Zebrafish as a Model for Anticancer Nanomedicine Studies
Abstract
:1. Introduction
2. Zebrafish Cancer Models
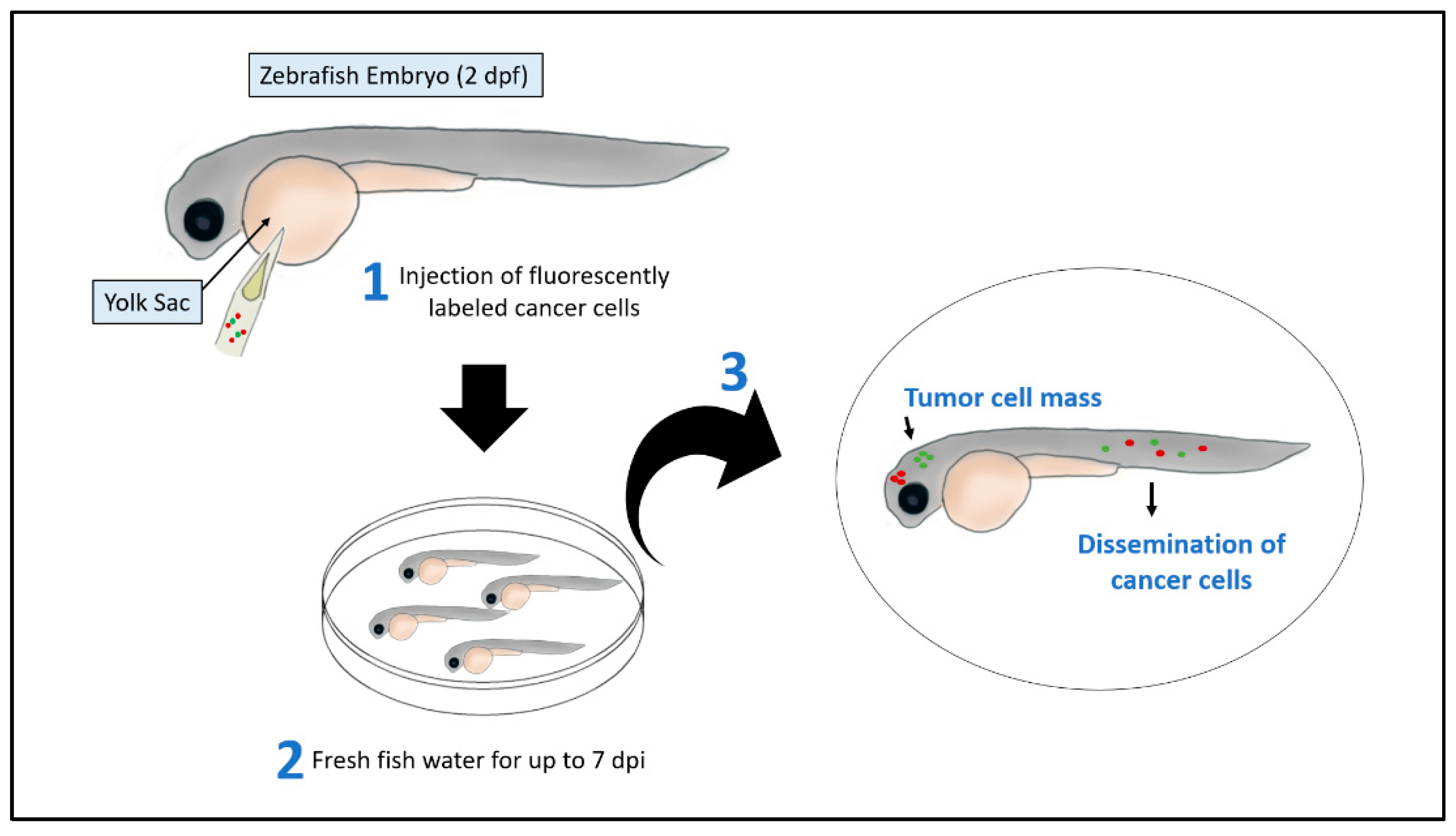
2.1. Xenograft Models
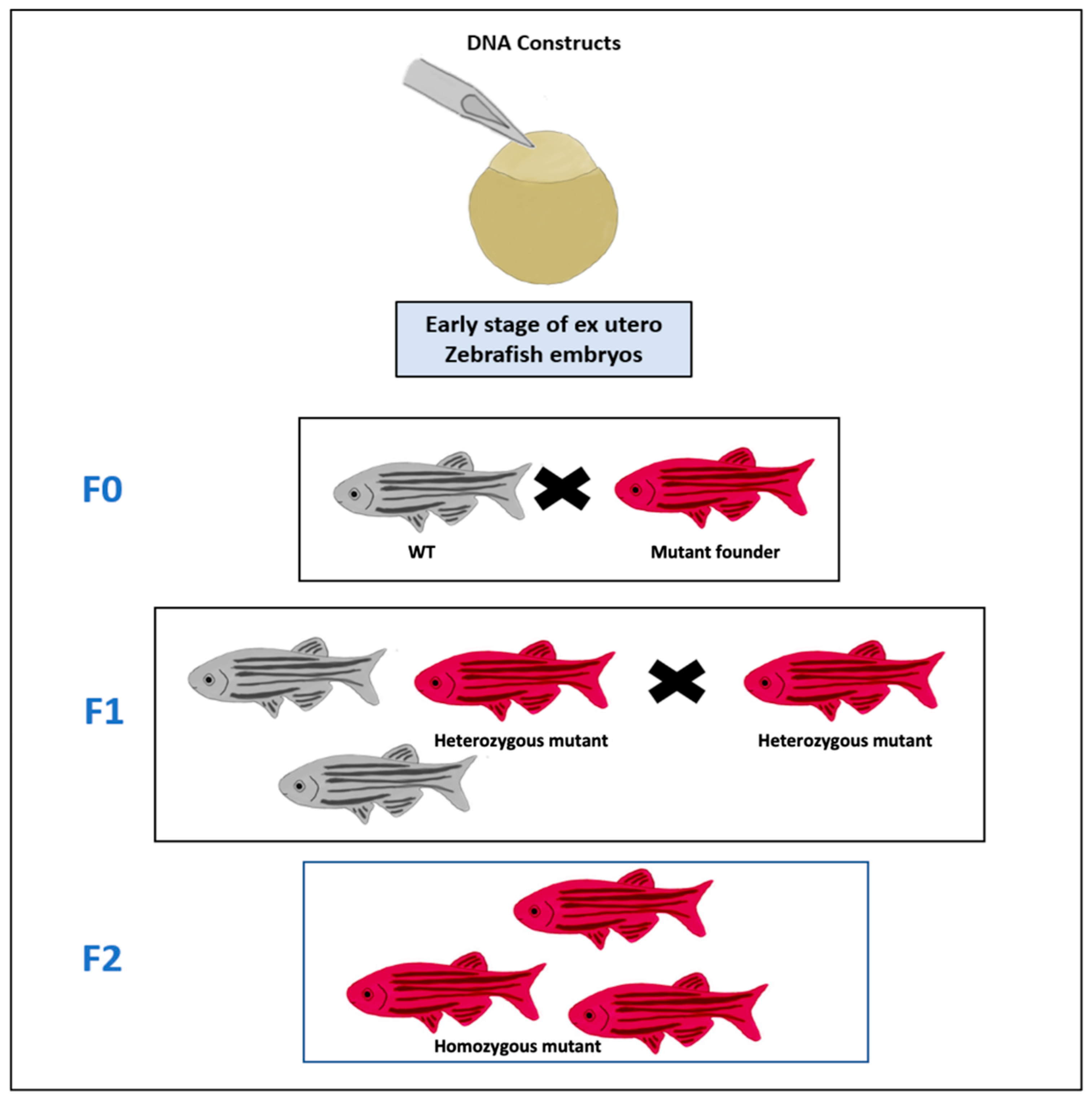
2.2. Genetic Models
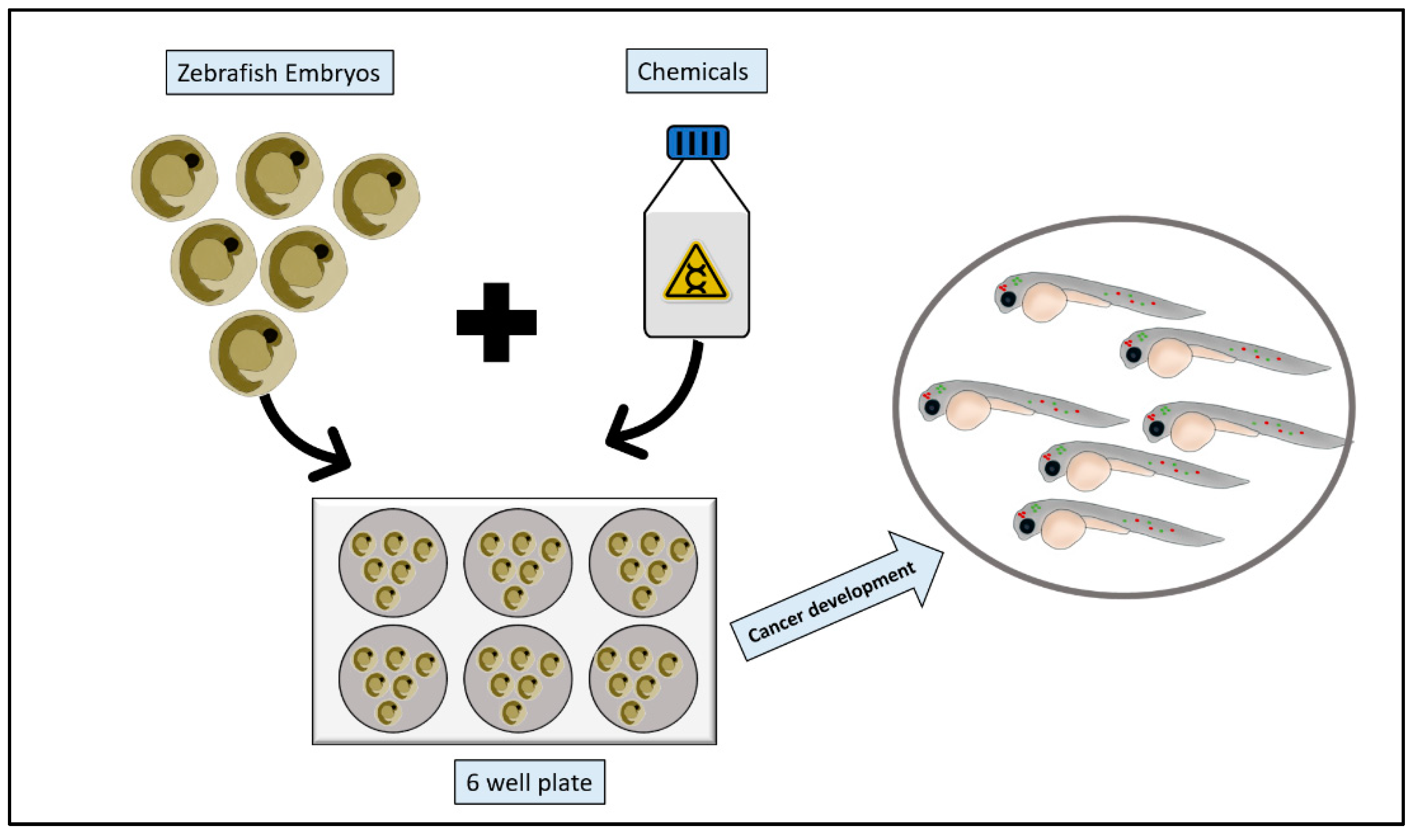
2.3. Chemical Models
3. Tumor Growth and Metastasis Assessment
4. Anti-Cancer Efficacy Assessment
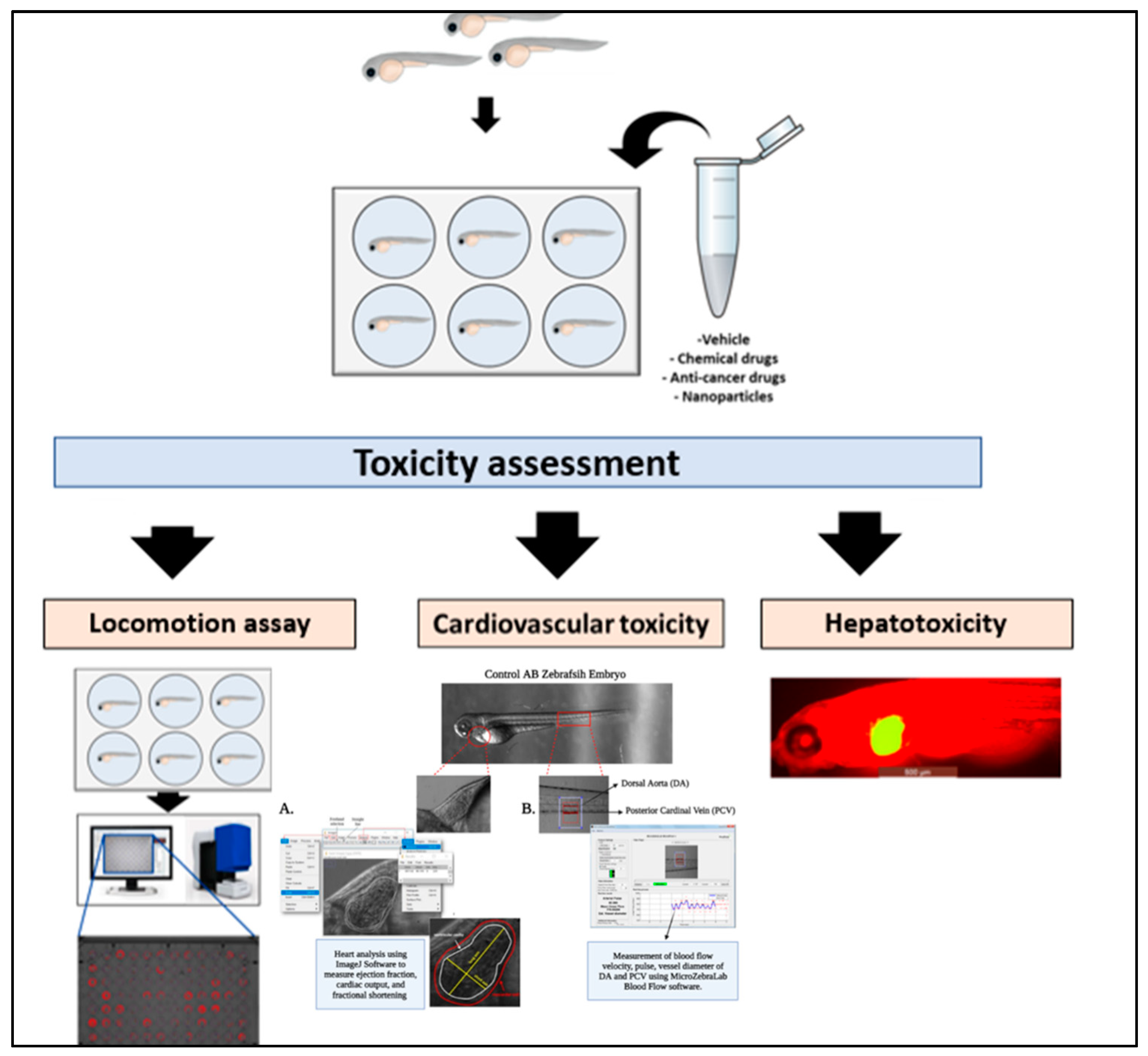
4.1. Toxicity Assessment
4.2. Behavioral and Neurotoxicity
4.3. Cardiovascular Toxicity
4.4. Hepatotoxicity
5. Anti-Cancer NPs Tested on Zebrafish
5.1. Gold and Platinum NPs
5.2. Dendrimers NPs
5.3. Polymersome NPs
5.4. Porphyrin-Based Bridged Silsesquioxane NPs
5.5. Hydroxyapatite NPs
| Anticancer NPs | Cancer Type: | Outcomes: | Toxicity: | References |
|---|---|---|---|---|
| Au and PT NPs | No previous studies |
| [141] | |
| Dendrimers NPs | Cervical Cancer | Inhibition of cancer cells proliferation |
| [143,144] |
| Polymersome NPs | Melanoma | Reduction in cancer cells |
| [145] |
| Porphyrin-based bridged silsesquioxane NPs | Breast Cancer | Reduction in tumor size & activation of apoptosis pathways |
| [147] |
| Hydroxyapatite NPs | Breast Cancer | Reduction in cancer cells’ survival |
| [148,149] |
| Silica NPs | Epithelial Cancers | Fast-targeting capability of epithelial tumors |
| [150,151] |
5.6. Silica NPs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- De Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with multiple properties for biomedical applications: A strategic guide. Nano Today 2016, 11, 435–463. [Google Scholar] [CrossRef]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. R. Soc. Interface 2009, 7, S119–S129. [Google Scholar] [CrossRef] [Green Version]
- McNamara, K.; Tofail, S.A. Nanoalloys: 10. Biomedical Applications of Nanoalloys; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Shurbaji, S.; Anlar, G.G.; Hussein, E.A.; Elzatahry, A.; Yalcin, H.C. Effect of Flow-Induced Shear Stress in Nanomaterial Uptake by Cells: Focus on Targeted Anti-Cancer Therapy. Cancers 2020, 12, 1916. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [Green Version]
- Haque, E.; Ward, A.C. Zebrafish as a Model to Evaluate Nanoparticle Toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Evensen, L.; Johansen, P.L.; Koster, G.; Zhu, K.; Herfindal, L.; Speth, M.; Fenaroli, F.; Hildahl, J.; Bagherifam, S.; Tulotta, C.; et al. Zebrafish as a model system for characterization of nanoparticles against cancer. Nanoscale 2015, 8, 862–877. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Kim, B.Y.S.; Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2018, 2, 797–809. [Google Scholar] [CrossRef]
- Amatruda, J.F.; Shepard, J.L.; Stern, H.M.; I Zon, L. Zebrafish as a cancer model system. Cancer Cell 2002, 1, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; Chua, H.; Gong, Z.; Lam, T.; Sin, Y. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- He, S.; Lamers, G.E.; Beenakker, J.M.; Cui, C.; Ghotra, V.P.; Danen, E.; Meijer, A.H.; Spaink, H.; Snaar-Jagalska, B.E. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012, 227, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Huang, J.; Ye, J. A fresh look at zebrafish from the perspective of cancer research. J. Exp. Clin. Cancer Res. 2015, 34, 80. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Jurewicz, A.; Ilyas, S.; Uppal, J.K.; Ivandic, I.; Korsching, S.; Mathur, S. Evaluation of Magnetite Nanoparticle-Based Toxicity on Embryo–Larvae Stages of Zebrafish (Danio rerio). ACS Appl. Nano Mater. 2020, 3, 1621–1629. [Google Scholar] [CrossRef]
- Dal, N.K.; Kocere, A.; Wohlmann, J.; Van Herck, S.; Bauer, T.A.; Resseguier, J.; Bagherifam, S.; Hyldmo, H.; Barz, M.; De Geest, B.G.; et al. Zebrafish Embryos Allow Prediction of Nanoparticle Circulation Times in Mice and Facilitate Quantification of Nanoparticle–Cell Interactions. Small 2020, 16, e1906719. [Google Scholar] [CrossRef]
- Dahm, R.; Geisler, R. Learning from Small Fry: The Zebrafish as a Genetic Model Organism for Aquaculture Fish Species. Mar. Biotechnol. 2006, 8, 329–345. [Google Scholar] [CrossRef]
- Khan, F.R.; Alhewairini, S.S. Zebrafish (Danio rerio) as a Model Organism. In Current Trends in Cancer Management; InTech: London, UK, 2019. [Google Scholar]
- Mimeault, M.; Batra, S.K. Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov. Today 2013, 18, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Currie, P. The role of zebrafish in chemical genetics. Curr. Med. Chem. 2007, 14, 2413–2420. [Google Scholar] [CrossRef]
- Kanungo, J.; Cuevas, E.; Ali, S.; Paule, M. Zebrafish Model in Drug Safety Assessment. Curr. Pharm. Des. 2014, 20, 5416–5429. [Google Scholar] [CrossRef]
- Gore, A.; Monzo, K.; Cha, Y.R.; Pan, W.; Weinstein, B.M. Vascular Development in the Zebrafish. Cold Spring Harb. Perspect. Med. 2012, 2, a006684. [Google Scholar] [CrossRef] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieschke, G.J. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 2001, 98, 3087–3096. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Ruzicka, L.; Howe, D.G.; Ramachandran, S.; Toro, S.; E Van Slyke, C.; Bradford, Y.M.; Eagle, A.; Fashena, D.; Frazer, K.; Kalita, P.; et al. The Zebrafish Information Network: New support for non-coding genes, richer Gene Ontology annotations and the Alliance of Genome Resources. Nucleic Acids Res. 2019, 47, D867–D873. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: Promoting preclinical applications. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [Green Version]
- McGrath, P.; Li, C.-Q. Zebrafish: A predictive model for assessing drug-induced toxicity. Drug Discov. Today 2008, 13, 394–401. [Google Scholar] [CrossRef]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Berghmans, S.; Jette, C.; Langenau, D.; Hsu, K.; Stewart, R.; Look, T.; Kanki, J.P. Making waves in cancer research: New models in the zebrafish. Biotechniques 2005, 39, 227–237. [Google Scholar] [CrossRef]
- Briggs, J.P. The zebrafish: A new model organism for integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R3–R9. [Google Scholar] [CrossRef] [Green Version]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Konantz, M.; Balci, T.; Hartwig, U.F.; Dellaire, G.; André, M.C.; Berman, J.N.; Lengerke, C. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. N. Y. Acad. Sci. 2012, 1266, 124–137. [Google Scholar] [CrossRef]
- Huang, H.; Vogel, S.S.; Liu, N.; A Melton, D.; Lin, S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol. Cell. Endocrinol. 2001, 177, 117–124. [Google Scholar] [CrossRef]
- Feitsma, H.; Cuppen, E. Zebrafish as a Cancer Model. Mol. Cancer Res. 2008, 6, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Muth-Köhne, E.; Sonnack, L.; Schlich, K.; Hischen, F.; Baumgartner, W.; Hund-Rinke, K.; Schäfers, C.; Fenske, M. The toxicity of silver nanoparticles to zebrafish embryos increases through sewage treatment processes. Ecotoxicology 2013, 22, 1264–1277. [Google Scholar] [CrossRef]
- Harfouche, R.; Basu, S.; Soni, S.; Hentschel, D.M.; Mashelkar, R.A.; Sengupta, S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis 2009, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Bill, B.R.; Petzold, A.; Clark, K.J.; Schimmenti, L.; Ekker, S.C. A Primer for Morpholino Use in Zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drabsch, Y.; Snaar-Jagalska, B.E.; Dijke, P.T. Fish tales: The use of zebrafish xenograft human cancer cell models. Histology Histopathology 2016, 32, 673–686. [Google Scholar] [CrossRef]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, B.; Jacquel, A.; Droin, N.; Auberger, P.; Bouscary, D.; Tamburini, J.; Muller, M.; Fontenay, M.; Chluba, J.; Solary, E. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica 2011, 96, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varas, M.A.; Muñoz-Montecinos, C.; Kallens, V.; Simon, V.; Allende, M.L.; Marcoleta, A.E.; Lagos, R. Exploiting Zebrafish Xenografts for Testing the in vivo Antitumorigenic Activity of Microcin E492 against Human Colorectal Cancer Cells. Front. Microbiol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Wertman, J.; Veinotte, C.J.; Dellaire, G.; Berman, J.N. The Zebrafish Xenograft Platform: Evolution of a Novel Cancer Model and Preclinical Screening Tool. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2016; Volume 916, pp. 289–314. [Google Scholar]
- Drabsch, Y.; He, S.; Zhang, L.; Snaar-Jagalska, B.E.; Dijke, P.T. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013, 15, R106. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Abubaker, K.; Castrechini, N.; Ward, A.C.; Liongue, C.; Dobill, F.; Kumar, J.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Cisplatin treatment of primary and metastatic epithelial ovarian carcinomas generates residual cells with mesenchymal stem cell-like profile. J. Cell. Biochem. 2011, 112, 2850–2864. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Foster, B.A.; Richards, M.; Bondioli, K.R.; Shah, G.; Green, C.C. Characterization of prostate cancer cell progression in zebrafish xenograft model. Int. J. Oncol. 2017, 52, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Weiss, F.U.; Marques, I.J.; Woltering, J.; Vlecken, D.H.; Aghdassi, A.; Partecke, L.I.; Heidecke, C.; Lerch, M.M.; Bagowski, C.P. Retinoic Acid Receptor Antagonists Inhibit miR-10a Expression and Block Metastatic Behavior of Pancreatic Cancer. Gastroenterol. 2009, 137, 2136–2145.e7. [Google Scholar] [CrossRef]
- Yu, C.-I.; Chen, C.-Y.; Liu, W.; Chang, P.-C.; Huang, C.-W.; Han, K.-F.; Lin, I.-P.; Lin, M.-Y.; Lee, C.-H. Sandensolide Induces Oxidative Stress-Mediated Apoptosis in Oral Cancer Cells and in Zebrafish Xenograft Model. Mar. Drugs 2018, 16, 387. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Y.; Huang, L.-T.; Wu, J.-Q.; He, M.-F.; Zhu, S.-H.; Zhan, P.; Lv, T.-F.; Song, Y. Zebrafish Xenograft Model of Human Lung Cancer for Evaluating Osimertinib Resistance. BioMed Res. Int. 2019, 2019, 3129748. [Google Scholar] [CrossRef]
- Xie, X.; Ross, J.L.; Cowell, J.K.; Teng, Y. The promise of zebrafish as a chemical screening tool in cancer therapy. Futur. Med. Chem. 2015, 7, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Schulte-Merker, S.; Walderich, B.; Plasterk, R.H.A. Target-Selected Inactivation of the Zebrafish rag1 Gene. Science 2002, 297, 99–102. [Google Scholar] [CrossRef]
- Ota, S.; Kawahara, A. Zebrafish: A model vertebrate suitable for the analysis of human genetic disorders. Congenit. Anom. 2014, 54, 8–11. [Google Scholar] [CrossRef]
- Heasman, J. Morpholino Oligos: Making Sense of Antisense? Dev. Biol. 2002, 243, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluskota, E.; Dowling, J.J.; Gordon, N.; Golden, J.A.; Szpak, D.; West, X.Z.; Nestor, C.; Ma, Y.-Q.; Bialkowska, K.; Byzova, T.; et al. The integrin coactivator Kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood 2011, 117, 4978–4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, C.-T.; Zhu, K.-Y.; Mi, J.-Q.; Liu, Y.-F.; Murray, S.T.; Fu, Y.-F.; Ren, C.-G.; Dong, Z.-W.; Liu, Y.-J.; Dong, M.; et al. An evolutionarily conserved PTEN-C/EBPα-CTNNA1 axis controls myeloid development and transformation. Blood 2010, 115, 4715–4724. [Google Scholar] [CrossRef] [Green Version]
- Wiellette, E.; Grinblat, Y.; Austen, M.; Hirsinger, E.; Amsterdam, A.; Walker, C.; Westerfield, M.; Sive, H. Combined haploid and insertional mutation screen in the zebrafish. Genes 2004, 40, 231–240. [Google Scholar] [CrossRef]
- Haramis, A.G.; Hurlstone, A.; Van Der Velden, Y.; Begthel, H.; Born, M.V.D.; A Offerhaus, G.J.; Clevers, H.C. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006, 7, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Doyon, Y.; McCammon, J.M.; Miller, J.C.; Faraji, F.; Ngo, C.; E Katibah, G.; Amora, R.; Hocking, T.D.; Zhang, L.; Rebar, E.J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsterdam, A. Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Mol. Cancer Res. 2009, 7, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.; Amsterdam, A.; Farrington, S.; Bronson, R.T.; Hopkins, N.; Lees, J.A. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Dev. Dyn. 2009, 238, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghmans, S.; Murphey, R.D.; Wienholds, E.; Neuberg, D.; Kutok, J.L.; Fletcher, C.D.M.; Morris, J.P.; Liu, T.X.; Schulte-Merker, S.; Kanki, J.P.; et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Langenau, D.M.; Feng, H.; Berghmans, S.; Kanki, J.P.; Kutok, J.L.; Look, A.T. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2005, 102, 6068–6073. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.H.; Jung, D.E.; Park, Y.N.; Song, S.Y.; Park, S.W. Aberrant Hedgehog Ligands Induce Progressive Pancreatic Fibrosis by Paracrine Activation of Myofibroblasts and Ductular Cells in Transgenic Zebrafish. PLoS ONE 2011, 6, e27941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.T. An inducible krasV12 transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis. Models Mech. 2012, 5, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goessling, W.; North, T.E.; Zon, L.I. New Waves of Discovery: Modeling Cancer in Zebrafish. J. Clin. Oncol. 2007, 25, 2473–2479. [Google Scholar] [CrossRef]
- Spitsbergen, J.M.; Tsai, H.-W.; Reddy, A.; Miller, T.; Arbogast, D.; Hendricks, J.D.; Bailey, G. Neoplasia in Zebrafish (Danio rerio) Treated with 7,12-Diniethylbenz[a]anthracene by Two Exposure Routes at Different Developmental Stages. Toxicol. Pathol. 2000, 28, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Spitsbergen, J.M.; Tsai, H.-W.; Reddy, A.; Miller, T.; Arbogast, D.; Hendricks, J.D.; Bailey, G. Neoplasia in Zebrafish (Danio rerio) Treated with N-methyl-N′nitro-N-nitrosoguanidine by Three Exposure Routes at ifferent Developmental Stages. Toxicol. Pathol. 2000, 28, 716–725. [Google Scholar] [CrossRef]
- Mizgireuv, I.V.; Revskoy, S.Y. Transplantable Tumor Lines Generated in Clonal Zebrafish. Cancer Res. 2006, 66, 3120–3125. [Google Scholar] [CrossRef] [Green Version]
- Mizgireuv, I.V.; Majorova, I.G.; Gorodinskaya, V.; Khudoley, V.V.; Revskoy, S.Y. Carcinogenic Effect of N-Nitrosodimethylamine on Diploid and Triploid Zebrafish (Danio rerio). Toxicol. Pathol. 2004, 32, 514–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepard, J.L.; Amatruda, J.F.; Finkelstein, D.; Ziai, J.; Finley, K.R.; Stern, H.M.; Chiang, K.; Hersey, C.; Barut, B.; Freeman, J.; et al. A mutation in separase causes genome instability and increased susceptibility to epithelial cancer. Genes Dev. 2007, 21, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Patron, L.A.; Agudelo-Dueñas, N.; Madrid-Wolff, J.; Venegas, J.A.; González, J.M.; Forero-Shelton, M.; Akle, V. Xenotransplantation of Human glioblastoma in Zebrafish larvae: In vivo imaging and proliferation assessment. Biol. Open 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Vittori, M.; Motaln, H.; Turnšek, T.L. The Study of Glioma by Xenotransplantation in Zebrafish Early Life Stages. J. Histochem. Cytochem. 2015, 63, 749–761. [Google Scholar] [CrossRef]
- Taylor, A.M.; Zon, L.I. Zebrafish Tumor Assays: The State of Transplantation. Zebrafish 2009, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Sainz, P.; Guerra-Varela, J.; Carreira, M.J.; Mariscal, J.; Roel, M.; Rubiolo, J.A.; Sciara, A.A.; Abal, M.; Botana, L.M.; López, R.; et al. Improving zebrafish embryo xenotransplantation conditions by increasing incubation temperature and establishing a proliferation index with ZFtool. BMC Cancer 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.; Astell, K.R.; Velikova, G.V.; Sieger, D. A Zebrafish Live Imaging Model Reveals Differential Responses of Microglia Toward Glioblastoma Cells In Vivo. Zebrafish 2016, 13, 523–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corkery, D.; Dellaire, G.; Berman, J.N. Leukaemia xenotransplantation in zebrafish—Chemotherapy response assay in vivo. Br. J. Haematol. 2011, 153, 786–789. [Google Scholar] [CrossRef]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.-D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-J.; Cui, W.; Gu, A.; Xu, C.; Yu, S.-C.; Li, T.-T.; Cui, Y.-H.; Zhang, X.; Bian, X.-W. A Novel Zebrafish Xenotransplantation Model for Study of Glioma Stem Cell Invasion. PLoS ONE 2013, 8, e61801. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Alhussain, H.; Hasan, A.; Ahmed, M.B.; Yalcin, H.C.; Al Moustafa, A.-E. A novel in ovo model to study cancer metastasis using chicken embryos and GFP expressing cancer cells. Bosn. J. Basic Med. Sci. 2019, 20, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, V.L.; Veinotte, C.J.; Corkery, D.; Pinder, J.B.; Leblanc, M.A.; Bedard, K.; Weng, A.P.; Berman, J.N.; Dellaire, G. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica 2014, 100, 70–76. [Google Scholar] [CrossRef]
- Ghotra, V.P.S.; He, S.; De Bont, H.; Van Der Ent, W.; Spaink, H.; Van De Water, B.; Snaar-Jagalska, B.E.; Danen, E.H.J. Automated Whole Animal Bio-Imaging Assay for Human Cancer Dissemination. PLoS ONE 2012, 7, e31281. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.C.; Hynes, R.O. Intravital imaging of metastasis in adult Zebrafish. BMC Cancer 2017, 17, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laggner, C.; Kokel, D.; Setola, V.; Tolia, A.; Lin, H.; Irwin, J.J.; Keiser, M.J.; Cheung, C.Y.J.; Minor, D.L.; Roth, B.L.; et al. Chemical informatics and target identification in a zebrafish phenotypic screen. Nat. Chem. Biol. 2011, 8, 144–146. [Google Scholar] [CrossRef]
- Terriente, J.; Pujades, C. Use of Zebrafish Embryos for Small Molecule Screening Related to Cancer. Dev. Dyn. 2013, 242, 97–107. [Google Scholar] [CrossRef]
- Ceol, C.J.; Houvras, Y.; Jane-Valbuena, J.; Bilodeau, S.; Orlando, D.A.; Battisti, V.; Fritsch, L.; Lin, W.M.; Hollmann, T.J.; Ferre’, F.; et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nat. Cell Biol. 2011, 471, 513–517. [Google Scholar] [CrossRef]
- Huiting, L.N.; Laroche, F.; Feng, H. The Zebrafish as a Tool to Cancer Drug Discovery. Austin J. Pharmacol. Ther. 2015, 3, 1069. [Google Scholar]
- Ridges, S.; Heaton, W.L.; Joshi, D.; Choi, H.; Eiring, A.; Batchelor, L.; Choudhry, P.; Manos, E.J.; Sofla, H.; Sanati, A.; et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood 2012, 119, 5621–5631. [Google Scholar] [CrossRef] [Green Version]
- MacRae, C.A.; Peterson, R.T. Zebrafish-based small molecule discovery. Chem. Biol. 2003, 10, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbughuni, M.M.; Jannetto, P.J.; Langman, L.J. Mass Spectrometry Applications for Toxicology. EJIFCC 2016, 27, 272–287. [Google Scholar] [PubMed]
- Kiper, K.G.; Freeman, J.L. Zebrafish as a Tool to Assess Developmental Neurotoxicity. In Animal Models of Drug Addiction; Springer: Berlin, Germany, 2019; pp. 169–193. [Google Scholar]
- Giordano, G.; Costa, L.G. Developmental Neurotoxicity: Some Old and New Issues. ISRN Toxicol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horzmann, A.K.; Freeman, J. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Matsoukas, T.; Desai, T.; Lee, K. Engineered Nanoparticles and Their Applications. J. Nanomater. 2015, 2015, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, E.; Soltantabar, P.; Webster, T.J. Nanotechnology and Picotechnology: A New Arena for Translational Medicine. In Biomaterials in Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–212. [Google Scholar]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Zebrafish as a Model Organism to Study Nanomaterial Toxicity. Emerg. Sci. J. 2019, 3, 195–208. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2016, 12, 218–224. [Google Scholar] [CrossRef]
- Cheng, D.; Shami, G.J.; Morsch, M.; Chung, R.; Braet, F. Ultrastructural Mapping of the Zebrafish Gastrointestinal System as a Basis for Experimental Drug Studies. BioMed Res. Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- MacPhail, R.C.; Hunter, D.L.; Irons, T.D.; Padilla, S. Locomotion and Behavioral Toxicity in Larval Zebrafish: Background, Methods, and Data. Zebrafish 2011, 151–164. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Han, X.; Huang, T. The Use of Zebrafish (Danio rerio) Behavioral Responses in Identifying Sublethal Exposures to Deltamethrin. Int. J. Environ. Res. Public Health 2014, 11, 3650–3660. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of Free Radicals in the Toxicity of Airborne Fine Particulate Matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Win-Shwe, T.-T.; Fujimaki, H. Nanoparticles and Neurotoxicity. Int. J. Mol. Sci. 2011, 12, 6267–6280. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, C.; Sarkar, B.K.; Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Shieh, P.C. Future prospects of nanoparticles on brain targeted drug delivery. J. Neuro-Oncol. 2009, 93, 285–286. [Google Scholar] [CrossRef]
- Li, X. SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sun, X.; Chen, L.; Lin, K.-F.; Dong, Q.-X.; Huang, C.-J.; Fu, R.-B.; Zhu, J. Toxicological effect of joint cadmium selenium quantum dots and copper ion exposure on zebrafish. Environ. Toxicol. Chem. 2012, 31, 2117–2123. [Google Scholar] [CrossRef]
- Sheng, L. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environ. Toxicol. 2016, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Parng, C.; Roy, N.M.; Ton, C.; Lin, Y.; McGrath, P. Neurotoxicity assessment using zebrafish. J. Pharmacol. Toxicol. Methods 2007, 55, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M.; Slotkin, T.A.; Seidler, F.J.; Badireddy, A.R.; Padilla, S. Silver nanoparticles alter zebrafish development and larval behavior: Distinct roles for particle size, coating and composition. Neurotoxicol. Teratol. 2011, 33, 708–714. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, G.M.T. Transient modulation of acetylcholinesterase activity caused by exposure to dextran-coated iron oxide nanoparticles in brain of adult zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 162, 77–84. [Google Scholar] [CrossRef]
- Zakaria, Z.Z.; Benslimane, F.; Nasrallah, G.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’As, S.I.; Yalcin, H.C. Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Benslimane, F.; Zakaria, Z.Z.; Shurbaji, S.; Abdelrasool, M.K.A.; Al-Badr, M.A.H.; Al Absi, E.S.K.; Yalcin, H.C. Cardiac function and blood flow hemodynamics assessment of zebrafish (Danio rerio) using high-speed video microscopy. Micron 2020, 136, 102876. [Google Scholar] [CrossRef]
- Yalcin, H.C. Hemodynamic Studies for Analyzing the Teratogenic Effects of Drugs in the Zebrafish Embryo. In Advanced Structural Safety Studies; Springer: Berlin, Germany, 2018; Volume 1797, pp. 487–495. [Google Scholar]
- Benslimane, F.; Alser, M.; Zakaria, Z.Z.; Sharma, A.; Abdelrahman, H.A.; Yalcin, H.C. Adaptation of a Mice Doppler Echocardiography Platform to Measure Cardiac Flow Velocities for Embryonic Chicken and Adult Zebrafish. Front. Bioeng. Biotechnol. 2019, 7, 96. [Google Scholar] [CrossRef]
- Da’As, S.I.; Yalcin, H.C.; Nasrallah, G.K.; Mohamed, I.A.; Nomikos, M.; Yacoub, M.H.; Fakhro, K.A. Functional characterization of human myosin-binding protein C3 variants associated with hypertrophic cardiomyopathy reveals exon-specific cardiac phenotypes in zebrafish model. J. Cell. Physiol. 2020, 235, 7870–7888. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Amindari, A.; Butcher, J.; Althani, A.; Yacoub, M. Heart function and hemodynamic analysis for zebrafish embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Salman, H.E.; Yalcin, H.C. Advanced blood flow assessment in Zebrafish via experimental digital particle image velocimetry and computational fluid dynamics modeling. Micron 2020, 130, 102801. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ichihara, G.; Shimada, Y.; Tada-Oikawa, S.; Kuroyanagi, J.; Zhang, B.; Suzuki, Y.; Sehsah, R.; Kato, M.; Tanaka, T.; et al. Copper Oxide Nanoparticles Reduce Vasculogenesis in Transgenic Zebrafish Through Down-Regulation of Vascular Endothelial Growth Factor Expression and Induction of Apoptosis. J. Nanosci. Nanotechnol. 2015, 15, 2140–2147. [Google Scholar] [CrossRef]
- Cui, B.; Ren, L.; Xu, Q.-H.; Yin, L.-Y.; Zhou, X.-Y.; Liu, J.-X. Silver_ nanoparticles inhibited erythrogenesis during zebrafish embryogenesis. Aquat. Toxicol. 2016, 177, 295–305. [Google Scholar] [CrossRef]
- Villacis, R.A.; Filho, J.S.; Piña, B.; Azevedo, R.B.; Pic-Taylor, A.; Mazzeu, J.; Grisolia, C.K. Integrated assessment of toxic effects of maghemite (γ-Fe2O3) nanoparticles in zebrafish. Aquat. Toxicol. 2017, 191, 219–225. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhang, G.; He, Z.; Wang, Y.; Cui, J. Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae. Int. J. Nanomed. 2016, ume 11, 905–918. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-P.; Fang, T.; Xiong, D.-W.; Zhu, W.-T.; Sima, X.-F. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, ˙OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975–1982. [Google Scholar] [CrossRef]
- Qin, X.; Laroche, F.F.J.; Peerzade, S.A.M.A.; Lam, A.; Sokolov, I.; Feng, H. In Vivo Targeting of Xenografted Human Cancer Cells with Functionalized Fluorescent Silica Nanoparticles in Zebrafish. J. Vis. Exp. 2020, 2020, e61187. [Google Scholar] [CrossRef]
- Singh, M.; Murriel, C.L.; Johnson, L. Genetically Engineered Mouse Models: Closing the Gap between Preclinical Data and Trial Outcomes: Figure 1. Cancer Res. 2012, 72, 2695–2700. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, E.F.; Fogh, J. Considerations in the use of nude mice for cancer research. Cancer Metastasis Rev. 1984, 3, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Baldi, A.; Narang, R.K. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.-X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomed. 2007, 2, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Gao, J. FePt@ CoS2 yolk− shell nanocrystals as a potent agent to kill HeLa cells. J. Am. Chem. Soc. 2007, 129, 1428–1433. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2010, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L. Dendrimer nanocompositer in medicine. Chim. Oggi 2002, 20, 35–40. [Google Scholar]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Chou, H.-Y.; Tsai, H.-C.; Anbazhagan, R.; Yuh, C.-H.; Yang, J.M.; Chang, Y.-H. Amino acid-modified PAMAM dendritic nanocarriers as effective chemotherapeutic drug vehicles in cancer treatment: A study using zebrafish as a cancer model. RSC Adv. 2020, 10, 20682–20690. [Google Scholar] [CrossRef]
- Kocere, A.; Resseguier, J.; Wohlmann, J.; Skjeldal, F.M.; Khan, S.; Speth, M.; Dal, N.-J.K.; Ng, M.Y.W.; Alonso-Rodriguez, N.; Scarpa, E.; et al. Real-time imaging of polymersome nanoparticles in zebrafish embryos engrafted with melanoma cancer cells: Localization, toxicity and treatment analysis. EBioMedicine 2020, 58, 102902. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoën, X.; Durand, J.-O.; Man, M.W.C.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Dib, S.; Aggad, D.; Jimenez, C.M.; Lakrafi, A.; Hery, G.; Nguyen, C.; Durand, D.; Morère, A.; El Cheikh, K.; Sol, V.; et al. Porphyrin-based bridged silsesquioxane nanoparticles for targeted two-photon photodynamic therapy of zebrafish xenografted with human tumor. Cancer Rep. 2019, 2, e1186. [Google Scholar] [CrossRef]
- Nadar, R.A.; Asokan, N.; Degli Esposti, L.; Curci, A.; Barbanente, A.; Schlatt, L.; Karst, U.; Iafisco, M.; Margiotta, N.; Brand, M.; et al. Preclinical evaluation of platinum-loaded hydroxyapatite nanoparticles in an embryonic zebrafish xenograft model. Nanoscale 2020, 12, 13582–13594. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.-L.; Song, C.; Wu, L.-L.; Gao, H.-W. Interactions of Hydroxyapatite with Proteins and Its Toxicological Effect to Zebrafish Embryos Development. PLoS ONE 2012, 7, e32818. [Google Scholar] [CrossRef] [PubMed]
- Peerzade, S.A.M.A.; Qin, X.; Laroche, F.J.F.; Palantavida, S.; Dokukin, M.; Peng, B.; Feng, H.; Sokolov, I. Ultrabright fluorescent silica nanoparticles for in vivo targeting of xenografted human tumors and cancer cells in zebrafish. Nanoscale 2019, 11, 22316–22327. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic Effects of Silica Nanoparticles on Zebrafish Embryos and Larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Thani, H.F.; Shurbaji, S.; Yalcin, H.C. Zebrafish as a Model for Anticancer Nanomedicine Studies. Pharmaceuticals 2021, 14, 625. https://doi.org/10.3390/ph14070625
Al-Thani HF, Shurbaji S, Yalcin HC. Zebrafish as a Model for Anticancer Nanomedicine Studies. Pharmaceuticals. 2021; 14(7):625. https://doi.org/10.3390/ph14070625
Chicago/Turabian StyleAl-Thani, Hissa F., Samar Shurbaji, and Huseyin C. Yalcin. 2021. "Zebrafish as a Model for Anticancer Nanomedicine Studies" Pharmaceuticals 14, no. 7: 625. https://doi.org/10.3390/ph14070625
APA StyleAl-Thani, H. F., Shurbaji, S., & Yalcin, H. C. (2021). Zebrafish as a Model for Anticancer Nanomedicine Studies. Pharmaceuticals, 14(7), 625. https://doi.org/10.3390/ph14070625







