Immunotherapeutic Efficacy of IgY Antibodies Targeting the Full-Length Spike Protein in an Animal Model of Middle East Respiratory Syndrome Coronavirus Infection
Abstract
:1. Introduction
2. Results
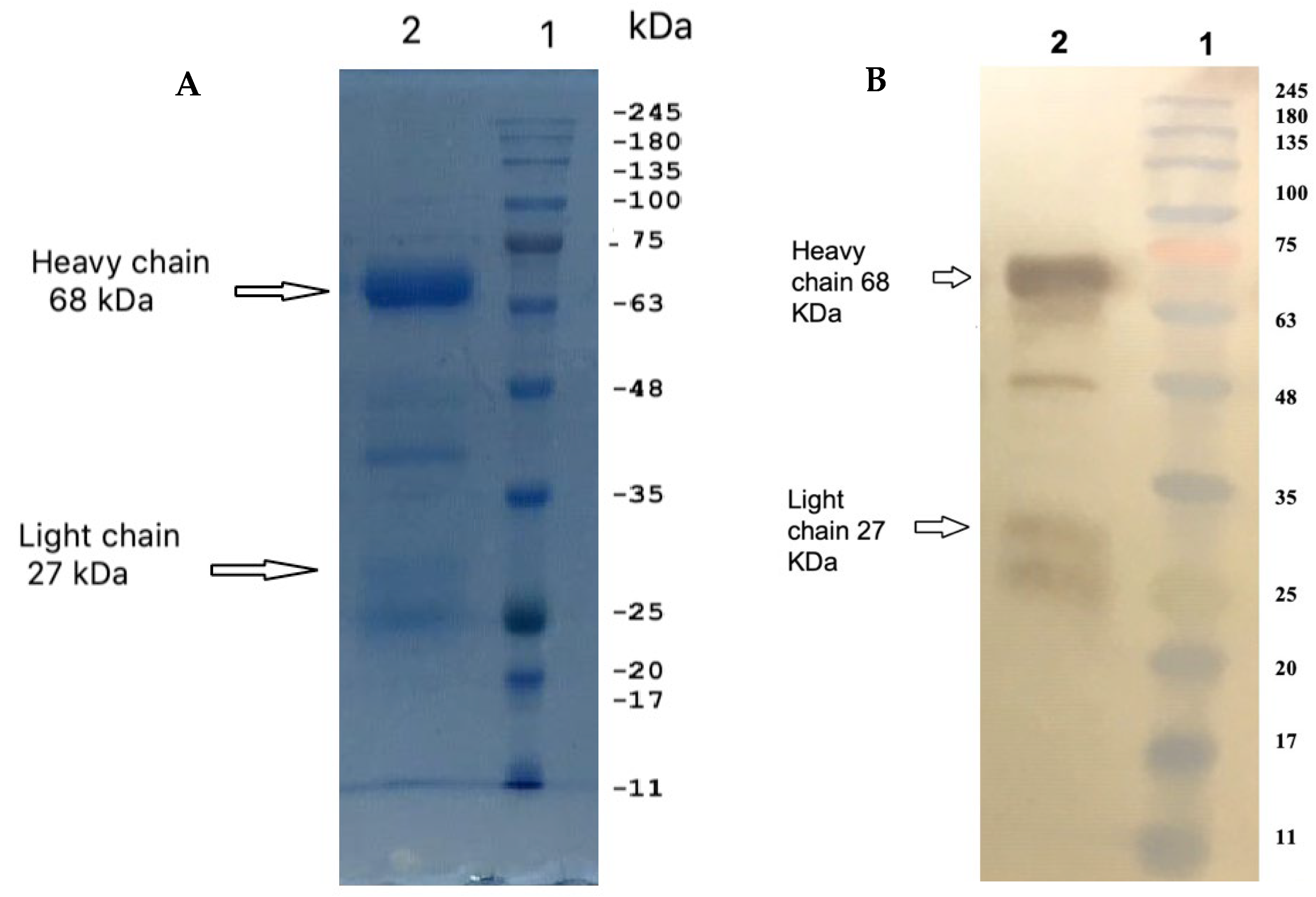
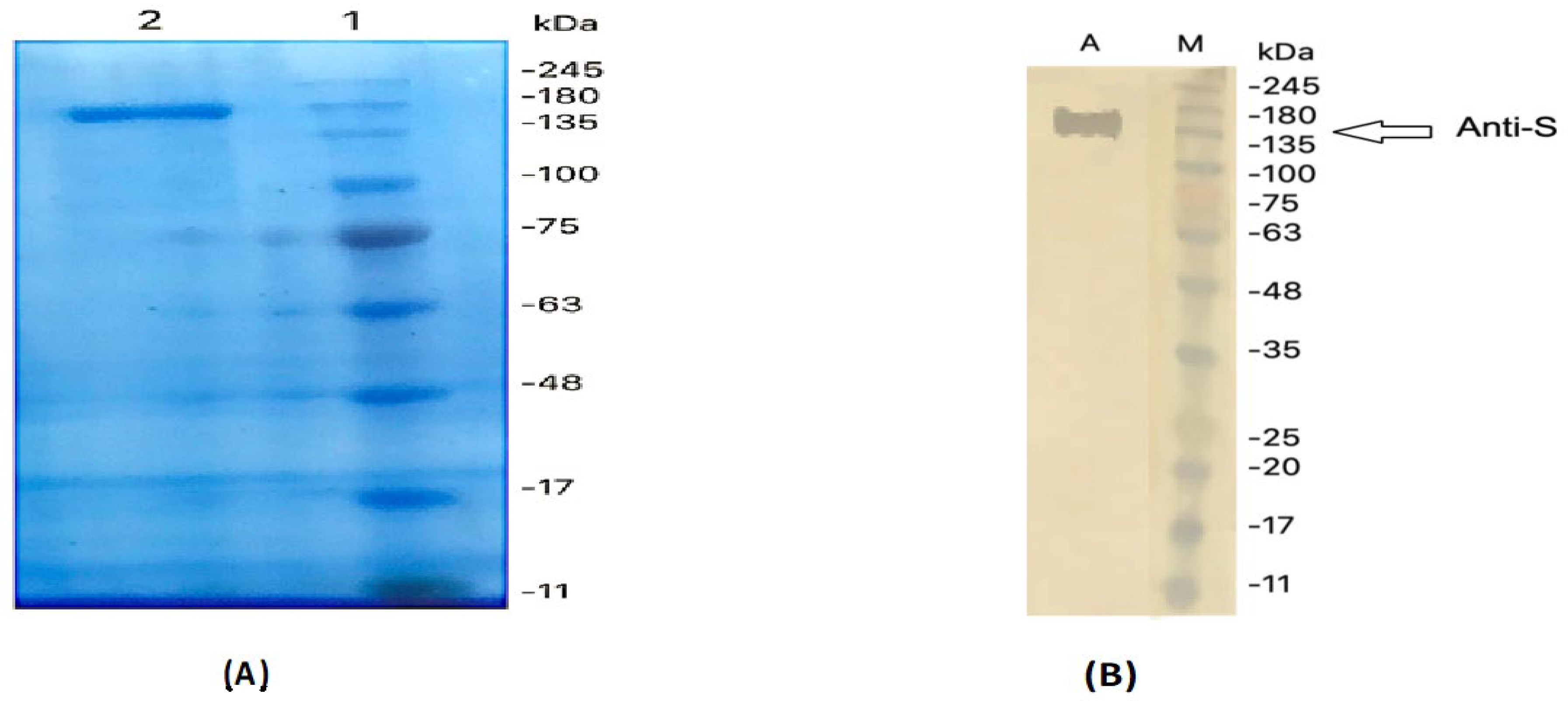
2.1. Isolation and Purification of IgY
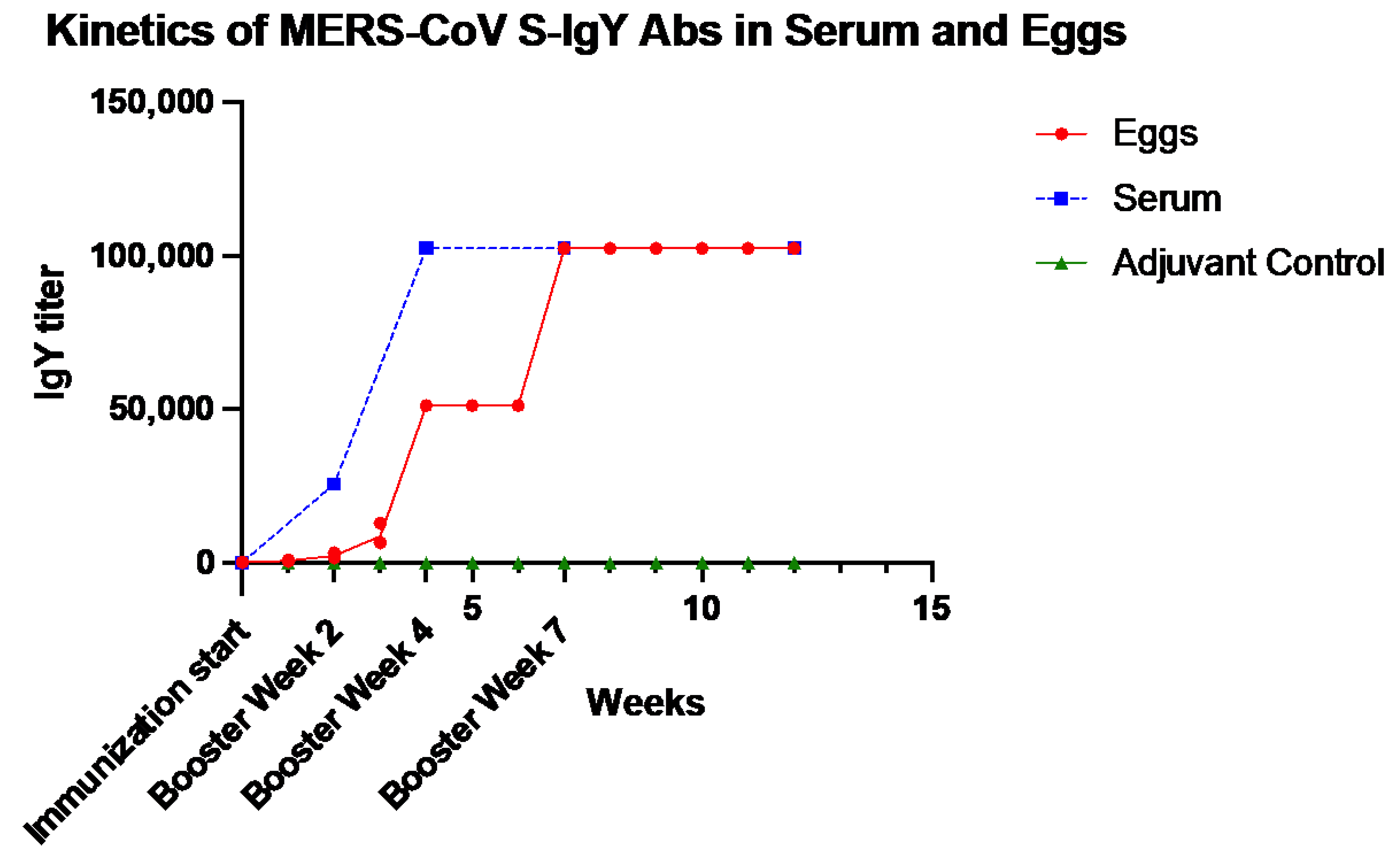
2.2. Dynamics of Anti-S IgY Antibodies in the Sera of Chickens and Egg Yolks
2.3. Immunoreactivity of Anti-S IgY of the MERS-COV
2.4. Dot Blotting
2.5. Anti-S IgY Neutralizes MERS-CoV
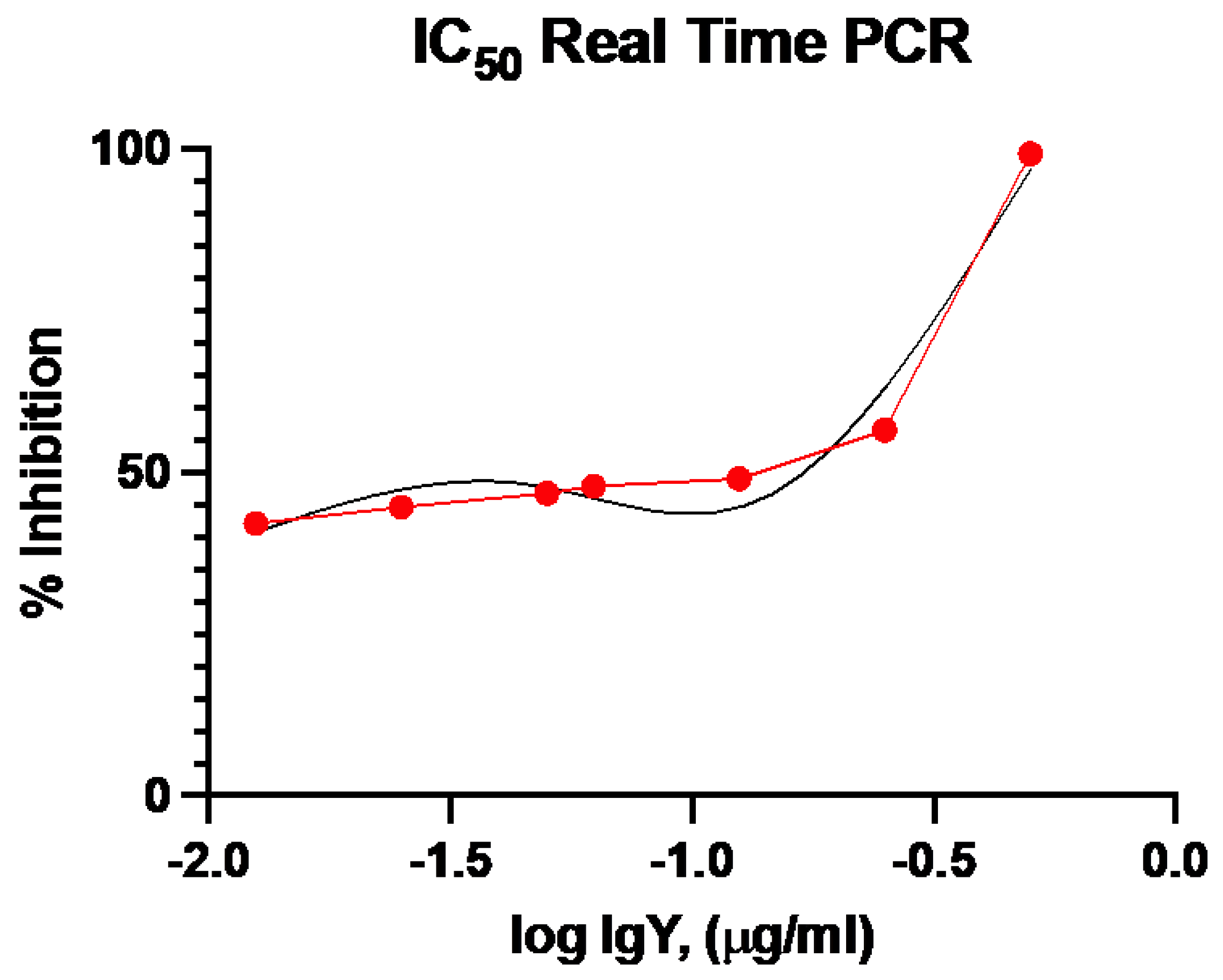
2.6. RT-qPCR-Based Neutralization Activity
2.7. IgY Confers In Vivo Protection in Virus-Challenged Mice
3. Discussion
4. Material and Methods
4.1. Immunization of Laying Hens
4.2. Isolation and Purification of Yolk IgY
4.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
4.4. Reactivity of Anti-S IgY Antibodies by ELISA
4.5. Western Blotting Assay
4.6. Dot-Blotting
4.7. Microneutralization Assay
4.8. Neutralization Using Real-Time qRT-PCR
4.9. Effect of Anti-S IgY Antibodies in Transgenic Mice Infected with MERS-CoV
4.10. Histopathology and Immunohistochemistry
4.11. Quantitative Analysis of Inflammation and Viral Antigen Positivity of Cells
4.12. Statistical Analysis
4.13. Ethics Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Widjaja, I.; Wang, C.; van Haperen, R.; Gutierrez-Alvarez, J.; van Dieren, B.; Okba, N.M.A.; Raj, V.S.; Li, W.; Fernandez-Delgado, R.; Grosveld, F.; et al. Towards a solution to MERS: Protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg. Microbes Infect. 2019, 8, 516–530. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Hui, D.S.; Perlman, S. Middle East respiratory syndrome. Lancet 2015, 386, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Middle East Respiratory Syndrome. Available online: https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1 (accessed on 21 May 2021).
- Ali, M.A.; Shehata, M.M.; Gomaa, M.R.; Kandeil, A.; El-Shesheny, R.; Kayed, A.S.; El-Taweel, A.N.; Atea, M.; Hassan, N.; Bagato, O.; et al. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes Infect. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzarano, D.; Kamissoko, B.; de Wit, E.; Maiga, O.; Cronin, J.; Samake, K.; Traore, A.; Milne-Price, S.; Munster, V.J.; Sogoba, N.; et al. Dromedary camels in northern Mali have high seropositivity to MERS-CoV. One Health 2017, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; El-Shesheny, R.; Kandeil, A.; Shehata, M.; Elsokary, B.; Gomaa, M.; Hassan, N.; El Sayed, A.; El-Taweel, A.; Sobhy, H.; et al. Cross-sectional surveillance of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels and other mammals in Egypt, August 2015 to January 2016. Euro Surveill. 2017, 22. [Google Scholar] [CrossRef]
- Sikkema, R.S.; Farag, E.; Himatt, S.; Ibrahim, A.K.; Al-Romaihi, H.; Al-Marri, S.A.; Al-Thani, M.; El-Sayed, A.M.; Al-Hajri, M.; Haagmans, B.L.; et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Infection in Camel Workers in Qatar During 2013–2014: A Case-Control Study. J. Infect. Dis. 2017, 215, 1702–1705. [Google Scholar] [CrossRef]
- Alhakeem, R.F.; Midgley, C.M.; Assiri, A.M.; Alessa, M.; Al Hawaj, H.; Saeed, A.B.; Almasri, M.M.; Lu, X.; Abedi, G.R.; Abdalla, O.; et al. Exposures among MERS Case-Patients, Saudi Arabia, January-February 2016. Emerg. Infect. Dis. 2016, 22, 2020–2022. [Google Scholar] [CrossRef] [Green Version]
- Yusof, M.F.; Queen, K.; Eltahir, Y.M.; Paden, C.R.; Al Hammadi, Z.; Tao, Y.; Li, Y.; Khalafalla, A.I.; Shi, M.; Zhang, J.; et al. Diversity of Middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, Abu Dhabi, United Arab Emirates. Emerg. Microbes Infect. 2017, 6, e101. [Google Scholar] [CrossRef] [Green Version]
- Madani, T.A.; Azhar, E.I.; Hashem, A.M. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014, 371, 1360. [Google Scholar] [CrossRef]
- Oh, M.D.; Park, W.B.; Park, S.W.; Choe, P.G.; Bang, J.H.; Song, K.H.; Kim, E.S.; Kim, H.B.; Kim, N.J. Middle East respiratory syndrome: What we learned from the 2015 outbreak in the Republic of Korea. Korean J. Intern. Med. 2018, 33, 233–246. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schafer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharbi, N.K.; Qasim, I.; Almasoud, A.; Aljami, H.A.; Alenazi, M.W.; Alhafufi, A.; Aldibasi, O.S.; Hashem, A.M.; Kasem, S.; Albrahim, R.; et al. Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels. Sci. Rep. 2019, 9, 16292. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Weber, H.; Elzayat, M.T.; Hoffmann, M.; Pohlmann, S. Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein. Sci. Rep. 2018, 8, 16597. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Zhao, J.; Pedotti, M.; Simonelli, L.; Agnihothram, S.; Fett, C.; Fernandez-Rodriguez, B.; Foglierini, M.; Agatic, G.; Vanzetta, F.; et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA 2015, 112, 10473–10478. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wan, Y.; Liu, P.; Zhao, J.; Lu, G.; Qi, J.; Wang, Q.; Lu, X.; Wu, Y.; Liu, W.; et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015, 25, 1237–1249. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, K.; Wohlford-Lenane, C.; Agnihothram, S.S.; Fett, C.; Zhao, J.; Gale, M.J., Jr.; Baric, R.S.; Enjuanes, L.; Gallagher, T.; et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4970–4975. [Google Scholar] [CrossRef] [Green Version]
- Sabir, J.S.; Lam, T.T.; Ahmed, M.M.; Li, L.; Shen, Y.; Abo-Aba, S.E.; Qureshi, M.I.; Abu-Zeid, M.; Zhang, Y.; Khiyami, M.A.; et al. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science 2016, 351, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Perera, R.A.; Kayali, G.; Meyerholz, D.; Perlman, S.; Peiris, M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J. Virol. 2015, 89, 6117–6120. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, C.; Qiu, B.; Li, C.; Wang, H.; Jin, H.; Gai, W.; Zheng, X.; Wang, T.; Sun, W.; et al. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antivir. Res. 2017, 137, 125–130. [Google Scholar] [CrossRef]
- Faith, R.E.; Clem, L.W. Passive cutaneous anaphylaxis in the chicken. Biological fractionation of the mediating antibody population. Immunology 1973, 25, 151–164. [Google Scholar] [PubMed]
- Gassmann, M.; Thommes, P.; Weiser, T.; Hubscher, U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990, 4, 2528–2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, L.; Qin, Z.; Lin, H.; Zhou, Y.; Li, J.; Xu, Z.; Babu, V.S.; Lin, L. Features of chicken egg yolk immunoglobulin (IgY) against the infection of red-spotted grouper nervous necrosis virus. Fish. Shellfish. Immunol. 2018, 80, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Azhar, E.I.A. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vaccines Immunother. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Tabll, A.A.; Hassan, A.M.; Iwata-Yoshikawa, N.; Nagata, N.; Azhar, E.I. Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model. Vaccines 2020, 8, 634. [Google Scholar] [CrossRef]
- Carlander, D.; Stalberg, J.; Larsson, A. Chicken antibodies: A clinical chemistry perspective. Upsala J. Med. Sci. 1999, 104, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, X.; Jin, L.; Zhen, Y.; Lu, Y.; Li, S.; You, J.; Wang, L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol. Adv. 2011, 29, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Dorner, M.; Zhang, X.; Hlinak, A.; Dorner, B.; Schade, R. Monitoring of laying capacity, immunoglobulin Y concentration, and antibody titer development in chickens immunized with ricin and botulinum toxins over a two-year period. Poult. Sci. 2009, 88, 281–290. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: A review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Gadde, U.; Rathinam, T.; Lillehoj, H.S. Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases—A review. Anim. Health Res. Rev. 2015, 16, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M. Introduction to poultry vaccines and immunity. Adv. Vet. Med. 1999, 41, 481–494. [Google Scholar] [PubMed]
- Ikemori, Y.; Peralta, R.C.; Kuroki, M.; Yokoyama, H.; Kodama, Y. Research note: Avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli fimbriae. Poult. Sci. 1993, 72, 2361–2365. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tumpey, T.M.; Park, H.J.; Byun, Y.H.; Tran, L.D.; Nguyen, V.D.; Kilgore, P.E.; Czerkinsky, C.; Katz, J.M.; Seong, B.L.; et al. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS ONE 2010, 5, e10152. [Google Scholar] [CrossRef]
- Yang, Y.E.; Wen, J.L.; Zhao, S.Q.; Zhang, K.; Zhou, Y.L. Prophylaxis and therapy of pandemic H1N1 virus infection using egg yolk antibody. J. Virol. Methods 2014, 206, 19–26. [Google Scholar] [CrossRef]
- Wallach, M.G.; Webby, R.J.; Islam, F.; Walkden-Brown, S.; Emmoth, E.; Feinstein, R.; Gronvik, K.O. Cross-Protection of Chicken Immunoglobulin Y Antibodies against H5N1 and H1N1 Viruses Passively Administered in Mice. Clin. Vaccine Immunol. 2011, 18, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, M.; Hiroi, S.; Adachi, K.; Kato, H.; Inai, M.; Konishi, I.; Tanaka, M.; Yamamoto, R.; Sawa, M.; Handharyani, E.; et al. Antibodies against swine influenza virus neutralize the pandemic influenza virus A/H1N1. Mol. Med. Rep. 2011, 4, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.L.; Zhao, S.Q.; He, D.G.; Yang, Y.N.; Li, Y.M.; Zhu, S.S. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antivir. Res. 2012, 93, 154–159. [Google Scholar] [CrossRef]
- Fu, C.Y.; Huang, H.; Wang, X.M.; Liu, Y.G.; Wang, Z.G.; Cui, S.J.; Gao, H.L.; Li, Z.; Li, J.P.; Kong, X.G. Preparation and evaluation of anti-SARS coronavirus IgY from yolks of immunized SPF chickens. J. Virol. Methods 2006, 133, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Ferella, A.; Bellido, D.; Chacana, P.; Wigdorovitz, A.; Santos, M.J.D.; Mozgovoj, M.V. Chicken egg yolk antibodies against bovine respiratory syncytial virus neutralize the virus in vitro. Procedia Vaccinol. 2012, 6, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Sudjarwo, S.A.; Eraiko, K.; Giftania Wardani Sudjarwo, K. The potency of chicken egg yolk immunoglobulin (IgY) specific as immunotherapy to Mycobacterium tuberculosis infection. J. Adv. Pharm. Technol Res. 2017, 8, 91–96. [Google Scholar] [CrossRef]
- Kollberg, H.; Carlander, D.; Olesen, H.; Wejaker, P.E.; Johannesson, M.; Larsson, A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: A phase I feasibility study. Pediatric Pulmonol. 2003, 35, 433–440. [Google Scholar] [CrossRef]
- Jahangiri, A.; Owlia, P.; Rasooli, I.; Salimian, J.; Derakhshanifar, E.; Naghipour Erami, A.; Darzi Eslam, E.; Darvish Alipour Astaneh, S. Specific egg yolk antibodies (IgY) confer protection against Acinetobacter baumannii in a murine pneumonia model. J. Appl. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Otterbeck, A.; Hanslin, K.; Lantz, E.L.; Larsson, A.; Stalberg, J.; Lipcsey, M. Inhalation of specific anti-Pseudomonas aeruginosa IgY antibodies transiently decreases P. aeruginosa colonization of the airway in mechanically ventilated piglets. Intensive Care Med. Exp. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- VanBlargan, L.A.; Goo, L.; Pierson, T.C. Deconstructing the antiviral neutralizing-antibody response: Implications for vaccine development and immunity. Microbiol. Mol. Biol. Rev. 2016, 80, 989–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, A.L.; Williams, K.L.; Harris, E.; Alvine, T.D.; Henderson, T.; Schiltz, J.; Nilles, M.L.; Bradley, D.S. Dengue virus specific IgY provides protection following lethal dengue virus challenge and is neutralizing in the absence of inducing antibody dependent enhancement. PLoS Negl. Trop. Dis. 2017, 11, e0005721. [Google Scholar] [CrossRef] [Green Version]
- Haese, N.; Brocato, R.L.; Henderson, T.; Nilles, M.L.; Kwilas, S.A.; Josleyn, M.D.; Hammerbeck, C.D.; Schiltz, J.; Royals, M.; Ballantyne, J. Antiviral biologic produced in DNA vaccine/goose platform protects hamsters against hantavirus pulmonary syndrome when administered post-exposure. PLoS Negl. Trop. Dis. 2015, 9, e0003803. [Google Scholar] [CrossRef] [Green Version]
- Brocato, R.; Josleyn, M.; Ballantyne, J.; Vial, P.; Hooper, J.W. DNA vaccine-generated duck polyclonal antibodies as a postexposure prophylactic to prevent hantavirus pulmonary syndrome (HPS). PLoS ONE 2012, 7, e35996. [Google Scholar] [CrossRef] [PubMed]
- Najdi, S.; Brujeni, G.N.; Sheikhi, N.; Chakhkar, S. Development of anti-Helicobacter pylori immunoglobulins Y (IgYs) in quail. Iran. J. Vet. Res. 2016, 17, 106. [Google Scholar]
- Lu, G.; Hu, Y.; Wang, Q.; Qi, J.; Gao, F.; Li, Y.; Zhang, Y.; Zhang, W.; Yuan, Y.; Bao, J.; et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 2013, 500, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Li, F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J. Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Du, L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines 2018, 17, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Slifka, M.K.; Amanna, I.J. Passive immunization. Plotkin’s Vaccines 2018, 84–95.e10. [Google Scholar] [CrossRef]
- Widagdo, W.; Okba, N.M.; Li, W.; de Jong, A.; de Swart, R.L.; Begeman, L.; van den Brand, J.M.; Bosch, B.-J.; Haagmans, B.L. Species-Specific Colocalization of Middle East Respiratory Syndrome Coronavirus Attachment and Entry Receptors. J. Virol. 2019, 93, e00107–e00119. [Google Scholar] [CrossRef] [Green Version]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?—A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Woolley, J.A.; Landon, J. Comparison of antibody production to human interleukin-6 (IL-6) by sheep and chickens. J. Immunol. Methods 1995, 178, 253–265. [Google Scholar] [CrossRef]
- Varada, J.C.; Teferedegne, B.; Crim, R.L.; Mdluli, T.; Audet, S.; Peden, K.; Beeler, J.; Murata, H. A neutralization assay for respiratory syncytial virus using a quantitative PCR-based endpoint assessment. Virol. J. 2013, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, S.; Choraria, A.; Antonysamy, M.; Zhang, X.-Y. Applications of Chicken Egg Yolk Antibodies (Igy) in Healthcare—A Review. Biomed. J. Sci. Tech. Res. 2018, 2, 2161–2163. [Google Scholar]
- Mackenzie, C.; Taylor, P.; Askonas, B. Rapid recovery of lung histology correlates with clearance of influenza virus by specific CD8+ cytotoxic T cells. Immunology 1989, 67, 375. [Google Scholar]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Duan, L. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Defang, G.; Luke, T.; Wu, H.; Zhao, J.; Channappanavar, R.; Coleman, C.M.; Jiao, J.A.; Matsushita, H.; Liu, Y.; Postnikova, E.N. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci. Transl. Med. 2016, 8, 326ra21. [Google Scholar]
- Zhou, L.; Ni, B.; Luo, D.; Zhao, G.; Jia, Z.; Zhang, L.; Lin, Z.; Wang, L.; Zhang, S.; Xing, L. Inhibition of infection caused by severe acute respiratory syndrome-associated coronavirus by equine neutralizing antibody in aged mice. Int. Immunopharmacol. 2007, 7, 392–400. [Google Scholar] [CrossRef]
- Lee, W.; Atif, A.S.; Tan, S.C.; Leow, C.H. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Van Nguyen, S.; Icatlo, F.C., Jr.; Umeda, K.; Kodama, Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Somasundaram, R.; Choraria, A.; Antonysamy, M. An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review. Int. Immunopharmacol. 2020, 85, 106654. [Google Scholar] [CrossRef]
- Spillner, E.; Braren, I.; Greunke, K.; Seismann, H.; Blank, S.; du Plessis, D. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals 2012, 40, 313–322. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Tian, Z.; Chen, S.; Schade, R. Chicken monoclonal IgY antibody: A novel antibody development strategy. Avian Biol. Res. 2010, 3, 97–106. [Google Scholar] [CrossRef]
- Munhoz, L.S.; Vargas, G.D.Á.; Fischer, G.; Lima, M.d.; Esteves, P.A.; Hübner, S.d.O. Avian IgY antibodies: Characteristics and applications in immunodiagnostic. Ciência Rural 2014, 44, 153–160. [Google Scholar] [CrossRef]
- Karlsson, M.; Kollberg, H.; Larsson, A. Chicken IgY: Utilizing the evolutionary advantage. World’s Poult. Sci. J. 2004, 60, 341–348. [Google Scholar] [CrossRef]
- Nguyen, H.; Tumpey, T.; Park, H.-J.; Han, G.-Y.; Lee, J.; Byun, Y.-H.; Song, J.-M.; Tran, L.; Nguyen, V.; Kilgore, P. Avian antibodies to combat potential H5N1 pandemic and seasonal influenza. Influenza Other Respir. Viruses 2011, 5, 233–236. [Google Scholar]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Constantin, C.; Neagu, M.; Supeanu, T.D.; Chiurciu, V.; Spandidos, D.A. IgY-turning the page toward passive immunization in COVID-19 infection. Exp. Ther. Med. 2020, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, A.; Fravalo, P.; Perron, A.; Lewandowski, S.L.; Letellier, A. Production and characterization of anti-Campylobacter jejuni IgY derived from egg yolks. Acta Vet. Scand. 2017, 59, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindawi, S.I.; Hashem, A.M.; Damanhouri, G.A.; El-Kafrawy, S.A.; Tolah, A.M.; Hassan, A.M.; Azhar, E.I. Inactivation of Middle East respiratory syndrome-coronavirus in human plasma using amotosalen and ultraviolet A light. Transfusion 2018, 58, 52–59. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Okamura, T.; Shimizu, Y.; Kotani, O.; Sato, H.; Sekimukai, H.; Fukushi, S.; Suzuki, T.; Sato, Y.; Takeda, M.; et al. Acute Respiratory Infection in Human Dipeptidyl Peptidase 4-Transgenic Mice Infected with Middle East Respiratory Syndrome Coronavirus. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kafrawy, S.A.; Abbas, A.T.; Sohrab, S.S.; Tabll, A.A.; Hassan, A.M.; Iwata-Yoshikawa, N.; Nagata, N.; Azhar, E.I. Immunotherapeutic Efficacy of IgY Antibodies Targeting the Full-Length Spike Protein in an Animal Model of Middle East Respiratory Syndrome Coronavirus Infection. Pharmaceuticals 2021, 14, 511. https://doi.org/10.3390/ph14060511
El-Kafrawy SA, Abbas AT, Sohrab SS, Tabll AA, Hassan AM, Iwata-Yoshikawa N, Nagata N, Azhar EI. Immunotherapeutic Efficacy of IgY Antibodies Targeting the Full-Length Spike Protein in an Animal Model of Middle East Respiratory Syndrome Coronavirus Infection. Pharmaceuticals. 2021; 14(6):511. https://doi.org/10.3390/ph14060511
Chicago/Turabian StyleEl-Kafrawy, Sherif A., Aymn T. Abbas, Sayed S. Sohrab, Ashraf A. Tabll, Ahmed M. Hassan, Naoko Iwata-Yoshikawa, Noriyo Nagata, and Esam I. Azhar. 2021. "Immunotherapeutic Efficacy of IgY Antibodies Targeting the Full-Length Spike Protein in an Animal Model of Middle East Respiratory Syndrome Coronavirus Infection" Pharmaceuticals 14, no. 6: 511. https://doi.org/10.3390/ph14060511
APA StyleEl-Kafrawy, S. A., Abbas, A. T., Sohrab, S. S., Tabll, A. A., Hassan, A. M., Iwata-Yoshikawa, N., Nagata, N., & Azhar, E. I. (2021). Immunotherapeutic Efficacy of IgY Antibodies Targeting the Full-Length Spike Protein in an Animal Model of Middle East Respiratory Syndrome Coronavirus Infection. Pharmaceuticals, 14(6), 511. https://doi.org/10.3390/ph14060511






