Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract
Abstract
:1. Introduction
2. Results
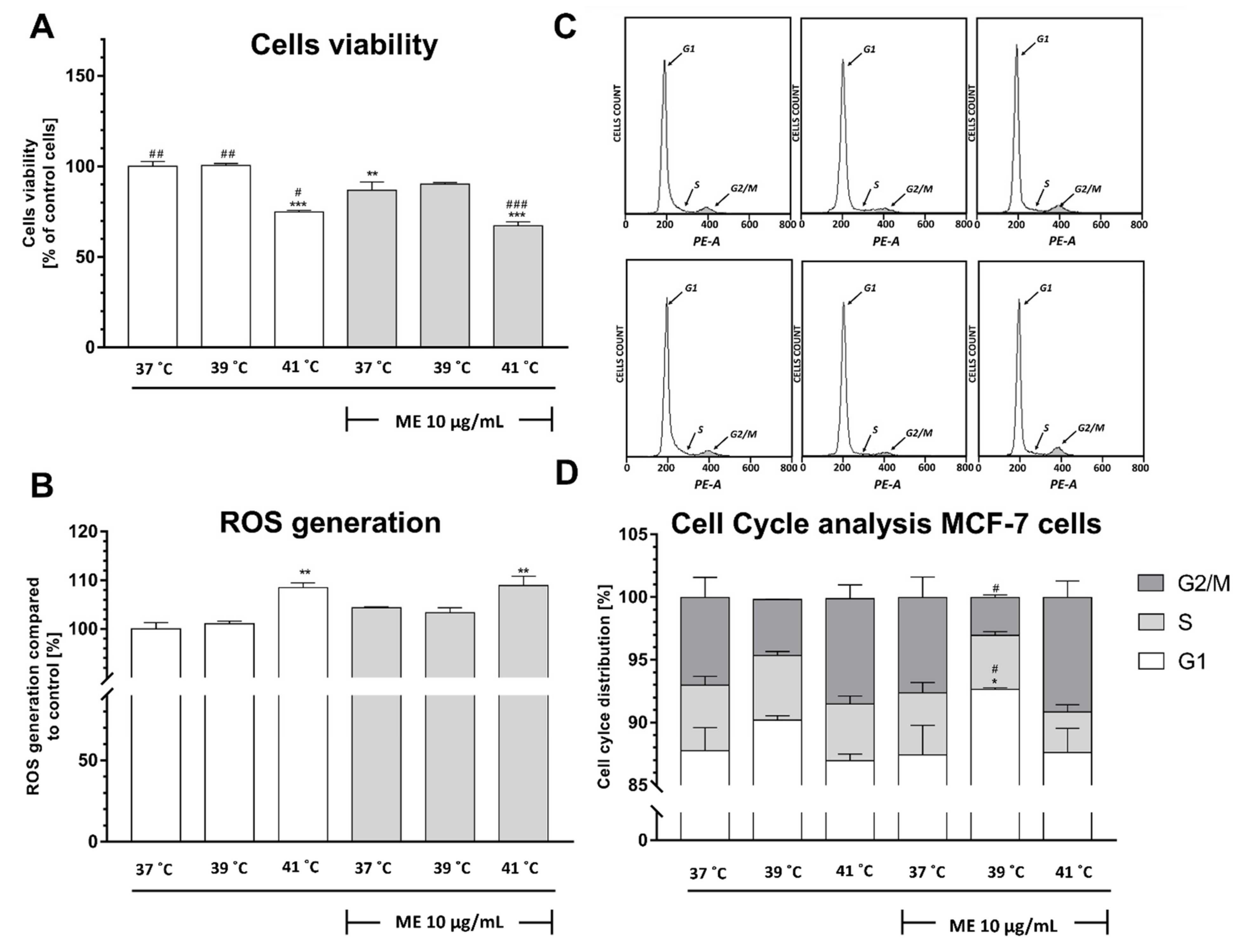
2.1. Exposure to Heat at 41 °C Decreased Viability of ME-Treated Mcf-7 Cells
2.2. Exposure to Heat at 41 °C Increased Reactive Oxygen Species Production in ME-Treated MCF-7 Cells
2.3. ME Combined with Heat Exposure to 39 °C Induced a Significant Cell Cycle Arrest at the G1 Phase in MCF-7 Cells
2.4. Exposure to Fever-Range Hyperthermia Decreased the Viability of ME-Treated 4T1 Cells
2.5. ME Enhances a Slight Increase in Reactive Oxygen Species Level
2.6. ME Combined with Heat Exposure to 41 °C Induced a Significant Cell Cycle Arrest at the S Phase in 4T1 Cells
2.7. Exposure to Temperatures of 39 °C or 41 °C Prevented the ME-Induced Decrease in RAW 264.7 Cell Viability
2.8. Exposure to Heat at 41 °C Increased Reactive Oxygen Species Generation in RAW 264.7 Cells
2.9. ME Treated RAW 264.7 Cells Demonstrate a Significant Arrest in the G2/M Phase after Heat Exposure
2.10. Exposure to Temperatures of 39 °C or 41 °C Inhibited ME-Induced mRNA Expression of IL-1β and IL-6 in MCF-7 Cells
2.11. Exposure of ME-Treated 4T1 Breast Cancer Cells to Heat at 41 °C Differentially Modulates mRNA Expression of IL-1β and IL-6
2.12. Exposure to Heat at 41 °C Enhanced mRNA Expression of IL-1β and IL-6 in ME-Treated RAW 264.7 Cells
2.13. ME Combined with Incubation at 41 °C Caused a Significant Increase in Expression of Cyclooxygenase-2 in RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Mistletoe Extract (ME)
4.2. MCF-7 Cell Line
4T1 Cell Line
4.3. RAW 264.7 Cell Line
4.4. Heat Exposure and Treatment with Mistletoe Extract
4.5. Assessment of Cell Viability Using the MTT Assay
4.6. Reactive Oxygen Species Determination Using Flow Cytometry
4.7. Cell Cycle Analysis by Flow Cytometry
4.8. Quantification of Cytokines and COX-2 mRNA Expression
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienle, G.S.; Berrino, F.; Büssing, A.; Portalupi, E.; Rosenzweig, S.; Kiene, H. Mistletoe in cancer—A systematic review on controlled clinical trials. Eur. J. Med. Res. 2003, 8, 109–119. [Google Scholar]
- Tröger, W.; Galun, D.; Reif, M.; Schumann, A.; Stanković, N.; Milićević, M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer 2013, 49, 3788–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and Clinical Effects of Mistletoe against Breast Cancer. Biomed. Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöffski, P.; Riggert, S.; Fumoleau, P.; Campone, M.; Bolte, O.; Marreaud, S.; Lacombe, D.; Baron, B.; Herold, M.; Zwierzina, H.; et al. Phase I trial of intravenous aviscumine (rViscumin) in patients with solid tumors: A study of the European Organization for Research and Treatment of Cancer New Drug Development Group. Ann. Oncol. 2004, 15, 1816–1824. [Google Scholar] [CrossRef]
- Kienle, G.S.; Glockmann, A.; Schink, M.; Kiene, H. Viscum album L. extracts in breast and gynaecological cancers: A systematic review of clinical and preclinical research. J. Exp. Clin. Cancer Res. 2009, 28, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienle, G.S.; Kiene, H. Review article: Influence of Viscum album L. (European mistletoe) extracts on quality of life in cancer patients: A systematic review of controlled clinical studies. Integr. Cancer Ther. 2010, 9, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Legnani, W. Mistletoe in conventional oncological practice: Exemplary cases. Integr. Cancer Ther. 2008, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Grugel, R.; Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans--systematic review of immune changes and safety parameters. BMC Complement. Altern. Med. 2011, 11, 72. [Google Scholar] [CrossRef] [Green Version]
- Hutt, N.; Kopferschmitt-Kubler, M.; Cabalion, J.; Purohit, A.; Alt, M.; Pauli, G. Anaphylactic reactions after therapeutic injection of mistletoe (Viscum album L.). Allergol. Immunopathol. 2001, 29, 201–203. [Google Scholar] [CrossRef]
- Schläppi, M.; Ewald, C.; Kuehn, J.J.; Weinert, T.; Huber, R. Fever Therapy with Intravenously Applied Mistletoe Extracts for Cancer Patients: A Retrospective Study. Integr. Cancer Ther. 2017, 16, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Reuter, U.R.M.; Oettmeier, R.; Hobohm, U. Safety of Therapeutic Fever Induction in Cancer Patients Using Approved PAMP Drugs. Transl. Oncol. 2018, 11, 330–337. [Google Scholar] [CrossRef]
- Blomqvist, A.; Engblom, D. Neural Mechanisms of Inflammation-Induced Fever. Neuroscientist 2018, 24, 381–399. [Google Scholar] [CrossRef]
- Zampronio, A.R.; Soares, D.M.; Souza, G.E.P. Central mediators involved in the febrile response: Effects of antipyretic drugs. Temperature 2015, 2, 506–521. [Google Scholar] [CrossRef] [Green Version]
- Kozak, W.; Wrotek, S.; Walentynowicz, K.; Waszak, P. Fever and symptoms of sickness are present in mice lacking functional B and T lyphocytes. Acta Biol. Crac. Ser. Zool. 2006, 48, 9–20. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Heiny, B.M.; Beuth, J. Mistletoe extract standardized for the galactoside-specific lectin (ML-1) induces beta-endorphin release and immunopotentiation in breast cancer patients. Anticancer Res. 1994, 14, 1339–1342. [Google Scholar]
- Büssing, A. Immune Modulation Using Mistletoe (Viscum album L.) Extracts Iscador. Arzneimittelforschung 2011, 56, 508–515. [Google Scholar] [CrossRef]
- Oei, S.L.; Thronicke, A.; Schad, F. Mistletoe and Immunomodulation: Insights and Implications for Anticancer Therapies. Evid. Based Complement. Alternat. Med. 2019, 2019, 5893017. [Google Scholar] [CrossRef]
- Kienle, G.S. Fever in Cancer Treatment: Coley’s Therapy and Epidemiologic Observations. Glob. Adv. Health Med. 2012, 1, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Wrotek, S.; Brycht, Ł.; Wrotek, W.; Kozak, W. Fever as a factor contributing to long-term survival in a patient with metastatic melanoma: A case report. Complement. Ther. Med. 2018, 38, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kozak, W.; Jedrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Wrotek, S. Toward Antitumor Immunity and Febrile Infections: Gamma/Delta (γδ) T Cells Hypothesis. Q. Rev. Biol. 2018, 93, 187–205. [Google Scholar] [CrossRef]
- Weissenstein, U.; Kunz, M.; Urech, K.; Baumgartner, S. Interaction of standardized mistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement. Altern. Med. 2014, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingbeil, M.G.; Xavier, F.C.A.; Sardinha, L.R.; Severino, P.; Mathor, M.B.; Rodrigues, R.V.; Pinto, D.S. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol. Rep. 2013, 30, 2316–2322. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Torres, M. Redox signaling in macrophages. Mol. Aspects Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Lasso, P.; Llano Murcia, M.; Sandoval, T.A.; Urueña, C.; Barreto, A.; Fiorentino, S. Breast Tumor Cells Highly Resistant to Drugs Are Controlled Only by the Immune Response Induced in an Immunocompetent Mouse Model. Integr. Cancer Ther. 2019. [Google Scholar] [CrossRef]
- Gayan, S.; Teli, A.; Dey, T. Inherent aggressive character of invasive and non-invasive cells dictates the in vitro migration pattern of multicellular spheroid. Sci. Rep. 2017, 7, 11527. [Google Scholar] [CrossRef] [Green Version]
- Comşa, S.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Lee, O.-Y.; Park, Y.; Seo, M.W.; Lee, D.-S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, S.; Wu, H.; Rong, X.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Dhabekar, G.; Dandekar, R.; Kingaonkar, A. Role of macrophages in malignancy. Ann. Maxillofac. Surg. 2011, 1, 150. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Hashemi Goradel, N.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage polarity in cancer: A review. J. Cell Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef]
- Veremeyko, T.; Yung, A.W.Y.; Anthony, D.C.; Strekalova, T.; Ponomarev, E.D. Early Growth Response Gene-2 Is Essential for M1 and M2 Macrophage Activation and Plasticity by Modulation of the Transcription Factor CEBPβ. Front. Immunol. 2018, 9, 2515. [Google Scholar] [CrossRef] [Green Version]
- Guzhova, I.V.; Shevtsov, M.A.; Abkin, S.V.; Pankratova, K.M.; Margulis, B.A. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int. J. Hyperthermia 2013, 29, 399–408. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Ang, B.; Cao, X.; Wan, T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 2011, 286, 30393–30400. [Google Scholar] [CrossRef] [Green Version]
- Harper, C.V.; Woodcock, D.J.; Lam, C.; Garcia-Albornoz, M.; Adamson, A.; Ashall, L.; Rowe, W.; Downton, P.; Schmidt, L.; West, S.; et al. Temperature regulates NF-κB dynamics and function through timing of A20 transcription. Proc. Natl. Acad. Sci. USA 2018, 115, E5243–E5249. [Google Scholar] [CrossRef] [Green Version]
- Weissenstein, U.; Kunz, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Interaction of a standardized mistletoe (Viscum album) preparation with antitumor effects of Trastuzumab in vitro. BMC Complement. Altern. Med. 2016, 16, 271. [Google Scholar] [CrossRef] [Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Lassche, G.; Crezee, J.; Van Herpen, C.M.L. Whole-body hyperthermia in combination with systemic therapy in advanced solid malignancies. Crit. Rev. Oncol. Hematol. 2019, 139, 67–74. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, H.M.; Pawlikowska, M.; Sobocińska, J.; Jędrzejewski, T.; Dzialuk, A.; Wrotek, S. Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract. Pharmaceuticals 2021, 14, 551. https://doi.org/10.3390/ph14060551
Kozłowski HM, Pawlikowska M, Sobocińska J, Jędrzejewski T, Dzialuk A, Wrotek S. Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract. Pharmaceuticals. 2021; 14(6):551. https://doi.org/10.3390/ph14060551
Chicago/Turabian StyleKozłowski, Henryk M., Małgorzata Pawlikowska, Justyna Sobocińska, Tomasz Jędrzejewski, Artur Dzialuk, and Sylwia Wrotek. 2021. "Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract" Pharmaceuticals 14, no. 6: 551. https://doi.org/10.3390/ph14060551
APA StyleKozłowski, H. M., Pawlikowska, M., Sobocińska, J., Jędrzejewski, T., Dzialuk, A., & Wrotek, S. (2021). Distinct Modulatory Effects of Fever-Range Hyperthermia on the Response of Breast Cancer Cells and Macrophages to Mistletoe (Viscum album L.) Extract. Pharmaceuticals, 14(6), 551. https://doi.org/10.3390/ph14060551





