Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections
Abstract
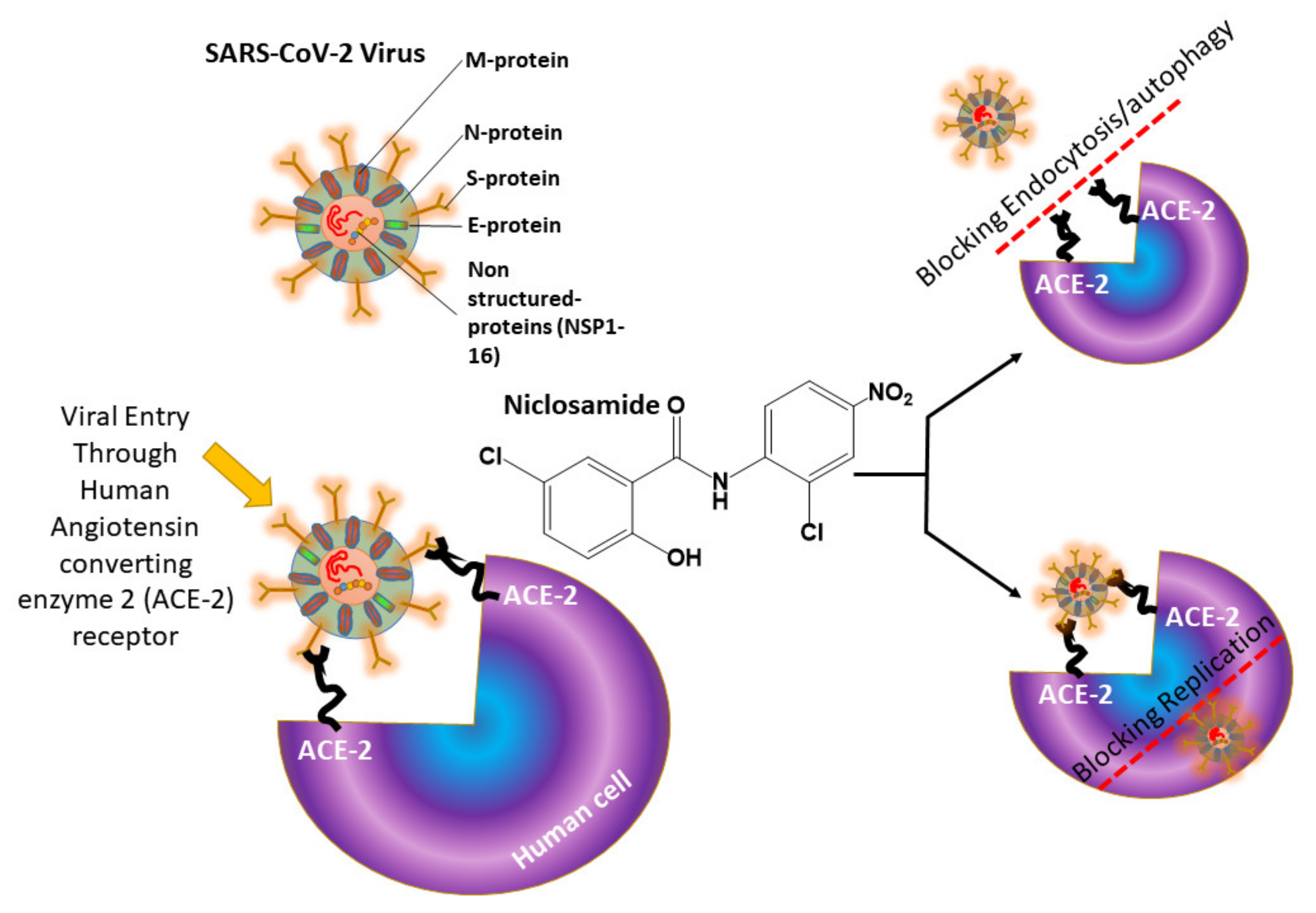
:1. Introduction
2. Results and Discussion
2.1. Powder X-ray Diffraction (PXRD) Analysis
2.2. Fourier Transform Infrared (FT-IR) Analysis
2.3. Surface Areas and Porosity Analysis
2.4. FE-SEM Analysis
2.5. Particle Size Analysis
2.6. NIC Contents in the Nanohybrids
2.7. In Vivo Pharmacokinetics of NIC
3. Clinical Perspectives
4. Materials and Methods
4.1. Materials
4.2. Preparation of DHT, NIC–DHT and Tween 60 (or HPMC)-Coated NIC–DHT
4.3. TGA
4.4. Characterization of NIC–DHT Hybrid
4.5. High Performance Liquid Chromatography
4.6. Pharmacokinetic Study in Rats
4.7. Quantification of Niclosamide in Rat’s Plasma
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, M.; Taylor-Robinson, D.; Barr, B. Poverty, health, and covid-19. BMJ 2021, 372, n376. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Dashey, S.; Stubbs, A.; Lai, F.Y.; Bird, P.W.; Badhwar, V.; Tang, J.W. Comparing SARS-CoV-2 and influenza A(H1N1)pdm09-infected patients requiring ECMO - A single-centre, retrospective observational cohort experience. J. Infect. 2020. [Google Scholar] [CrossRef]
- Standl, F.; Jockel, K.H.; Brune, B.; Schmidt, B.; Stang, A. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis 2021, 21, e77. [Google Scholar] [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Chua, A.Q.; Al Knawy, B.; Grant, B.; Legido-Quigley, H.; Lee, W.C.; Leung, G.M.; Looi, M.K.; Maurer-Stroh, S. How the lessons of previous epidemics helped successful countries fight covid-19. BMJ 2021, 372, n486. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Jan, J.-T.; Chen, C.-M.; Hsieh, H.-P.; Hwang, D.-R.; Liu, H.-W.; Liu, C.-Y.; Huang, H.-W.; Chen, S.-C.; Hong, C.-F.; et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother 2004, 48, 2693–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mösbauer, K.; Zellner, A.; et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 5770. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob Agents Chemother 2020, 64, e00819-20. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [Green Version]
- Amaral, M.; Pereiro, A.B.; Gaspar, M.M.; Reis, C.P. Recent advances in ionic liquids and nanotechnology for drug delivery. Nanomedicine 2021, 16, 63–80. [Google Scholar] [CrossRef]
- Dhadde, S.B.; Patil, J.S.; Chandakavathe, B.N.; Thippeswamy, B.S.; Kavatekar, M.G. Relevance of Nanotechnology in Solving Oral Drug Delivery Challenges: A Perspective Review. Crit Rev. Ther. Drug Carr. Syst. 2020, 37, 407–434. [Google Scholar] [CrossRef]
- Egorov, E.; Pieters, C.; Korach-Rechtman, H.; Shklover, J.; Schroeder, A. Robotics, microfluidics, nanotechnology and AI in the synthesis and evaluation of liposomes and polymeric drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.R.; Senel, S. Nanotechnology and Veterinary Drug/Vaccine Delivery. Pharm. Nanotechnol. 2021, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Guven, E. Nanotechnology-based drug delivery systems in orthopedics. Jt. Dis. Relat. Surg. 2021, 32, 267–273. [Google Scholar] [CrossRef]
- Hsu, J.F.; Chu, S.M.; Liao, C.C.; Wang, C.J.; Wang, Y.S.; Lai, M.Y.; Wang, H.C.; Huang, H.R.; Tsai, M.H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers (Basel) 2021, 13, 195. [Google Scholar] [CrossRef]
- Kaur, I.; Kumar, A.; Behl, T.; Setia, D. Recent Advances in Nanotechnology-Based Drug Delivery Approaches for Alzheimer disease. Curr. Drug Targets 2021. [Google Scholar] [CrossRef]
- Luo, M.X.; Hua, S.; Shang, Q.Y. Application of nanotechnology in drug delivery systems for respiratory diseases. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Nie, Y. Nanotechnology will Transform the Drug Delivery System in Ways of Obvious Benefit to Animals. Pharm Nanotechnol 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Goyal, A.; Ghosh, G.; Rath, G. Recent Advancement in Nanotechnology-Based Drug Delivery System Against Viral Infections. Aaps Pharmscitech 2021, 22, 47. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Singh, V.; Redhu, R.; Verma, R.; Mittal, V.; Kaushik, D. Anti-acne Treatment using Nanotechnology based on Novel Drug Delivery System and Patents on Acne Formulations: A Review. Recent Pat. Nanotechnol. 2020. [Google Scholar] [CrossRef]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.G.M.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and evaluation of cyclodextrin complexes for improved anticancer activity of repurposed drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Mohiyuddin, S.; Gopinath, P. Niclosamide loaded biodegradable chitosan nanocargoes: An in vitro study for potential application in cancer therapy. Royal Soc. Open Sci. 2017, 4, 170611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoxha, A.; Gillam, D.G.; Bushby, A.J.; Agha, A.; Patel, M.P. Layered Double Hydroxide Fluoride Release in Dental Applications: A Systematic Review. Dent. J. (Basel) 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Xu, M.; Yang, S.; Omonov, S.; Huang, S.; Zhao, J.; Ruan, H.; Zeng, M. Novel bio-inspired three-dimensional nanocomposites based on montmorillonite and chitosan. Int J. Biol Macromol 2020, 165, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, X.; Li, H.; Zhou, D.; Wu, Q. Bio-Composites Consisting of Cellulose Nanofibers and Na(+) Montmorillonite Clay: Morphology and Performance Property. Polymers (Basel) 2020, 12, 1448. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Bio nanocomposites based on cationic starch reinforced with montmorillonite and cellulose nanocrystals: Fundamental properties and biodegradability study. Int J. Biol Macromol 2020, 146, 374–386. [Google Scholar] [CrossRef]
- Zakuwan, S.Z.; Ahmad, I. Effects of Hybridized Organically Modified Montmorillonite and Cellulose Nanocrystals on Rheological Properties and Thermal Stability of K-Carrageenan Bio-Nanocomposite. Nanomaterials (Basel) 2019, 9, 1547. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Yu, Y.; Chang, M.; Chang, J. Preparation and Characterization of Bio-oil Phenolic Foam Reinforced with Montmorillonite. Polymers (Basel) 2019, 11, 1471. [Google Scholar] [CrossRef] [Green Version]
- Llanos, J.H.R.; Tadini, C.C. Preparation and characterization of bio-nanocomposite films based on cassava starch or chitosan, reinforced with montmorillonite or bamboo nanofibers. Int J. Biol Macromol 2018, 107, 371–382. [Google Scholar] [CrossRef]
- Jahanizadeh, S.; Yazdian, F.; Marjani, A.; Omidi, M.; Rashedi, H. Curcumin-loaded chitosan/carboxymethyl starch/montmorillonite bio-nanocomposite for reduction of dental bacterial biofilm formation. Int. J. Biol. Macromol. 2017, 105, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Incoronato, A.L.; Buonocore, G.G.; Conte, A.; Lavorgna, M.; Nobile, M.A. Active systems based on silver-montmorillonite nanoparticles embedded into bio-based polymer matrices for packaging applications. J. Food Prot. 2010, 73, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Mokarram, N.; Solati-Hashjin, M.; Moaddel, H. Novel high-performance nanohybrid polyelectrolyte membranes based on bio-functionalized montmorillonite for fuel cell applications. Chem. Commun. 2010, 46, 6500–6502. [Google Scholar] [CrossRef]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D.; Gorga, R.E. Effect of type and content of modified montmorillonite on the structure and properties of bio-nanocomposite films based on soy protein isolate and montmorillonite. J. Food Sci. 2010, 75, N46–N56. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Choy, Y.B.; Oh, J.-M.; Kim, J.Y.; Hwang, S.-J.; Choy, J.-H. Controlled release of donepezil intercalated in smectite clays. Int. J. Pharm. 2008, 359, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, H.-M.; Choy, Y.B.; Hwang, S.-J.; Choy, J.-H. Itraconazole–Laponite: Kinetics and mechanism of drug release. Appl. Clay Sci. 2008, 40, 99–107. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, J.H.; Lee, J.H.; Choi, G.; Park, D.H.; Jo, M.R.; Choi, S.J.; Choy, J.H. 2D Inorganic–Antimalarial Drug–Polymer Hybrid with pH-Responsive Solubility. Chem. Asian J. 2015, 10, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Lee, J.-H.; Oh, Y.-J.; Choy, Y.B.; Park, M.C.; Chang, H.C.; Choy, J.-H. Inorganic-polymer nanohybrid carrier for delivery of a poorly-soluble drug, ursodeoxycholic acid. Int. J. Pharm. 2010, 402, 117–122. [Google Scholar] [CrossRef]
- Choi, S.-J.; Choi, G.E.; Oh, J.-M.; Oh, Y.-J.; Park, M.-C.; Choy, J.-H. Anticancer drug encapsulated in inorganic lattice can overcome drug resistance. J. Mater. Chem. 2010, 20, 9463–9469. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Cai, X.; Yan, Z.; Yang, C.; Hu, X.; Chen, B. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.-W.; Song, K.G.; Choi, K.; Lee, Y.J.; Jung, K.-W. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis. Compos. Part B Eng. 2019, 176, 107209. [Google Scholar] [CrossRef]
- Sulmonetti, T.P.; Hu, B.; Lee, S.; Agrawal, P.K.; Jones, C.W. Reduced Cu–Co–Al mixed metal oxides for the ring-opening of furfuryl alcohol to produce renewable diols. ACS Sustain. Chem. Eng. 2017, 5, 8959–8969. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Zhao, F.; Cui, F.; Li, X.; Xia, C.; Chen, J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1, 2-and 1, 5-pentanediols over a non-precious Cu–Mg 3 AlO 4.5 bifunctional catalyst. Catal. Sci. Technol. 2016, 6, 668–671. [Google Scholar] [CrossRef]
- Choi, G.; Jeon, I.-R.; Piao, H.; Choy, J.-H. Drug Delivery: Highly Condensed Boron Cage Cluster Anions in 2D Carrier and Its Enhanced Antitumor Efficiency for Boron Neutron Capture Therapy (Adv. Funct. Mater. 27/2018). Adv. Funct. Mater. 2018, 28, 1870182. [Google Scholar] [CrossRef]
- Park, D.H.; Cho, J.; Kwon, O.J.; Yun, C.O.; Choy, J.H. Biodegradable Inorganic Nanovector: Passive versus Active Tumor Targeting in siRNA Transportation. Angew. Chem. Int. Ed. Engl. 2016, 55, 4582–4586. [Google Scholar] [CrossRef]
- Kim, M.H.; Hur, W.; Choi, G.; Min, H.S.; Choi, T.H.; Choy, Y.B.; Choy, J.-H. Theranostic Bioabsorbable Bone Fixation Plate with Drug-Layered Double Hydroxide Nanohybrids. Adv. Healthc. Mater. 2016, 5, 2765–2775. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, H.; Ding, X.; Zhang, Q.Y. Contributions of Hepatic and Intestinal Metabolism to the Disposition of Niclosamide, a Repurposed Drug with Poor Bioavailability. Drug Metab. Dispos. 2019, 47, 756–763. [Google Scholar] [CrossRef]
- Rocha, J.; Del Arco, M.; Rives, V.; Ulibarri, M.A. Reconstruction of layered double hydroxides from calcined precursors: A powder XRD and 27 Al MAS NMR study. J. Mater. Chem. 1999, 9, 2499–2503. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Ahmed, A. Use of Alginate/Montmorillonite Nanocomposites as Drug Delivery System for Curcumin. Ph.D. Thesis, American University in Cairo, Cairo, Egypt, 2015. [Google Scholar]
- Yu, S.; Piao, H.; Rejinold, N.S.; Jin, G.; Choi, G.; Choy, J.-H. Niclosamide–Clay Intercalate Coated with Nonionic Polymer for Enhanced Bioavailability toward COVID-19 Treatment. Polymers 2021, 13, 1044. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, M.W.; Shul, Y.G.; Choy, J.H. Sepiocite, sepiolite-like nanoclay derived from hydrotalcite-like layered double hydroxide. J. Nanosci. Nanotechnol. 2011, 11, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Meng, L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg–Al layered double hydroxide. J. Chem. Eng. Data 2011, 56, 4217–4225. [Google Scholar] [CrossRef]
- Oh, J.M.; Choi, S.J.; Kim, S.T.; Choy, J.H. Cellular uptake mechanism of an inorganic nanovehicle and its drug conjugates: Enhanced efficacy due to clathrin-mediated endocytosis. Bioconjug. Chem. 2006, 17, 1411–1417. [Google Scholar] [CrossRef]
- Jara, M.O.; Warnken, Z.N.; Williams, R.O., 3rd. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 2021, 13, 97. [Google Scholar] [CrossRef]
- Anjani, D.C.; Sai, K.M.; Sanjana, N.; Sampath, K.N.S. Nanotechnology: An emerging approach to combat COVID-19. Emergent Mater. 2021, 1–12. [Google Scholar] [CrossRef]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021, 1–25. [Google Scholar] [CrossRef]
- Tharayil, A.; Rajakumari, R.; Chirayil, C.J.; Thomas, S.; Kalarikkal, N. A short review on nanotechnology interventions against COVID-19. Emergent Mater. 1007, 1–11. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Dan, S.; Girdhar, M.; Rastogi, K. Recent Advancement in SARS-CoV-2 Diagnosis, Treatment, and Vaccine Formulation: A New Paradigm of Nanotechnology in Strategic Combating of COVID-19 Pandemic. Curr. Pharm. Rep. 2021, 1–14. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Mehta, V.N.; Koduru, J.R.; Basu, H.; Singhal, R.K.; Murthy, Z.V.P.; Park, T.J. An overview of molecular biology and nanotechnology based analytical methods for the detection of SARS-CoV-2: Promising biotools for the rapid diagnosis of COVID-19. Analyst 2021, 146, 1489–1513. [Google Scholar] [CrossRef]
- Yang, D. Application of Nanotechnology in the COVID-19 Pandemic. Int J. Nanomed. 2021, 16, 623–649. [Google Scholar] [CrossRef]
- Musyuni, P.; Nagpal, M.; Singh, M.; Goyal, R.K.; Aggarwal, G. Nanotechnology Enabled Solutions to Combat Covid-19: Prevention, Treatment and Diagnosis. Curr. Pharm. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Ghaffari, M.; Mollazadeh-Bajestani, M.; Moztarzadeh, F.; Uludag, H.; Hardy, J.G.; Mozafari, M. An overview of the use of biomaterials, nanotechnology, and stem cells for detection and treatment of COVID-19: Towards a framework to address future global pandemics. Emergent Mater. 2021, -, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Abduljauwad, S.N.; Habib, T.; Ahmed, H.-u.-R. Nano-clays as Potential Pseudo-antibodies for COVID-19. Nanoscale Res. Lett. 2020, 15, 173. [Google Scholar] [CrossRef] [PubMed]







| Samples | SBET (m2/g) a | Vp (cm3/g) b |
|---|---|---|
| HT | 9.05 | 0.061 |
| DHT | 62.18 | 0.099 |
| NIC–DHT | 6.85 | 0.035 |
| Parameters | ** Yomesan® (50 mg/kg) | NIC–DHT/Tween 60 (50 mg/kg) | NIC–DHT/Tween 60 (200 mg/kg) | NIC–DHT/HPMC (200 mg/kg) |
|---|---|---|---|---|
| AUC(last) (ng·h/mL) | 1096.81 ± 359.28 | 1823.83 ± 305.26 | 6819.30 ± 2530.40 | 30,430.35 ± 10,921.98 |
| Cmax (ng /mL) | 155.27 ± 39.92 | 1350.37 ± 613.98 | 6316.60 ± 4270.00 | 18,928.63 ± 2934.34 |
| Tmax (h) | 4.00 ± 0.89 | 0.25 ± 0.00 | 0.25 ± 1.65 | 0.38 ± 0.14 |
| T1/2 (h) | 5.72 ± 6.09 | 3.19 ± 0.43 | 2.69 ± 1.46 | 6.75 ± 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, G.; Piao, H.; Rejinold, N.S.; Yu, S.; Kim, K.-y.; Jin, G.-w.; Choy, J.-H. Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections. Pharmaceuticals 2021, 14, 486. https://doi.org/10.3390/ph14050486
Choi G, Piao H, Rejinold NS, Yu S, Kim K-y, Jin G-w, Choy J-H. Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections. Pharmaceuticals. 2021; 14(5):486. https://doi.org/10.3390/ph14050486
Chicago/Turabian StyleChoi, Goeun, Huiyan Piao, N. Sanoj Rejinold, Seungjin Yu, Ki-yeok Kim, Geun-woo Jin, and Jin-Ho Choy. 2021. "Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections" Pharmaceuticals 14, no. 5: 486. https://doi.org/10.3390/ph14050486
APA StyleChoi, G., Piao, H., Rejinold, N. S., Yu, S., Kim, K.-y., Jin, G.-w., & Choy, J.-H. (2021). Hydrotalcite–Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections. Pharmaceuticals, 14(5), 486. https://doi.org/10.3390/ph14050486








