.; Fidyt, K.; Braniewska, A.; Sas, Z.; Kotula, I.; Rygiel, T.P.; Boon, L.; Golab, J.; et al. Inhibition of IDO leads to IL-6-dependent systemic inflammation in mice when combined with photodynamic therapy. Cancer Immunol. Immunother. 2020, 69, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunwald, V.; Voss, M.H.; Rini, B.I.; Powles, T.; Albiges, L.; Giles, R.H.; Jonasch, E. Axitinib plus immune checkpoint inhibitor: Evidence- and expert-based consensus recommendation for treatment optimisation and management of related adverse events. Br. J. Cancer 2020, 123, 898–904. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulieres, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Mo, D.C.; Luo, P.H.; Huang, S.X.; Wang, H.L.; Huang, J.F. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: A systematic review. Int. Immunopharmacol. 2021, 91, 107281. [Google Scholar] [CrossRef] [PubMed]
- Solban, N.; Selbo, P.K.; Sinha, A.K.; Chang, S.K.; Hasan, T. Mechanistic investigation and implications of photodynamic therapy induction of vascular endothelial growth factor in prostate cancer. Cancer Res. 2006, 66, 5633–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosharskyy, B.; Solban, N.; Chang, S.K.; Rizvi, I.; Chang, Y.; Hasan, T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Res. 2006, 66, 10953–10958. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.B.; Sweeney, E.E.; Ramanujam, A.S.; Fernandes, R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int. J. Hyperthermia 2020, 37, 34–49. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, Z.; Duan, Y.; Fu, Z.; Bin, L. Reprogramming Tumor Microenvironment with Photothermal Therapy. Bioconjug Chem 2020, 31, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Saneja, A.; Kumar, R.; Arora, D.; Kumar, S.; Panda, A.K.; Jaglan, S. Recent advances in near-infrared light-responsive nanocarriers for cancer therapy. Drug Discov. Today 2018, 23, 1115–1125. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, W.; Cheng, Z.; Yao, K.; Yang, Y.; Xu, B.; Su, G. Targeted Delivery of siRNA with pH-Responsive Hybrid Gold Nanostars for Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 2029. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chi, C.; Wang, S.; Wang, L.; Liang, P.; Liu, F.; Shang, W.; Wang, W.; Zhang, F.; Li, S.; et al. A Comparative Study of Clinical Intervention and Interventional Photothermal Therapy for Pancreatic Cancer. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, C.; Wu, X.; He, M.; Zhang, Y.; Zhang, R.; Dong, C.M. Polymeric photothermal agents for cancer therapy: Recent progress and clinical potential. J. Mater. Chem. B 2021, 9, 1478–1490. [Google Scholar] [CrossRef]
- Ju, Y.; Zhang, H.; Yu, J.; Tong, S.; Tian, N.; Wang, Z.; Wang, X.; Su, X.; Chu, X.; Lin, J.; et al. Monodisperse Au-Fe2C Janus Nanoparticles: An Attractive Multifunctional Material for Triple-Modal Imaging-Guided Tumor Photothermal Therapy. ACS Nano 2017, 11, 9239–9248. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shen, H.; Yang, T.; Wang, L.; Fu, H.; Chen, H.; Zhang, Z. Indocyanine Green Loaded Magnetic Carbon Nanoparticles for Near Infrared Fluorescence/Magnetic Resonance Dual-Modal Imaging and Photothermal Therapy of Tumor. ACS Appl. Mater. Interfaces 2017, 9, 9484–9495. [Google Scholar] [CrossRef] [PubMed]
- Mioc, A.; Mioc, M.; Ghiulai, R.; Voicu, M.; Racoviceanu, R.; Trandafirescu, C.; Dehelean, C.; Coricovac, D.; Soica, C. Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy. Curr. Med. Chem. 2019, 26, 6493–6513. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.R.; Nordquist, R.E.; Chen, W.R. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Kibanov Solomonov, V.V.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. Int. J. Toxicol. 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Movahedi, M.M.; Alamzadeh, Z.; Hosseini-Nami, S.; Shakeri-Zadeh, A.; Taheripak, G.; Ahmadi, A.; Zare-Sadeghi, A.; Ghaznavi, H.; Mehdizadeh, A. Investigating the mechanisms behind extensive death in human cancer cells following nanoparticle assisted photo-thermo-radiotherapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101600. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, E.E.; Cano-Mejia, J.; Fernandes, R. Photothermal Therapy Generates a Thermal Window of Immunogenic Cell Death in Neuroblastoma. Small 2018, 14, e1800678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, B.; Middha, E.; Huang, Z.; Hu, Q.; Fu, Z.; Liu, B. Microfluidics-Prepared Uniform Conjugated Polymer Nanoparticles for Photo-Triggered Immune Microenvironment Modulation and Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 11167–11176. [Google Scholar] [CrossRef]
- Yu, G.T.; Rao, L.; Wu, H.; Yang, L.L.; Bu, L.L.; Deng, W.W.; Wu, L.; Nan, X.L.; Zhang, W.F.; Zhao, X.Z.; et al. Myeloid-Derived Suppressor Cell Membrane-Coated Magnetic Nanoparticles for Cancer Theranostics by Inducing Macrophage Polarization and Synergizing Immunogenic Cell Death. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Jia, X.; Cai, S.; Liang, W.; Qin, Y.; Yang, R.; Wang, C. CpG loaded MoS2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale 2017, 9, 5927–5934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wei, C.; Lin, A.; Pan, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J.; et al. Responsive functionalized MoSe2 nanosystem for highly efficient synergistic therapy of breast cancer. Colloids Surf. B Biointerfaces 2020, 189, 110820. [Google Scholar] [CrossRef]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Song, J.; Wang, M.; Wang, X.; Wang, J.; Howard, E.W.; Zhou, F.; Qu, J.; Chen, W.R. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale 2018, 10, 21640–21647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, S.; Song, S.; Chen, W.R.; Resasco, D.E.; Xing, D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials 2012, 33, 3235–3242. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef]
- Liu, Y.; Maccarini, P.; Palmer, G.M.; Etienne, W.; Zhao, Y.; Lee, C.T.; Ma, X.; Inman, B.A.; Vo-Dinh, T. Synergistic Immuno Photothermal Nanotherapy (SYMPHONY) for the Treatment of Unresectable and Metastatic Cancers. Sci. Rep. 2017, 7, 8606. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, C.; Yin, H.; Jiang, M.; Zhang, J.; Qin, B.; Luo, Z.; Yuan, X.; Yang, J.; Li, W.; et al. Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for the Treatment of Metastatic Tumors. ACS Nano 2018, 12, 7647–7662. [Google Scholar] [CrossRef]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Y.; Xu, J.; Shi, Y.; Yang, J.; Zhang, R.; Song, J.; Bai, X.; Wu, X.; Bao, Y.; et al. Combination of MAPK inhibition with photothermal therapy synergistically augments the anti-tumor efficacy of immune checkpoint blockade. J. Control. Release 2021, 332, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, X.; Li, W.; Li, R.; Chen, J.; Zhou, L.; Qiang, S.; Wu, W.; Shi, S.; Dong, C. M2-Like TAMs Function Reversal Contributes to Breast Cancer Eradication by Combination Dual Immune Checkpoint Blockade and Photothermal Therapy. Small 2021, 17, e2007051. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Shi, K.; Xiao, Y.; Liu, Q.; Han, R.; Wei, X.; Qian, Z. Rationally designed peptide-conjugated gold/platinum nanosystem with active tumor-targeting for enhancing tumor photothermal-immunotherapy. J. Control. Release 2019, 308, 29–43. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.; Zhu, X.; Wang, X.; Liu, L.; Sun, H.; Wang, C.; Kong, D.; Ma, G. Nanoscale Reduced Graphene Oxide-Mediated Photothermal Therapy Together with IDO Inhibition and PD-L1 Blockade Synergistically Promote Antitumor Immunity. ACS Appl. Mater. Interfaces 2019, 11, 1876–1885. [Google Scholar] [CrossRef]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-Activatable Fe3O4 Superparticle Nanodrug Carriers with PD-L1 Immune Checkpoint Blockade for Anti-metastatic Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef] [PubMed]
- Cano-Mejia, J.; Burga, R.A.; Sweeney, E.E.; Fisher, J.P.; Bollard, C.M.; Sandler, A.D.; Cruz, C.R.Y.; Fernandes, R. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine 2017, 13, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Lauber, K.; Niyazi, M.; Friedl, A.A.; Li, M.; Maihofer, C.; Schuttrumpf, L.; Ernst, A.; Niemoller, O.M.; Belka, C. Current concepts in clinical radiation oncology. Radiat. Environ. Biophys. 2014, 53, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef]
- Bartzsch, S.; Corde, S.; Crosbie, J.C.; Day, L.; Donzelli, M.; Krisch, M.; Lerch, M.; Pellicioli, P.; Smyth, L.M.L.; Tehei, M. Technical advances in x-ray microbeam radiation therapy. Phys. Med. Biol 2020, 65, 02TR01. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Thompson, C.B. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 2007, 13, 7271–7279. [Google Scholar] [CrossRef] [Green Version]
- Rouschop, K.M.; Ramaekers, C.H.; Schaaf, M.B.; Keulers, T.G.; Savelkouls, K.G.; Lambin, P.; Koritzinsky, M.; Wouters, B.G. Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiother. Oncol. 2009, 92, 411–416. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Martins, I.; Brenner, C.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Modulating Both Tumor Cell Death and Innate Immunity Is Essential for Improving Radiation Therapy Effectiveness. Front. Immunol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Bhardwaj, N.; McBride, W.H.; Formenti, S.C. Combining radiotherapy and immunotherapy: A revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, B.; Rubner, Y.; Wunderlich, R.; Weiss, E.M.; Pockley, A.G.; Fietkau, R.; Gaipl, U.S. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—Implications for cancer therapies. Curr. Med. Chem. 2012, 19, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Burnette, B.; Weichselbaum, R.R. Radiation as an immune modulator. Semin. Radiat. Oncol. 2013, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.B.; Peters, L.J.; Milas, L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J. Natl. Cancer Inst. 1979, 63, 1229–1235. [Google Scholar] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.X.; Auh, S.L. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Bloy, N.; Garcia, P.; Laumont, C.M.; Pitt, J.M.; Sistigu, A.; Stoll, G.; Yamazaki, T.; Bonneil, E.; Buque, A.; Humeau, J.; et al. Immunogenic stress and death of cancer cells: Contribution of antigenicity vs adjuvanticity to immunosurveillance. Immunol. Rev. 2017, 280, 165–174. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.P.; Yamazaki, T.; Zhang, T.; Charpentier, M.; Galluzzi, L.; Dephoure, N.; Clement, C.C.; Santambrogio, L.; Zhou, X.K.; et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Goldszmid, R.S.; Idoyaga, J.; Bravo, A.I.; Steinman, R.; Mordoh, J.; Wainstok, R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J. Immunol. 2003, 171, 5940–5947. [Google Scholar] [CrossRef] [Green Version]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Vandenberk, L.; Belmans, J.; Van Woensel, M.; Riva, M.; Van Gool, S.W. Exploiting the Immunogenic Potential of Cancer Cells for Improved Dendritic Cell Vaccines. Front. Immunol. 2015, 6, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Chee, C.A.; Ilbery, P.L.; Rickinson, A.B. Depression of lymphocyte replicating ability in radiotherapy patients. Br. J. Radiol. 1974, 47, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Order, S.E. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer 1977, 39, 737–743. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasmim, M.; Noman, M.Z.; Messai, Y.; Bordereaux, D.; Gros, G.; Baud, V.; Chouaib, S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-beta1. J. Immunol. 2013, 191, 5802–5806. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, H.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics (Sao Paulo) 2018, 73, e557s. [Google Scholar] [CrossRef]
- Herter-Sprie, G.S.; Koyama, S.; Korideck, H.; Hai, J.; Deng, J.; Li, Y.Y.; Buczkowski, K.A.; Grant, A.K.; Ullas, S.; Rhee, K.; et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016, 1, e87415. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1085–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Wu, M.O.; De la Maza, L.; Yun, Z.; Yu, J.; Zhao, Y.; Cho, J.; de Perrot, M. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015, 6, 12468–12480. [Google Scholar] [CrossRef] [Green Version]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar] [PubMed]
- Pfannenstiel, L.W.; McNeilly, C.; Xiang, C.; Kang, K.; Diaz-Montero, C.M.; Yu, J.S.; Gastman, B.R. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology 2019, 8, e1507669. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Luo, Y.; Tian, X.; Ma, S.; Sun, Y.; You, C.; Gong, Y.; Xie, C. Impact of Radiotherapy Concurrent with Anti-PD-1 Therapy on the Lung Tissue of Tumor-Bearing Mice. Radiat. Res. 2019, 191, 271–277. [Google Scholar] [CrossRef]
- Ruckert, M.; Deloch, L.; Frey, B.; Schlucker, E.; Fietkau, R.; Gaipl, U.S. Combinations of Radiotherapy with Vaccination and Immune Checkpoint Inhibition Differently Affect Primary and Abscopal Tumor Growth and the Tumor Microenvironment. Cancers 2021, 13, 714. [Google Scholar] [CrossRef]
- Chuong, M.; Chang, E.T.; Choi, E.Y.; Mahmood, J.; Lapidus, R.G.; Davila, E.; Carrier, F. Exploring the Concept of Radiation “Booster Shot” in Combination with an Anti-PD-L1 mAb to Enhance Anti-Tumor Immune Effects in Mouse Pancreas Tumors. J. Clin. Oncol. Res. 2017, 5, 1058. [Google Scholar]
- Oweida, A.; Lennon, S.; Calame, D.; Korpela, S.; Bhatia, S.; Sharma, J.; Graham, C.; Binder, D.; Serkova, N.; Raben, D.; et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 2017, 6, e1356153. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.H.; Lee, S.J.; Lee, E.J.; Shin, E.C.; Seong, J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget 2017, 8, 41242–41255. [Google Scholar] [CrossRef] [Green Version]
- Son, C.H.; Bae, J.H.; Shin, D.Y.; Lee, H.R.; Choi, Y.J.; Jo, W.S.; Ho Jung, M.; Kang, C.D.; Yang, K.; Park, Y.S. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J. Immunother. 2014, 37, 1–7. [Google Scholar] [CrossRef]
- Belcaid, Z.; Phallen, J.A.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.; Kellett, M.; Ruzevick, J.; Jackson, C.; et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsrud, A.J.; Jenkins, S.V.; Jamshidi-Parsian, A.; Quick, C.M.; Galhardo, E.P.; Dings, R.P.M.; Vang, K.B.; Narayanasamy, G.; Makhoul, I.; Griffin, R.J. Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations. Radiat. Res. 2020, 194, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Helm, A.; Tinganelli, W.; Simoniello, P.; Kurosawa, F.; Fournier, C.; Shimokawa, T.; Durante, M. Reduction of Lung Metastases in a Mouse Osteosarcoma Model Treated with Carbon Ions and Immune Checkpoint Inhibitors. Int. J. Radiat Oncol. Biol Phys. 2021, 109, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769–776. [Google Scholar] [CrossRef] [PubMed]
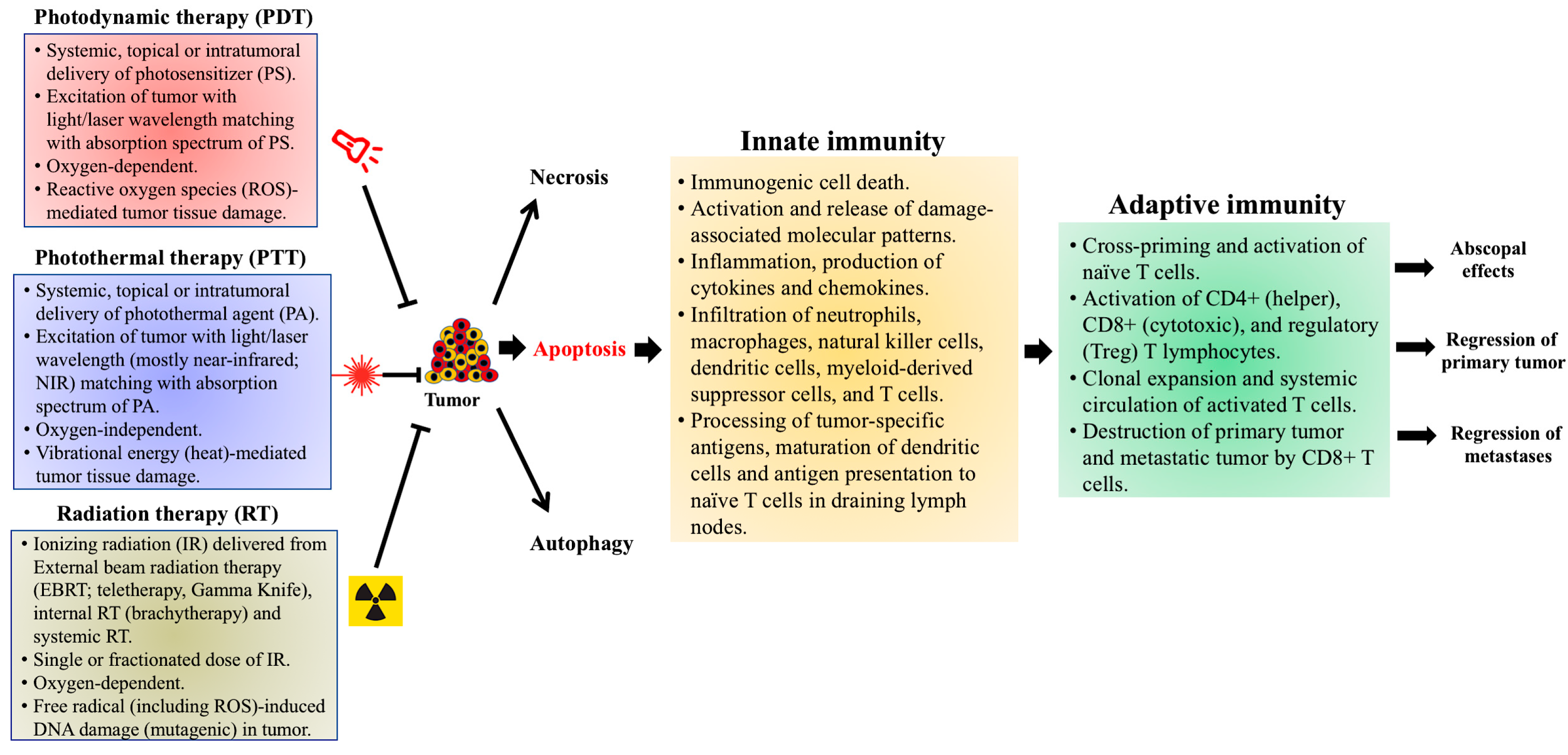
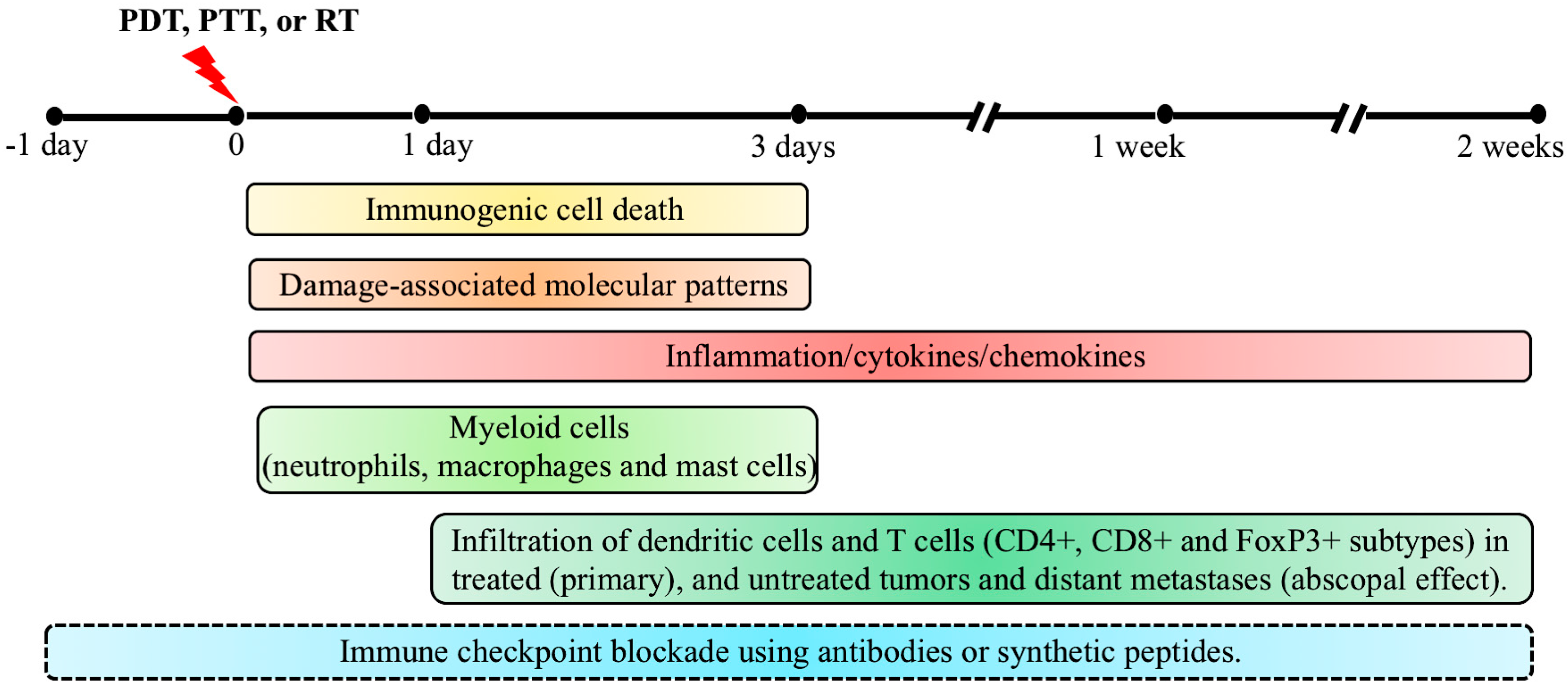
| Checkpoint Inhibitor Target | Photosensitizer/Construct | Murine Tumor Model | Immune Effector Cells | Cytokines | Therapeutic/Immune Response | Ref. |
|---|---|---|---|---|---|---|
| PD-1 | αvβ6 integrin-specific phthalocyanine dye labeled probe | 4T1 breast tumor | DC, CD8+ T cells | IL-1β, IL-12 | Reduced primary tumor growth and lung metastasis. Abscopal effect. | [98] |
| PD-1 | Pheophorbide A, given together with a tumor-specific peptide vaccine adjuvanted with TLR5 antagonist | BF16-F10 murine melanoma model | DC, CD8+ T cells | IFNγ | Reduced primary tumor growth and lung metastasis | [101] |
| PD-L1 | IRD700, conjugated to Fab fragment of anti-αCD276 antibody | 4T1 breast tumor | CD8+ T cells | Not analyzed | Reduced primary tumor growth and lung metastasis | [104] |
| PD-L1 | EGFR-targeted porphyrin-containing nanoliposomes conjugated with IRDye800CW and DOTA-Gd | Subcutaneous CT26 colon cancer | Not analyzed | Not analyzed | Tumor regression | [100] |
| PD-L1 | Verteporfin | 4T1 breast tumor | DC, CD8+ T cells | Not analyzed | Regression of primary tumors by destruction of tumor-associated lymphatic vessels | [105] |
| PD-L1 and BMS202 PD1/PDL1 inhibitor | Chlorin 6 NPs | 4T1 breast tumor | DC, CD8+ T cells | IFNγ, IL-6, TNFα | Regression of primary tumors, reduced lung metastases | [103] |
| PD1 + PD-L1 | WST11 | Renal cell carcinoma line that develops lung metastases | CD8+, CD4+FoxP3-T cells | Not analyzed | Regression of primary tumors, reduced lung metastases | [99] |
| CTLA4 | Bremachlorin | Subcutaneous MC38 and CT26 colon cancer double tumor model | CD8+ T cells | Not analyzed | Significant improvement of therapeutic efficacy and survival, abscopal effect | [106] |
| CTLA4 | Nanoparticles simultaneously loaded with chlorin e6 (photosensitizer) and imiquimod (Toll-like receptor-7 agonist) | Subcutaneous CT26 colon cancer | DCs, CD8+, CD4+FoxP3+ T cells | IFNγ, IL-12, TNFα | Therapeutic efficacy with abscopal effect. Prevented tumor recurrence, via immune memory effects | [107] |
| CTLA4 | OR141 | Ab1 and Ab12 mesothelioma murine model | CD4+ and CD8+ T cells, DCs | Not analyzed | Inhibition of mesothelioma cell growth | [102] |
| IDO | Chlorin-based nanoscale metal–organic framework (nMOF) | Subcutaneous B16F10 melanoma and CT26 colon cancer double tumor model. | CD4+ and CD45+ T cells, neutrophils, and B cells | Not analyzed | Local and distant tumor rejection and T cell infiltration of TME. Compensatory roles of neutrophils and B cells in presenting TAAs to T cells | [108] |
| IDO | Verteporfin | 4T1 breast tumor | Myeloid cells | IL-6 | Tumor regression | [109] |
| E0771 breast tumor |
| Checkpoint Inhibitor Target | Photothermal Agent/Construct | Murine Tumor Model | Immune Effector Cells | Cytokines | Therapeutic/Immune Response | Ref. |
|---|---|---|---|---|---|---|
| PD1 | Hollow gold nanoshell (HAuNS) | 4T1 breast tumor Colon cancer CT26 | CD4+ and CD8+ T cells | IFNγ, IL-2, TNFα | Reduced primary tumor growth and distant metastasis. | [148] |
| B cells | ||||||
| PD1 | Black phosphorus quantum dots (BPQDs) | BF16-F10 murine melanoma | DCs, CD4+ and CD8+ T cells | IFNγ, TNFα | Reduced primary tumor growth and inhibition of lung metastasis. | [149] |
| 4T1 breast tumor | ||||||
| PD1 | A triple-layer nano-system AuNC@mSiO2@ copolymer∩vemurafenib (ASP∩V) | SMM103 melanoma tumors | CD3+, CD4+ and CD8+ T cells | Not analyzed | Primary tumor regression and distant tumor regression by abscopal effect. | [150] |
| PD1 | ZIF-PQ-PDA-AUN | 4T1 breast tumor | CD4+ and CD8+ T cells | Not analyzed | Primary tumor regression. | [151] |
| CD47 | TAMs polarization from M2 to M1 | |||||
| PDL1 | Gold nanostar | Murine bladder cancer | CD4+ and CD8+ T cells | Not analyzed | Reduced primary tumor growth and distant metastasis. Long-term immunity in re-challenge experiments. | [145] |
| MB49 | B cells | |||||
| PDL1 | Au@Pt nanoparticles | 4T1 breast tumor | CD4+ and CD8+ T cells | IFNγ, IL-6, IL-12, TNFα | Regression of primary and distal tumors, inhibition of metastasis. | [152] |
| PDL1 and IDO | Reduced graphene oxide-based nanosheets | CT26 murine colon cancer | DCs, NK cells, CD45+ leukocytes, CD4+ and CD8+ T cells | IFNγ | Primary tumor regression and distant tumor regression by abscopal effect. | [153] |
| PDL1 and R837 | Fe3O4-R837 spherical superparticles | 4T1 breast tumor | DCs, NK cells, B cells, CD4+ and CD8+ T cells | IFNγ, IL-6, TNFα | Primary tumor regression and distant tumor regression by abscopal effect. | [154] |
| CTLA4 | Single-walled nanotubes (SWNTs) | BF16-F10 murine melanoma | DCs, CD4+, CD8+, CD20+ T cells | IL-6, IL-12, IL-1β, TNFα | Reduced primary tumor growth and distant metastasis. | [146] |
| 4T1 breast tumor | ||||||
| CTLA4 | Prussian blue nanoparticles (PBNP) | Murine neuroblastoma cell Neuro2a | CD4+ and CD8+ T cells | Not analyzed | Lower tumor burden, synergistic effect on enhanced survival, development of immune memory in re-challenge experiments. | [155] |
| CTLA4 and R837 | Indocyanine green and R837 co-encapsulated by poly (lactic-co-glycolic) acid (PLGA) | 4T1 breast tumor | DCs, CD4+, CD8+ T cells, memory T cells | IL-6, IL-12, IL-1β TNFα, IFNγ | Primary tumor regression and distant tumor regression by abscopal effect; inhibition of metastasis. | [147] |
| Colon cancer CT26 |
| Checkpoint Inhibitor Target | Radiation Therapy Dose (Fractions) | Murine Tumor Model | Immune Effector Cells | Cytokines | Therapeutic/Immune response | Ref. |
|---|---|---|---|---|---|---|
| PD1 | 8 Gy (4 fractions) | Metastatic melanoma in the brain | CD8+ T cells | Not analyzed | Reduced tumor growth and systemic immunity by abscopal effect | [187] |
| PD1 | 24 Gy (3 fractions) | Non-small-cell lung carcinoma | Neutrophils, CD4+ and CD8+ T cells | IL-5, IFNγ, TNFα | Higher lung injury score, increased inflammatory response | [188] |
| PD1 | 16 Gy (2 fractions) | B16-F10 melanoma TS/A mammary adeno-carcinoma | DCs, monocytes, macrophages and CD8+ T cells | IFNβ upregulated in abscopal tumors | Reduced tumor growth and systemic immunity by abscopal effect | [189] |
| PDL1 | 12 Gy | Pancreatic cancer | CD4+ and CD8+ T cells, myeloid-derived suppressor cells, tumor-associated macrophages | Not analyzed | Reduced primary tumor growth and systemic immunity by abscopal effect | [190] |
| PDL1 | 10 Gy | Head and neck squamous cell carcinoma | CD4+ and CD8+ T cells | Not analyzed | Enhanced tumor control and improved survival | [191] |
| PDL1 | 10 Gy | Hepatocellular carcinoma | CD8+ T cells | Not analyzed | Significant suppression of tumor growth and improved survival | [192] |
| CTLA4 along with immature dendritic cells (iDCs) | 10 Gy | Colon cancer CT26 | IFNγ-secreting T cells, CD8+ CTLs | IFNγ | Suppression of tumor growth and improved survival of tumor-bearing mice | [193] |
| CTLA4 | 10 Gy | Orthotopic glioma | CD4+ and CD8+ T cells | Not analyzed | Improved survival of treated mice | [194] |
| PD1 + CTLA4 | 20 Gy (either single dose or in fractions) | 4T1 mammary carcinoma | APCs, CD4+ and CD8+ cells | IFNγ | Primary tumor regression, abscopal effect in fractionated dose | [195] |
| PD1 + CTLA4 | 10 Gy | LM8 osteosarcoma | CD8+ T cells | Not analyzed | Reduced primary tumor growth and lung metastasis, systemic immunity by abscopal effect | [196] |
| Checkpoint Molecule Targeted for ICI | ICI Agent Used | Disease | Radiation Therapy Dose (Fractions) | Additional Drugs Used | Estimated Patient Accrual (n) | Timing of Radiotherapy | ClinicalTrials.gov for ICI Identifier * |
|---|---|---|---|---|---|---|---|
| PD1 | Nivolumab | Glioblastoma | 2 Gy × 30 | Temozolomide | 693 | n/s | NCT02667587 |
| PD1 | Nivolumab | Glioblastoma | not specified | Temozolomide | 550 | n/s | NCT02617589 |
| PD1 | Pembrolizumab | HNSCC, locally advanced | 2 Gy × 35 | Cisplatin | 780 | ICI then RT (RT at cycle 2 of ICI) | NCT03040999 |
| PD1 | Nivolumab | HNSCC, locally advanced | n/s | Cisplatin, Cetuximab | 1046 | n/s | NCT03349710 |
| PD1 | Pembrolizumab | Breast cancer, triple negative | n/s | chemotherapy | 1000 | RT then ICI | NCT02954874 |
| PD1 | Nivolumab | NSCLC, Stage IV | 4 Gy × 5 | none | 130 | ICI then RT | NCT03044626 |
| PD1 | Pembrolizumab | Breast cancer, localized | 8 Gy × 3 (alternate days) | ± Flt3 ligand (CDX-301) | 100 | n/s | NCT03804944 |
| PD1 | Nivolumab | Pancreatic cancer (PDAC) | 6.6 Gy × 5 | ± CCR2/CCR5 dual antagonist; ± GVAX | 30 | RT then ICI | NCT03767582 |
| PD-L1 | Durvalumab | Glioblastoma, recurrent | 8 Gy × 3 once daily | none | 62 | RT then ICI (ICI starts on last day of RT) | NCT02866747 |
| PD-L1 | Durvalumab | Breast cancer, luminal B | SBRT 8 Gy × 2 fractions preoperatively | chemotherapy, ± anti-CD73 (oleclumab) | 147 | RT then ICI | NCT03875573 |
| PD-L1 | Avelumab | Hepatobiliary malignancy(advanced) | Hypofractionated in 5 fractions | DNA-PK inhibitor | 92 | RT then ICI | NCT04068194 |
| PD-L1 | Avelumab | Various advanced solid tumors | 30 Gy in 10 fractions over 2 weeks | DNA-PK inhibitor | 54 | RT and ICI together (1st dose), then ICI continues | NCT03724890 |
| CTLA4 | Ipilimumab | Prostate cancer (metastatic) | n/s | none | 988 | RT then ICI | NCT00861614 |
| PD1, and PD-L1 | Nivolumab, and atezolizumab | RCC Stage IV, or UC Stage IV | 3 Gy × 10 | none | 112 | RT begins ±24 h of ICI start | NCT03115801 |
| PD1, and CTLA4 | Nivolumab, and Ipilimumab | NSCLC, Stage IV | n/s | none | 270 | ICI then RT | NCT03391869 |
| PD-L1, and CTLA4 | Durvalumab, and tremelimumab | NSCLC and colon cancer | High dose: 1 daily fraction × 3 days; Low dose: 2 fx daily on weeks 2, 6, 10, and 14 | none | 180 | ICI then RT | NCT02888743 |
| PD-L1, and CTLA4 | Durvalumab, and tremelimumab | SCLC, relapsed | SBRT or hypofractionated RT over 3–5 days | none | 20 | RT then ICI | NCT02701400 |
| PD-L1, and CTLA4 | Durvalumab, and tremelimumab | SCLC, advanced stage | 30 Gy in 10 fractions over 2 weeks | PARP inhibitor (olaparib) | 54 | RT then ICI | NCT03923270 |
| PD-L1, and CTLA4 | Durvalumab, and tremelimumab | Esophageal cancer, Stage III–IV | n/s | chemotherapy | 75 | ICI then RT | NCT02735239 |
| Any ICI target | Any approved agent | Any metastatic cancer, with a lesion treatable with SBRT | SBRT 9.5 Gy × 3 | none | 146 | ICI then RT | NCT02843165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, S.; Chan, T.A.; Hasan, T.; Maytin, E.V. Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals 2021, 14, 447. https://doi.org/10.3390/ph14050447
Anand S, Chan TA, Hasan T, Maytin EV. Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals. 2021; 14(5):447. https://doi.org/10.3390/ph14050447
Chicago/Turabian StyleAnand, Sanjay, Timothy A. Chan, Tayyaba Hasan, and Edward V. Maytin. 2021. "Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review)" Pharmaceuticals 14, no. 5: 447. https://doi.org/10.3390/ph14050447
APA StyleAnand, S., Chan, T. A., Hasan, T., & Maytin, E. V. (2021). Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals, 14(5), 447. https://doi.org/10.3390/ph14050447






