Treatment with an Anti-CX3CL1 Antibody Suppresses M1 Macrophage Infiltration in Interstitial Lung Disease in SKG Mice
Abstract
:1. Introduction
2. Results
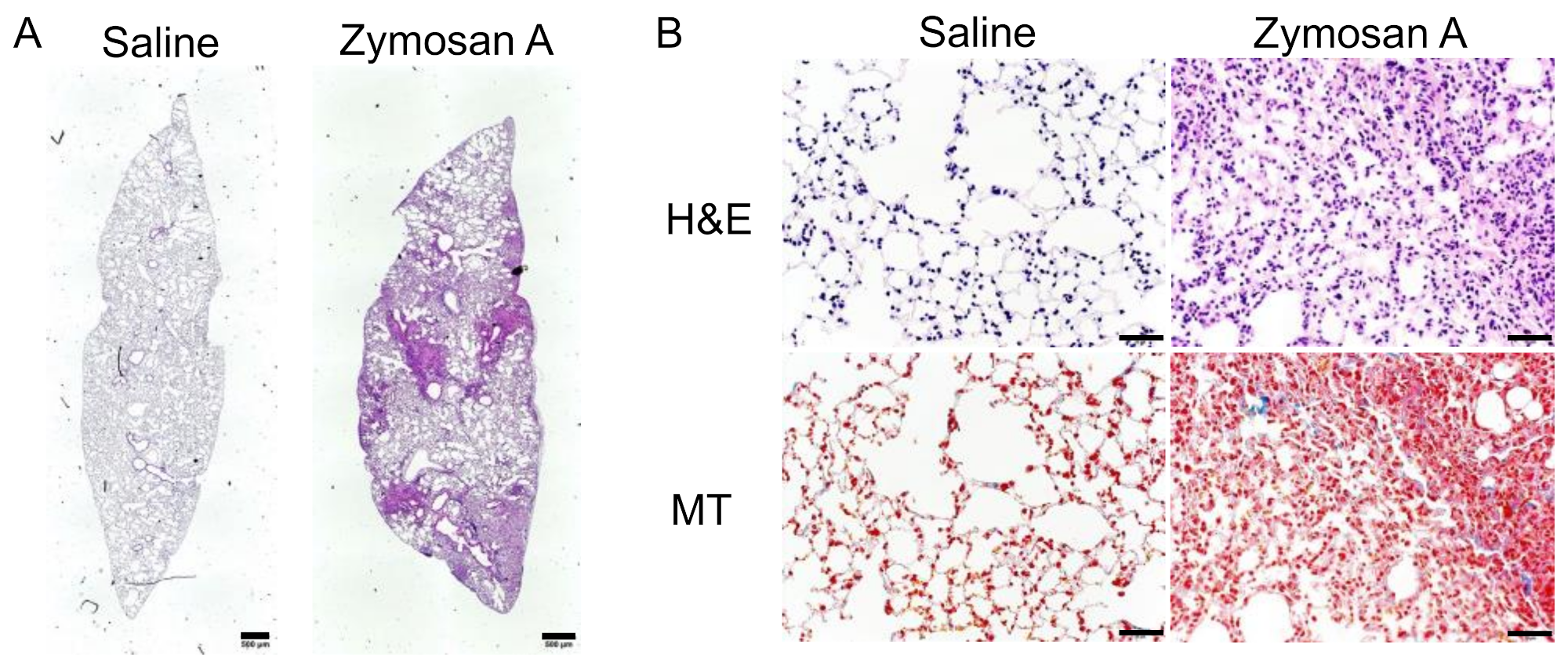
2.1. Histopathological Findings of SKG-ILD
2.2. Expression of CX3CL1 and CX3CR1 in Lungs with ILD in SKG Mice
2.3. Minimal Effects of the Blockade of CX3CL1 in the Lung Pathology of SKG-ILD
2.4. Flow Cytometric Analysis of Bronchoalveolar Fluid (BALF) Cells in SKG-ILD
2.5. Effects of the Blockade of CX3CL1 on Alveolar Macrophages in SKG-ILD
2.6. High Expression Levels of CX3CR1 in Alveolar M1 Macrophages
3. Discussion
4. Materials and Methods
4.1. SKG Mice
4.2. Histopathological Investigation
4.3. Flow Cytometric Analysis of BALF
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) for BALF
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Redente, E.F.; Aguilar, M.A.; Black, B.P.; Edelman, B.L.; Bahadur, A.N.; Humphries, S.M.; Lynch, D.A.; Wollin, L.; Riches, D.W.H. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L998–L1009. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am. J. Respir Crit. Care Med. 2011, 183, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, T.J.; Dellaripa, P.F.; Batra, K.; Frits, M.L.; Iannaccone, C.K.; Hatabu, H.; Nishino, M.; Weinblatt, M.E.; Ascherman, D.P.; Washko, G.R.; et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest 2014, 146, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Robbe, P.; Draijer, C.; Borg, T.R.; Luinge, M.; Timens, W.; Wouters, I.M.; Melgert, B.N.; Hylkema, M.N. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L358–L367. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Filardi, E.; Nieto, C.; Dominguez-Soto, A.; Barroso, R.; Sanchez-Mateos, P.; Puig-Kroger, A.; Lopez-Bravo, M.; Joven, J.; Ardavin, C.; Rodriguez-Fernandez, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Filardi, E.; Vega, M.A.; Sanchez-Mateos, P.; Corbi, A.L.; Puig-Kroger, A. Heme Oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology 2010, 215, 788–795. [Google Scholar] [CrossRef]
- Lu, H.L.; Huang, X.Y.; Luo, Y.F.; Tan, W.P.; Chen, P.F.; Guo, Y.B. Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Xie, N.; Cui, H.; Ge, J.; Banerjee, S.; Guo, S.; Dubey, S.; Abraham, E.; Liu, R.M.; Liu, G. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L834–L844. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Zhang, Z.; He, L.; Zhu, J.; Zhang, M.; He, X.; Cheng, Z.; Ao, Q.; Cao, Y.; et al. Chop Deficiency Protects Mice Against Bleomycin-induced Pulmonary Fibrosis by Attenuating M2 Macrophage Production. Mol. Ther. 2016, 24, 915–925. [Google Scholar] [CrossRef]
- Florez-Sampedro, L.; Song, S.; Melgert, B.N. The diversity of myeloid immune cells shaping wound repair and fibrosis in the lung. Regeneration 2018, 5, 3–25. [Google Scholar] [CrossRef]
- Moore, B.B.; Kolodsick, J.E.; Thannickal, V.J.; Cooke, K.; Moore, T.A.; Hogaboam, C.; Wilke, C.A.; Toews, G.B. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am. J. Pathol. 2005, 166, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Louie, M.C.; Vannella, K.M.; Wilke, C.A.; LeVine, A.M.; Moore, B.B.; Shanley, T.P. New concepts of IL-10-induced lung fibrosis: Fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L341–L353. [Google Scholar] [CrossRef] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [Green Version]
- McCubbrey, A.L.; Barthel, L.; Mohning, M.P.; Redente, E.F.; Mould, K.J.; Thomas, S.M.; Leach, S.M.; Danhorn, T.; Gibbings, S.L.; Jakubzick, C.V.; et al. Deletion of c-FLIP from CD11b(hi) Macrophages Prevents Development of Bleomycin-induced Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 66–78. [Google Scholar] [CrossRef]
- Kim, K.W.; Vallon-Eberhard, A.; Zigmond, E.; Farache, J.; Shezen, E.; Shakhar, G.; Ludwig, A.; Lira, S.A.; Jung, S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 2011, 118, e156–e167. [Google Scholar] [CrossRef] [Green Version]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Tsou, C.L.; Haskell, C.A.; Charo, I.F. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001, 276, 44622–44626. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Umehara, H.; Nakayama, T.; Yoneda, O.; Hieshima, K.; Kakizaki, M.; Dohmae, N.; Yoshie, O.; Imai, T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J. Immunol. 2002, 168, 6173–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruth, J.H.; Volin, M.V.; Haines, G.K., 3rd; Woodruff, D.C.; Katschke, K.J., Jr.; Woods, J.M.; Park, C.C.; Morel, J.C.; Koch, A.E. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001, 44, 1568–1581. [Google Scholar] [CrossRef]
- Koizumi, K.; Saitoh, Y.; Minami, T.; Takeno, N.; Tsuneyama, K.; Miyahara, T.; Nakayama, T.; Sakurai, H.; Takano, Y.; Nishimura, M.; et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J. Immunol. 2009, 183, 7825–7831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaschke, S.; Koziolek, M.; Schwarz, A.; Benohr, P.; Middel, P.; Schwarz, G.; Hummel, K.M.; Muller, G.A. Proinflammatory role of fractalkine (CX3CL1) in rheumatoid arthritis. J. Rheumatol. 2003, 30, 1918–1927. [Google Scholar]
- Nanki, T.; Urasaki, Y.; Imai, T.; Nishimura, M.; Muramoto, K.; Kubota, T.; Miyasaka, N. Inhibition of fractalkine ameliorates murine collagen-induced arthritis. J. Immunol. 2004, 173, 7010–7016. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeuchi, T.; Yamanaka, H.; Nanki, T.; Umehara, H.; Yasuda, N.; Tago, F.; Kitahara, Y.; Kawakubo, M.; Torii, K.; et al. Efficacy and Safety of E6011, an Anti-Fractalkine Monoclonal Antibody, in Patients With Active Rheumatoid Arthritis With Inadequate Response to Methotrexate: Results of a Randomized, Double-Blind, Placebo-Controlled Phase II Study. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeuchi, T.; Umehara, H.; Nanki, T.; Yasuda, N.; Tago, F.; Kawakubo, M.; Kitahara, Y.; Hojo, S.; Kawano, T.; et al. Safety, pharmacokinetics, and efficacy of E6011, an antifractalkine monoclonal antibody, in a first-in-patient phase 1/2 study on rheumatoid arthritis. Mod. Rheumatol. 2018, 28, 58–65. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Herrera, I.; Salgado-Aguayo, A.; Buendia-Roldan, I.; Becerril, C.; Cisneros, J. CX3CL1 and CX3CR1 could be a relevant molecular axis in the pathophysiology of idiopathic pulmonary fibrosis. Int. J. Med. Sci. 2020, 17, 2357–2361. [Google Scholar] [CrossRef]
- Yamada, S.; Miyoshi, S.; Nishio, J.; Mizutani, S.; Yamada, Z.; Kusunoki, N.; Sato, H.; Kuboi, Y.; Hoshino-Negishi, K.; Ishii, N.; et al. Effects of CX3CL1 inhibition on murine bleomycin-induced interstitial pneumonia. Eur. J. Inflamm. 2020, 18. [Google Scholar] [CrossRef]
- Suzuki, F.; Kubota, T.; Miyazaki, Y.; Ishikawa, K.; Ebisawa, M.; Hirohata, S.; Ogura, T.; Mizusawa, H.; Imai, T.; Miyasaka, N.; et al. Serum level of soluble CX3CL1/fractalkine is elevated in patients with polymyositis and dermatomyositis, which is correlated with disease activity. Arthritis Res. Ther. 2012, 14, R48. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann-Vold, A.M.; Weigt, S.S.; Palchevskiy, V.; Volkmann, E.; Saggar, R.; Li, N.; Midtvedt, O.; Lund, M.B.; Garen, T.; Fishbein, M.C.; et al. Augmented concentrations of CX3CL1 are associated with interstitial lung disease in systemic sclerosis. PLoS ONE 2018, 13, e0206545. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef]
- Ishida, Y.; Kimura, A.; Nosaka, M.; Kuninaka, Y.; Hemmi, H.; Sasaki, I.; Kaisho, T.; Mukaida, N.; Kondo, T. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci. Rep. 2017, 7, 16833. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, N.; Takahashi, T.; Hata, H.; Nomura, T.; Tagami, T.; Yamazaki, S.; Sakihama, T.; Matsutani, T.; Negishi, I.; Nakatsuru, S.; et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 2003, 426, 454–460. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Yoshitomi, H.; Hata, H.; Takahashi, T.; Nomura, T. Spontaneous development of autoimmune arthritis due to genetic anomaly of T cell signal transduction: Part 1. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2006; Volume 18, pp. 199–206. [Google Scholar]
- Shiomi, A.; Usui, T.; Ishikawa, Y.; Shimizu, M.; Murakami, K.; Mimori, T. GM-CSF but not IL-17 is critical for the development of severe interstitial lung disease in SKG mice. J. Immunol. 2014, 193, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, F.; Nanki, T.; Imai, T.; Kikuchi, H.; Hirohata, S.; Kohsaka, H.; Miyasaka, N. Inhibition of CX3CL1 (fractalkine) improves experimental autoimmune myositis in SJL/J mice. J. Immunol. 2005, 175, 6987–6996. [Google Scholar] [CrossRef]
- Hoshino-Negishi, K.; Ohkuro, M.; Nakatani, T.; Kuboi, Y.; Nishimura, M.; Ida, Y.; Kakuta, J.; Hamaguchi, A.; Kumai, M.; Kamisako, T.; et al. Role of Anti-Fractalkine Antibody in Suppression of Joint Destruction by Inhibiting Migration of Osteoclast Precursors to the Synovium in Experimental Arthritis. Arthritis Rheumatol. 2019, 71, 222–231. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, S.; Nishio, J.; Kondo, K.; Motomura, K.; Yamada, Z.; Masuoka, S.; Yamada, S.; Muraoka, S.; Ishii, N.; Kuboi, Y.; et al. Treatment with an Anti-CX3CL1 Antibody Suppresses M1 Macrophage Infiltration in Interstitial Lung Disease in SKG Mice. Pharmaceuticals 2021, 14, 474. https://doi.org/10.3390/ph14050474
Mizutani S, Nishio J, Kondo K, Motomura K, Yamada Z, Masuoka S, Yamada S, Muraoka S, Ishii N, Kuboi Y, et al. Treatment with an Anti-CX3CL1 Antibody Suppresses M1 Macrophage Infiltration in Interstitial Lung Disease in SKG Mice. Pharmaceuticals. 2021; 14(5):474. https://doi.org/10.3390/ph14050474
Chicago/Turabian StyleMizutani, Satoshi, Junko Nishio, Kanoh Kondo, Kaori Motomura, Zento Yamada, Shotaro Masuoka, Soichi Yamada, Sei Muraoka, Naoto Ishii, Yoshikazu Kuboi, and et al. 2021. "Treatment with an Anti-CX3CL1 Antibody Suppresses M1 Macrophage Infiltration in Interstitial Lung Disease in SKG Mice" Pharmaceuticals 14, no. 5: 474. https://doi.org/10.3390/ph14050474
APA StyleMizutani, S., Nishio, J., Kondo, K., Motomura, K., Yamada, Z., Masuoka, S., Yamada, S., Muraoka, S., Ishii, N., Kuboi, Y., Sendo, S., Mikami, T., Imai, T., & Nanki, T. (2021). Treatment with an Anti-CX3CL1 Antibody Suppresses M1 Macrophage Infiltration in Interstitial Lung Disease in SKG Mice. Pharmaceuticals, 14(5), 474. https://doi.org/10.3390/ph14050474






