Abstract
Inside cells, the immunomodulator methotrexate (MTX) undergoes the addition of glutamates to form methotrexate polyglutamates (MTX-Glu)—promising biomarkers of systemic exposure and treatment response to MTX in rheumatology. MTX-Glu are underexplored in Inflammatory Bowel Disease (IBD), with no data in pediatrics. In this cross-sectional secondary analysis, we assessed the relationships between MTX-Glu and MTX dose and treatment response in pediatric IBD. Twenty-one children with IBD, receiving maintenance therapy with infliximab (IFX) and MTX, had MTX-Glu1–6 concentrations and IFX troughs/antibodies measured and disease activity assessed for comparison in remission vs. active IBD using non-parametric tests, with associations explored using Spearman’s correlation (ρ) and regression analyses; SASv9.4 (α = 0.05). Total and long-chain MTX-Glu correlated with MTX dose (ρ = 0.51 and 0.56, respectively; p ≤ 0.02). In children with Crohn’s disease (n = 19), short-chain MTX-Glu1–2 were 2.5-fold higher in remission vs. active disease, approaching statistical significance (p = 0.066), with no statistical differences in IFX trough (p = 0.549) between groups. Our study highlights a potential role for long-chain MTX-Glu in the therapeutic drug monitoring of MTX in IBD. It is the first study in pediatric IBD and, although statistical significance was not reached, our findings also suggest that higher short-chain MTX-Glu levels may be associated with IBD treatment response to MTX in children.
1. Introduction
Methotrexate (MTX) is a folic acid analog used as monotherapy, or in combination with anti-TNF-α agents, for the treatment of autoimmune disorders [1]. To exert its action, MTX enters cells via folate transporters and receptors, and undergoes the addition of glutamic acid (Glu) residues by folylpolyglutamate synthetase to form methotrexate polyglutamates (MTX-Glun) [2], where n represents the number of glutamic acid residues, with MTX-Glu1 denoting the parent form of MTX. The addition of glutamic acid to MTX occurs sequentially, and the metabolites formed are classified based on the length of the glutamate side chain, where glutamate Glu1 and 2 are considered short-chain and Glu3–6 long-chain [3]. Unlike MTX-Glu1–2, MTX-Glu3–6 are not effluxed from the cell efficiently and, therefore, reflect steady-state intracellular methotrexate concentration. In addition, the polyglutamated metabolites of MTX have a differential activity profile compared to the parent drug, with similar inhibitory activity against dihydrofolate reductase, but increased direct inhibitory activity against folate-dependent enzymes involved in purine and pyrimidine biosynthesis [4]. In rheumatology, erythrocyte concentrations of MTX-Glun have been associated with systemic exposure to MTX and MTX treatment efficacy [3,5,6,7]. Data are lacking in inflammatory bowel disease (IBD), where erythrocyte MTX-Glun levels could offer a clinically useful biomarker for MTX responsiveness and/or a measure for therapeutic drug monitoring—currently unavailable for this drug despite its frequent use. The aim of this prospective, single-center, cross-sectional, secondary analysis of pediatric patients receiving stable doses of infliximab (IFX) and MTX for IBD was to explore the relationships between MTX-Glun and MTX dose and treatment response in IBD.
2. Results
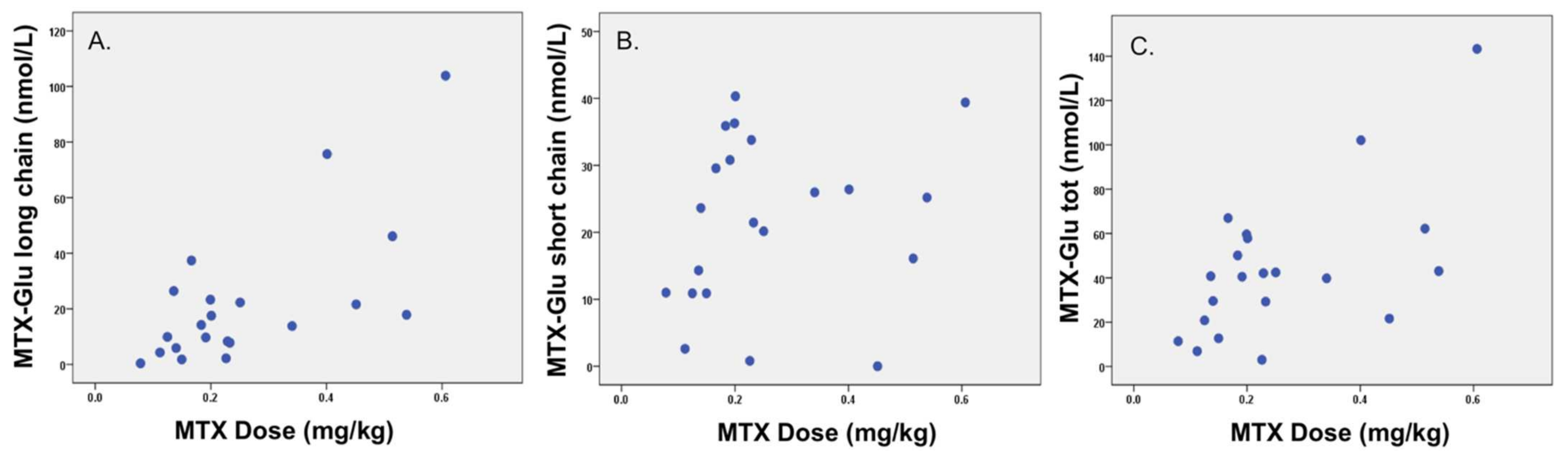
Children included in this secondary analysis completed a convenience-sampling cross-sectional investigation of IFX pharmacokinetics across different pediatric autoimmunity diagnoses [8]. Of the 21 children enrolled (median age 16 years (IQR 12, 17), 38% F, 90% Crohn’s disease), 10 had active disease (six mild, three moderate, and one severe) and 11 were in remission (Paris Disease Classification in Table 1). One child with active disease (mild), and undetectable IFX trough, had anti-IFX antibodies. The two study groups were otherwise comparable in age, at 16.0 (13.0, 17.0) vs. 14.5 (9.0, 19.0) years, and disease duration, at 2.5 (1.8, 5.2) vs. 3.8 (1.1, 4.5) years; both p ≥ 0.8. Clinically prescribed MTX doses for patients varied from 5 to 25 mg weekly, with 86% oral administration. To account for inter-individual variability in MTX dose, as well as variability in patient age and size in our pediatric cohort (5–21-year-olds), the MTX dose was adjusted for total body weight (mg/kg). Adjusted for weight (mg/kg), the MTX dose correlated significantly with long-chain MTX-Glu3–5 (ρ = 0.56; p = 0.009) and MTX-GluTotal (ρ = 0.51; p = 0.018), but not short-chain Glu1–2 (ρ = 0.27, p = 0.244); see Figure 1. MTX-Glu6 was undetectable across the study population.

Table 1.
Paris Classification. Age at diagnosis: A1a (0–<10 years); A1b (10–<17 years). Location: L1: distal 1/3 ileum +/− limited cecal disease; L2: colonic; L3: ileocolonic; L4a: upper disease proximal to ligament of Treitz; L4b: upper disease distal to ligament of Treitz and proximal to distal 1/3 ileum. Extent: E1: ulcerative proctitis; E2: Left-sided UC (distal to splenic flexure); E3: extensive (hepatic flexure distally); E4: pancolitis (proximal to hepatic flexure). Behavior: B1: nonstricturing nonpenetrating; B2: structuring; B3: penetrating; B2B3: both penetrating and stricturing disease, either at the same or different times; p: perianal disease modifier. Severity: S0: never severe; S1: ever severe. Growth: G0: no evidence of growth delay; G1: growth delay.
Figure 1.
Spearman’s correlation (ρ) between methotrexate polyglutamate (MTX-Glu) concentrations and methotrexate (MTX) dose for long-chain MTX-Glu3–5, ρ = 0.56; p = 0.009 (A); short-chain Glu1–2, ρ = 0.27; p = 0.244 (B); and total MTX-Glu (MTX-Glutot), ρ = 0.51; p = 0.018 (C).
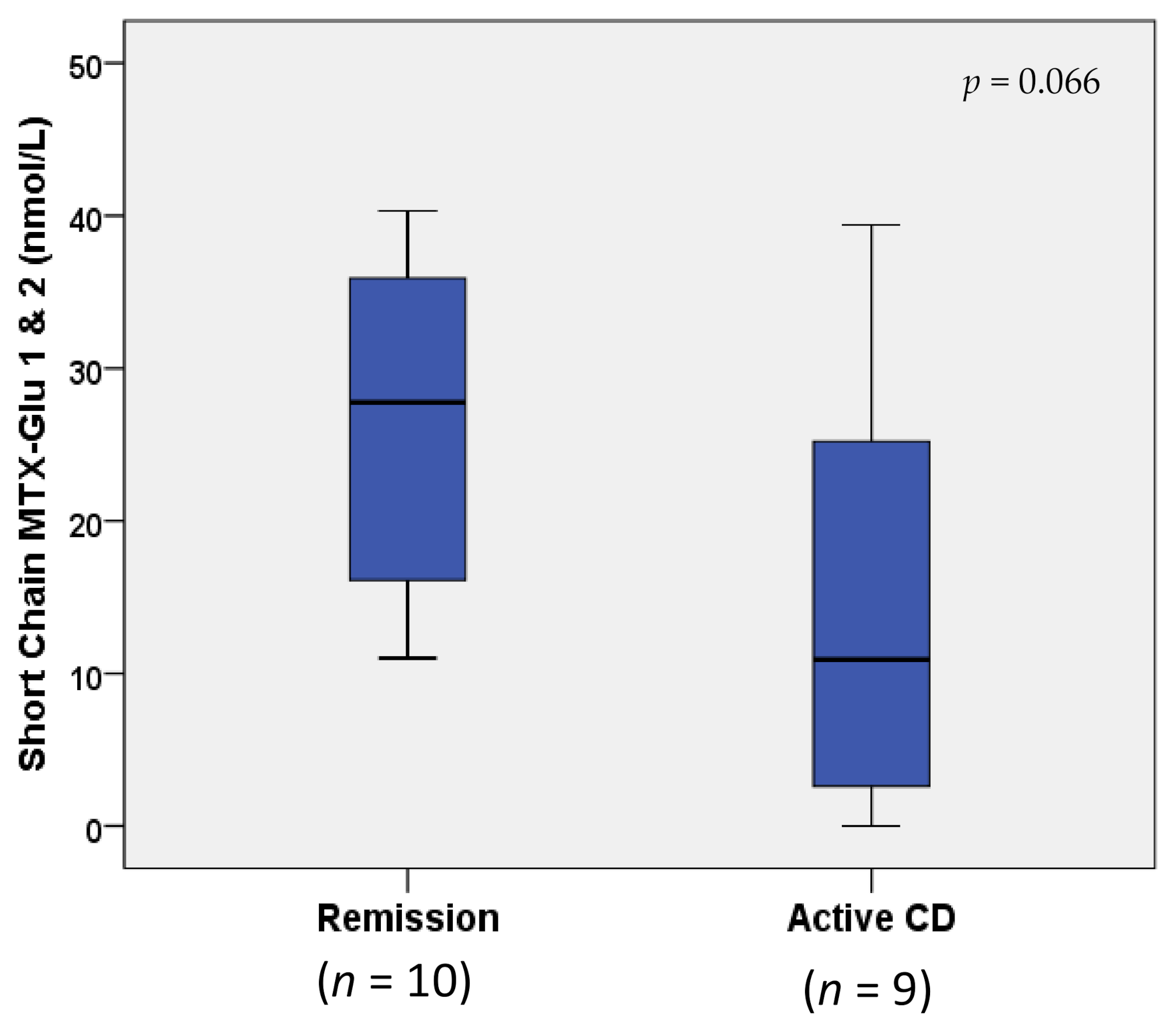
MTX-Glun concentrations were not statistically different between children with IBD in remission vs. active disease (p > 0.1; data not shown). However, when MTX-Glun concentrations were compared only in children with Crohn’s disease (n = 10 remission vs. n = 9 active), an increase greater than two-fold in short-chain MTX-Glu1–2 concentrations (nmol/L) was observed in remission (27.77 (16.10, 35.90)) vs. active Crohn’s disease (10.90 (2.60, 25.20)), approaching statistical significance (p = 0.066; Figure 2). The two Crohn’s disease study groups were receiving comparable dosing of both MTX (0.20 (0.17, 0.23) vs. 0.23 (0.15, 0.45) mg/kg weekly) and IFX (9.24 (7.45, 10.04) mg/kg every 4.0 (4.0, 6.0) weeks vs. 9.56 (8.49, 10.03) mg/kg every 5.0 (4.0, 6.0) weeks); p ≥ 0.554 (Table 2). Median and IQR IFX troughs (μg/mL) were within therapeutic range (i.e., ≥3–7 μg/mL [9]) in both groups, and not statistically different between groups (25.28 (5.35, 38.96) vs. 15.54 (7.11, 24.15)); p = 0.540 (Table 2). However, when IFX was included as a covariate in a logistic regression model, the differences in short-chain MTX-Glun between remission and active Crohn’s disease became less apparent (p = 0.130).
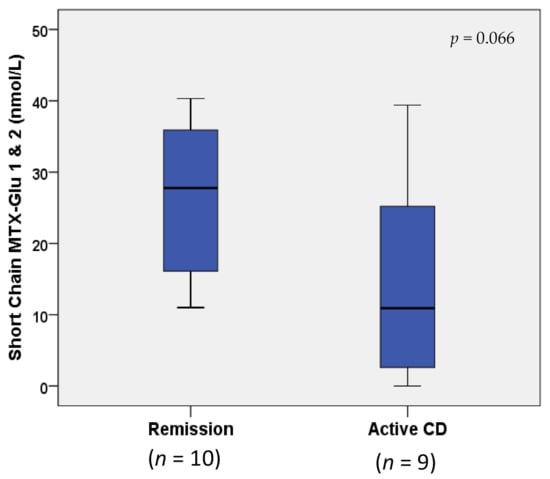
Figure 2.
Box and whisker plot comparison of erythrocyte MTX-Glu1–2 concentrations in remission vs. active Crohn’s disease. Horizontal line in boxes represents median value, with whiskers representing the range.

Table 2.
Patient characteristics for children with Crohn’s disease: IFX: infliximab, MTX: methotrexate, adjIFX: dose or concentration adjusted for mg/kg IFX received and time since drug administration. All patients received weekly MTX.
Cut-off values for MTX-Glun concentrations previously associated with treatment response in rheumatology [5] were probed, and none were found to discriminate remission from active disease in IBD or in the IBD subset with Crohn’s disease only (data not shown).
3. Discussion
To the best of our knowledge, this is the first study of MTX-Glun levels in pediatric IBD and it offers valuable insights for future investigations. First, our data demonstrate that long-chain MTX-Glun levels correlate with methotrexate dose and could, therefore, serve as markers of systemic MTX exposure, offering a therapeutic drug monitoring option for MTX. Second, in children with Crohn’s disease, short-chain MTX-Glun levels were over two-fold higher in patients with disease in remission, compared to active disease, suggesting that short-chain MTX-Glu may have a role in Crohn’s disease treatment responsiveness to MTX.
Although our observation of higher short-chain MTX-Glu levels in CD in remission compared to active CD did not reach statistical significance (p = 0.066), this is likely due to limitations in the sample size and further obscured by participants receiving concomitant IFX therapy, as suggested by our multivariable regression analysis. Nevertheless, the observed trend in short-chain MTX-Glu suggests that efficient intracellular drug uptake and/or primary intracellular conversion of MTX-Glu1 to short-chain MTX-Glu2 may be associated with the treatment responsiveness of pediatric Crohn’s disease to MTX. An alternative explanation for this observation is that primary polyglutamation may be impaired in active disease, perhaps through altered intracellular drug uptake or inflammatory cytokine-mediated modulation of enzymes in the polyglutamation pathway (e.g., folypolyglutamate synthase or gamma glutamyl hydrolase, responsible for glutamation and deglutamation, respectively) [10]. Future studies should be aimed at understanding such sources and implications of inter-individual variability in polyglutamation for the treatment of pediatric IBD, where equipoise remains regarding up-front identification of patients who benefit most from MTX therapy (alone or in combination with biologics).
Ours is the first study of MTX-Glun in pediatric IBD and our findings regarding long-chain MTX-Glun echo findings in juvenile idiopathic arthritis (JIA) and rheumatoid arthritis (RA)—two chronic, autoimmune, inflammatory conditions also treated with MTX. Becker et al. previously demonstrated increased MTX-Glu3–5 formation as a function of MTX dose, route of administration, and duration of drug exposure in patients with JIA. Specifically, MTX dose escalation resulted in a preferential increase in long-chain MTX-Glun, at the expense of short-chain MTX-Glun [11]. A study in RA also demonstrated a positive association of MTX dose with long-chain MTX-Glu3, MTX-Glu4, MTX-Glu5, and MTX-Glu3–5, while observing a weaker association between dose and total MTX-Glu1–5 [12], analogous to the observations in our study.
Previous studies in rheumatology have also commented on the relationship between MTX-Glun and treatment response [3,5,6,7]. No statistically significant differences in individual or total MTX-Glun concentrations were observed in children with IBD in remission vs. active disease in our study; however, MTX therapy is felt to be less effective for ulcerative colitis than Crohn’s disease [13,14]. Looking only at children with Crohn’s disease (n = 19), a positive trend toward higher short-chain MTX-Glun concentrations was observed in children with remission vs. active disease, approaching statistical significance (p = 0.066). The two Crohn’s disease study groups were otherwise comparable for age and disease duration, without statistically significant differences in IFX troughs between groups. Although a trend toward greater IFX troughs (both absolute and adjusted for clinical variability in IFX dose and interval) was observed in children with remission vs. active Crohn’s disease, this trend did not approach statistical significance (p = 0.549), and mean and median IFX troughs for both groups fell well within the therapeutic range (i.e., ≥3–7 μg/mL [9]); see Table 2. Nevertheless, we acknowledge that concomitant IFX therapy may be a confounding variable in our MTX-Glu analyses, as supported by observations of a less obvious difference in short-chain MTX-Glun between Crohn’s disease in remission and active disease after incorporating IFX into a multivariable logistic regression model. While some previous studies of IFX and MTX have shown a questionable benefit of combination therapy for Crohn’s disease [15], our findings raise the possibility that the benefit of combination therapy may be related to the intracellular MTX-Glun concentration achieved, emphasizing the need for further investigation of MTX-Glun monitoring in IBD.
Although published rheumatology data to date are felt insufficient to recommend the definite implementation of MTX-Glun therapeutic drug monitoring in clinical practice [16], a systematic review of the rheumatology literature from 2015 provides supportive evidence for the role of MTX-Glun as biomarkers of autoimmune disease response to MTX treatment [7]. In a study of MTX-naïve patients with RA, short-chain MTX-Glu2 correlated with an improvement in clinical assessments over 16 weeks of MTX therapy. [3] In another study of RA, de Rotte et al. proposed concentrations of MTX-Glu2 > 22 nmol/L and MTX-GluTotal > 74 nmol/L as predictors of moderate/good clinical response to MTX. [5] Cut-off values to discriminate MTX responders from non-responders could not be established in our study, but this is likely secondary to limitations in sample size.
Previous studies of MTX-Glun in IBD are sparse and limited to adult observational studies smaller than ours, which may explain why prior studies also failed to demonstrate a statistically significant association between MTX-Glun and IBD treatment response. [17,18]. In a cross-sectional study of 12 adults, Fischer et al. observed higher long-chain MTX-Glu3–5 in patients with Crohn’s disease in remission vs. active disease, but the trend did not reach statistical significance. [17] Brooks et al. found long-chain MTX-Glu4–5 to correlate inversely with MTX efficacy in 18 adults with Crohn’s disease, with higher levels noted in patients experiencing gastrointestinal adverse effects [18]. However, findings of an inverse relationship between MTX-Glun and MTX efficacy are not substantiated in most rheumatology literature [5,6,7], except for one study that found a positive correlation for MTX-Glun with MTX dose, but a negative correlation with treatment outcomes in RA [19].
Studies of MTX-Glun in children are rare and, to the best of our knowledge, ours is the first pediatric study in IBD. In a pediatric study of JIA, higher concentrations of long-chain MTX-Glun (MTX-Glu3–5) were associated with lower disease activity at 3 months and 1 year of MTX therapy. [6] The cross-sectional nature of our pediatric study does not allow for a longitudinal assessment. However, despite the significant positive correlation of long-chain MTX-Glun with MTX dose in our study (ρ = 0.56; p = 0.01), we did not observe a relationship with disease response, suggesting that the relationship between long-chain MTX-Glun and treatment response may be disease-specific and could differ between JIA and pediatric IBD.
Although this is the largest study of MTX-Glun in IBD to date, the limitations of our study reside with its small sample size and cross-sectional design. The present study design does not allow us to comment on the relationship between maintenance MTX-Glun concentrations and long-term disease outcomes in IBD. The decision to study patients receiving combination therapy with MTX and IFX may be viewed as another study limitation; however, at our institution, MTX is most often used in combination with biologics, rather than as monotherapy, for the treatment of IBD. Thus, the selected study population is more generalizable to the patient population at large, which is important for translating study results into clinical practice. To account for potential confounders from concomitant IFX treatment, IFX troughs were measured at the time of MTX-Glun assessment, using a single CLIA-approved assay, and incorporated into our statistical analyses. Future investigations should be aimed at longitudinal, combination, and monotherapy MTX studies, to build on our observations of the potential role of long-chain MTX-Glun in the therapeutic drug monitoring of systemic exposure to MTX, and short-chain MTX-Glun as potential biomarkers of IBD responsiveness to MTX treatment. Prospective, multicenter, dose-controlled trials of MTX could help to identify therapeutic target ranges for MTX-Glun in IBD.
4. Materials and Methods
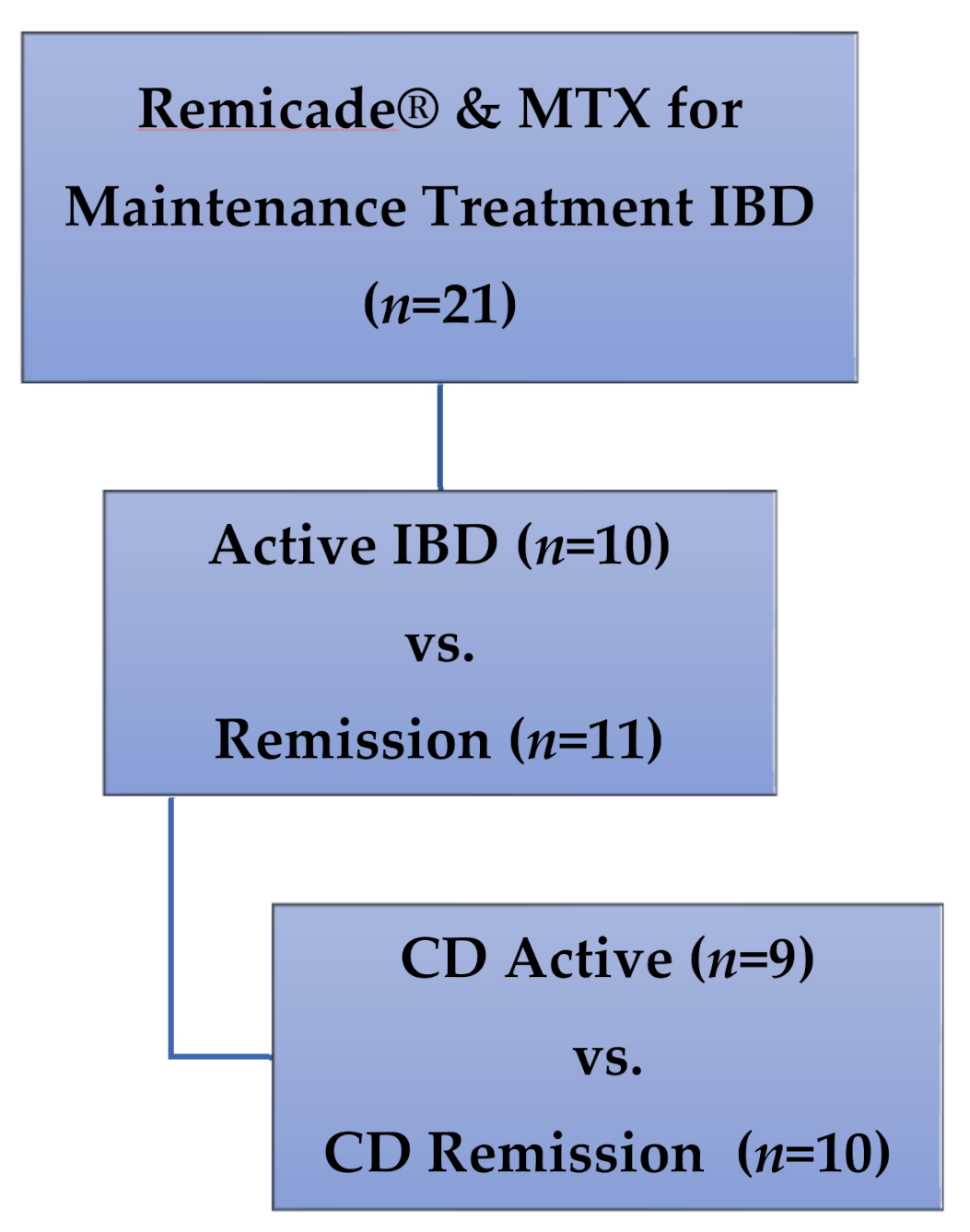
Patients: Children enrolled in a cross-sectional study of IFX monotherapy vs. combination IFX therapy with concurrent immunomodulators, and receiving medication doses in accordance with prescriber preference for clinical care of IBD [20], were included in this secondary analysis. All participants were enrolled at a single outpatient infusion center at the Children’s Mercy Hospital (Kansas City, MO, USA). Only patients receiving therapy with both IFX (Remicade®) and MTX were included in this secondary analysis (Figure 3), with maintenance dosing defined as no changes in dose or interval of either drug for at least two IFX infusion cycles.
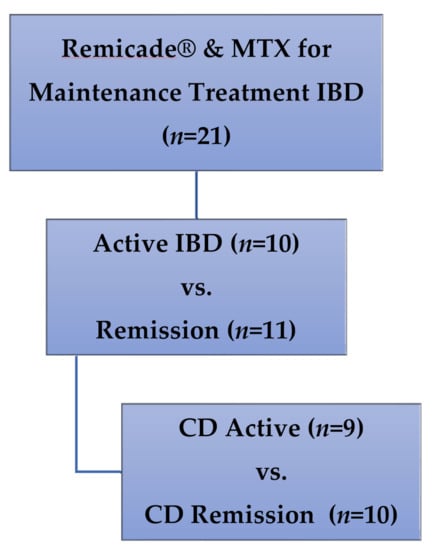
Figure 3.
Patients included in this secondary analysis of methotrexate polyglutamates in pediatric IBD. MTX: methotrexate, IBD: inflammatory bowel disease, CD: Crohn’s disease.
Clinical parameters: Clinical parameters were assessed using Physician Global Assessment (PGA) scores via agreement by two independent pediatric gastroenterologists. Due to limitations in sample size, PGA mild, moderate, and severe were grouped together as active disease (active) and compared to quiescent disease (remission).
Analytical techniques and validation: MTX-Glu1–6 concentrations were measured in erythrocytes, using high-performance liquid chromatography/tandem mass spectrometry utilizing a previously established assay [20]. IFX troughs and anti-IFX antibodies (anti-IFX) were measured using a CLIA-approved NF-kB luciferase gene-reporter assay, with lower and upper limits of quantification at 0.65 and 40 μg/mL, respectively (ARUP Laboratories, Salt Lake City, Utah, UT, USA).
Statistical analysis: Nonparametric tests (e.g., Wilcoxon Rank Sum and Fisher’s Exact tests) were used to compare MTX-Glu, IFX, anti-IFX, laboratory, and demographic data in children with remission vs. active disease. Spearman’s correlation (ρ) was used to look for relationships between continuous variables, and multivariable logistic regression models were used to look for differences between study groups, while controlling for covariates. Previously reported cut-off values in rheumatology of MTX-GluTotal > 74 nmol/L and MTX-Glu2 > 22 nmol/L were probed to discriminate remission from active disease in IBD [5]. A significance level of 0.05 in SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), was used for all analyses. Unless otherwise specified, data are reported as median (IQR).
Author Contributions
R.M.: Performed data collection, and data analysis and interpretation. Reviewed current literature in both rheumatology and gastroenterology with regard to use of methotrexate polyglutamates for clinical purposes. Wrote the manuscript and participated in manuscript edits and revisions. R.F.: Participated in study design and provided expertise on methotrexate polyglutamates and IFX analytics. Participated in data analysis and interpretation. Revised and edited manuscript. M.B.: Provided expertise on methotrexate polyglutamates and IFX analytics. Participated in data analysis and interpretation. Revised and edited manuscript. A.S.: Provided expertise in statistical methods and analyses. Participated in data analysis and interpretation. Provided revisions and edits for manuscript. L.V.H.: Provided expertise on analytical techniques of measuring methotrexate polyglutamates in erythrocytes with HPLC/MS. Participated in data analysis and interpretation. Provided revisions and edits for manuscript. T.H.: Independently reviewed clinical data, assigned Paris classifications, and PGA scores. Provided revisions and edits for manuscript. R.C.: Participated in study design, data collection, and data analysis and interpretation. Provided revisions and edits for manuscript. V.S.: Participated in study design, data collection, and data analysis and interpretation. Provided expertise with regard to pharmacotherapy in patients with IBD. Participated in and provided oversight for manuscript preparation. Provided revisions and edits for the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
RSF is supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (# KL2TR002367), and a COBRE grant from NIGMS awarded to the Kansas Institute for Precision Medicine (# P20GM130423).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Children’s Mercy Kansas City (IRB# 14100454, date of approval 2 June 2015).
Informed Consent Statement
Informed consent and permission/assent was obtained from all subjects involved in the study prior to any study related procedures.
Data Availability Statement
Data are not publicly archived, but deidentified data can be made available upon request.
Acknowledgments
The authors are grateful for analytical support from Julio Delgado and ARUP Laboratories for measuring infliximab trough concentrations, philanthropic contribution to Children’s Mercy Kansas City from Todd and Emily Novicoff, and investigator support through L40 TR000598 from NCATS (VS PI)—a CTSA grant from NCATS (# KL2TR002367) awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute, and a COBRE grant from NIGMS (# P20GM130423) awarded to the Kansas Institute for Precision Medicine (RSF PI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rampton, D.S. Methotrexate in Crohn′s Disease. Gut 2001, 48, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Egan, L.J.; Sandborn, W.J. Methotrexate for Inflammatory Bowel Disease: Pharmacology and Preliminary Results. Mayo Clin. Proc. 1996, 71, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hobl, E.-L.; Jilma, B.; Erlacher, L.; Duhm, B.; Mustak, M.; Bröll, H.; Högger, P.; Rizovski, B.; Mader, R.M. A Short-Chain Methotrexate Polyglutamate as Outcome Parameter in Rheumatoid Arthritis Patients Receiving Methotrexate. Clin. Exp. Rheumatol. 2012, 30, 156–163. [Google Scholar] [PubMed]
- Smoleńska, Ż.; Kaznowska, Z.; Zarówny, D.; Simmonds, H.A.; Smoleński, R.T. Effect of Methotrexate on Blood Purine and Pyrimidine Levels in Patients with Rheumatoid Arthritis. Rheumatology 1999, 38, 997–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Rotte, M.C.F.J.; Den Boer, E.; De Jong, P.H.P.; Pluijm, S.M.F.; Ćalasan, M.B.; Weel, A.E.; Huisman, A.M.; Gerards, A.H.; Van Schaeybroeck, B.; Wulffraat, N.M.; et al. Methotrexate Polyglutamates in Erythrocytes are Associated with Lower Disease Activity in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2013, 74, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ćalasan, M.B.; Den Boer, E.; De Rotte, M.C.F.J.; Vastert, S.J.; Kamphuis, S.; De Jonge, R.; Wulffraat, N.M. Methotrexate Polyglutamates in Erythrocytes Are Associated with Lower Disease Activity in Juvenile Idiopathic Arthritis Patients. Ann. Rheum. Dis. 2013, 74, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.J.; Sorich, M.J.; Kowalski, S.M.; McKinnon, R.; Proudman, S.M.; Cleland, L.; Wiese, M.D. The Role and Utility of Measuring Red Blood Cell Methotrexate Polyglutamate Concentrations in Inflammatory Arthropathies—A Systematic Review. Eur. J. Clin. Pharmacol. 2015, 71, 411–423. [Google Scholar] [CrossRef]
- Funk, R.S.; Shakhnovich, V.; Cho, Y.K.; Polireddy, K.; Jausurawong, T.; Gress, K.; Becker, M.L. Factors Associated with Reduced Infliximab Exposure in the Treatment of Pediatric Autoimmune Disorders: A Cross-Sectional Prospective Convenience Sampling Study. Pediatr. Rheumatol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Casteele, N.V.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steenet, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough Concentrations of Infliximab Guide Dosing for Patients With Inflammatory Bowel Disease. Gastroenterology 2015, 148, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Gaedigk, R.; Van Haandel, L.; Thomas, B.; Lasky, A.; Hoeltzel, M.; Dai, H.; Stobaugh, J.; Leeder, J.S. The Effect of Genotype on Methotrexate Polyglutamate Variability in Juvenile Idiopathic Arthritis and Association with Drug Response. Arthritis Rheum. 2010, 63, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.L.; Van Haandel, L.; Gaedigk, R.; Lasky, A.; Hoeltzel, M.; Stobaugh, J.; Leeder, J.S. Analysis of Intracellular Methotrexate Polyglutamates in Patients with Juvenile Idiopathic Arthritis: Effect of Route of Administration on Variability in Intracellular Methotrexate Polyglutamate Concentrations. Arthritis Rheum. 2010, 62, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; O’Donnell, J.L.; Chapman, P.T.; Zhang, M.; Frampton, C.; James, J.; Barclay, M.L. Determinants of Red Blood Cell Methotrexate Polyglutamate Concentrations in Rheumatoid Arthritis Patients Receiving Long-Term Methotrexate Treatment. Arthritis Rheum. 2009, 60, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: A systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 2020, 20, 100271. [Google Scholar] [CrossRef] [PubMed]
- Herfarth, H.; Barnes, E.L.; Valentine, J.F.; Hanson, J.; Higginset, P.D.R.; Isaacs, K.L.; Jackson, S.; Osterman, M.T.; Anton, K.; Ivanova, A.; et al. Methotrexate Is Not Superior to Placebo in Maintaining Steroid-Free Response or Remission in Ulcerative Colitis. Gastroenterology 2018, 155, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; McDonald, J.W.; Panaccione, R.; Enns, R.A.; Bernstein, C.N.; Ponich, T.P.; Bourdages, R.; MacIntosh, D.G.; Dallaire, C.; Cohen, A.; et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014, 146, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Mastrangelo, G.; Barone, P.; La Torre, F.; Martino, S.; Pappagallo, G.; Ravelli, A.; Taddio, A.; Zulian, F.; Cimaz, R. Methotrexate in Juvenile Idiopathic Arthritis: Advice and Recommendations from the MARAJIA Expert Consensus Meeting. Pediatr. Rheum. 2018, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Siva, S.; Cook, G.K.; Jones, D.R.; Fadda, H.M. Methotrexate Polyglutamate Monitoring in Patients With Crohn′s Disease. Clin. Pharmacol. Drug Dev. 2017, 6, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Begg, E.J.; Zhang, M.; Frampton, C.M.; Barclay, M.L. Red Blood Cell Methotrexate Polyglutamate Concentrations in Inflammatory Bowel Disease. Ther. Drug Monit. 2007, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; O’Donnell, J.L.; Chapman, P.T.; Zhang, M.; James, J.; Frampton, C.; Barclay, M.L. Methotrexate Polyglutamate Concentrations Are Not Associated with Disease Control in Rheumatoid Arthritis Patients Receiving Long-Term Methotrexate Therapy. Arthritis Rheum. 2010, 62, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Van Haandel, L.; Becker, M.L.; Williams, T.D.; Leeder, J.S.; Stobaugh, J.F. Measurement of Methotrexate Polyglutamates in Human Erythrocytes by Ion-Pair UPLC–MS/MS. Bioanalysis 2011, 3, 2783–2796. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).