Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives
Abstract
:1. Introduction
2. Epidemiology and Disease Burden
3. Cells and Mediators
4. Clinical Signs and Symptoms
5. Clinicopathological Correlations
6. Management
7. Therapeutic Targets for Pharmacotherapy
8. Therapeutic Targets for Immunomodulators
9. Therapeutic Targets for Immunobiologics
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu El-Asrar, A.M.; Van Aelst, I.; Al-Mansouri, S.; Missotten, L.; Opdenakker, G.; Geboes, K. Gelatinase B in vernal keratoconjunctivitis. Arch. Ophthalmol. 2001, 119, 1505–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Busca, F.; Motterle, L.; Cavarzeran, F.; Fregona, I.A.; Plebani, M.; Secchi, A.G. Case series of 406 vernal keratoconjunctivitis patients: A demographic and epidemiological study. Acta Ophthalmol. Scand. 2006, 84, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Sathe, S.; Bortolotti, M.; Beaton, A.; Sack, R. Cytokines, matrix metalloproteases, angiogenic and growth factors in tears of normal subjects and vernal keratoconjunctivitis patients. Allergy 2009, 64, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Strologo, M.D.; Pichi, F.; Luccarelli, S.V.; De Cillà, S.; Serafino, M.; Nucci, P. Dry Eye in Vernal Keratoconjunctivitis: A Cross-Sectional Comparative Study. Medicine 2015, 94, e1648. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A. Management of Vernal Keratoconjunctivitis. Ophthalmol. Ther. 2013, 2, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zicari, A.M.; Mora, B.; Lollobrigida, V.; Occasi, F.; Marcelli, A.C.; Megiorni, F.; Pizzuti, A.; Nebbioso, M.; Duse, M. Immunogenetic investigation in vernal keratoconjunctivitis. Pediatr. Allergy Immunol. 2014, 25, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Minchiotti, S.; Leonardi, A.; Secchi, A.; Rolando, M.; Calabria, G.; Orsoni, J.; Zola, E.; Ferreri, G.; Aragona, P.; et al. Prospective, Multicenter Demographic and Epidemiological Study on Vernal Keratoconjunctivitis: A Glimpse of Ocular Surface in Italian Population. Ophthalmic Epidemiol. 2009, 16, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Coutu, R.B. Treatment of Vernal Keratoconjunctivitis: A Retrospective Clinical Case Study. Optom. Vis. Sci. 1991, 68, 561–564. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Sandrasekaramudaly-Brown, S. Ocular surface disease: A case of vernal keratoconjunctivitis. Contact Lens Anterior Eye 2011, 34, 39–44. [Google Scholar] [CrossRef]
- Leonardi, A.; Secchi, A.G. Vernal Keratoconjunctivitis. Int. Ophthalmol. Clin. 2003, 43, 41–58. [Google Scholar] [CrossRef]
- Tuft, S.J.; Cree, I.A.; Woods, M.; Yorston, D. Limbal vernal keratoconjunctivitis in the tropics. Ophthalmology 1998, 105, 1489–1493. [Google Scholar] [CrossRef]
- Feizi, S.; Javadi, M.A.; Alemzadeh-Ansari, M.; Arabi, A.; Shahraki, T.; Kheirkhah, A. Management of corneal complications in vernal keratoconjunctivitis: A review. Ocul. Surf. 2020. [Google Scholar] [CrossRef]
- Bonini, S.; Bonini, S.; Lambiase, A.; Marchi, S.; Pasqualetti, P.; Zuccaro, O.; Rama, P.; Magrini, L.; Juhas, T.; Bucci, M.G. Vernal keratoconjunctivitis revisited: A case series of 195 patients with long-term followup. Ophthalmology 2000, 107, 1157–1163. [Google Scholar] [CrossRef]
- Bonini, S.; Coassin, M.; Aronni, S.; Lambiase, A. Vernal keratoconjunctivitis. Eye 2004, 18, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Iovieno, A.; Lambiase, A.; Sacchetti, M.; Stampachiacchiere, B.; Micera, A.; Bonini, S. Preliminary evidence of the efficacy of probiotic eye-drop treatment in patients with vernal keratoconjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 435–441. [Google Scholar] [CrossRef]
- Chigbu, D.I. The management of allergic eye diseases in primary eye care. Contact Lens Anterior Eye 2009, 32, 260–272. [Google Scholar] [CrossRef]
- Kumar, S. Vernal keratoconjunctivitis: A major review. Acta Ophthalmol. 2009, 87, 133–147. [Google Scholar] [CrossRef]
- Ukponmwan, C.U. Vernal Keratoconjunctivitis in Nigerians: 109 Consecutive Cases. Trop. Dr. 2003, 33, 242–245. [Google Scholar] [CrossRef]
- Saboo, U.S.; Jain, M.; Reddy, J.C.; Sangwan, V.S. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J. Ophthalmol. 2013, 61, 486. [Google Scholar] [CrossRef]
- Leonardi, A.; Castegnaro, A.; Valerio AL, G.; Lazzarini, D. Epidemiology of allergic conjunctivitis: Clinical appearance and treatment patterns in a population-based study. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 482–488. [Google Scholar] [CrossRef]
- McMoli, T.; Assonganyi, T. Limbal vernal kerato-conjunctivitis in Yaounde, Cameroon. A clinico-immunology study. Rev. Int. Trach. Pathol. Ocul. Trop. Subtrop. Sante Publique 1991, 68, 157–170. [Google Scholar]
- O’Shea, J.G. A survey of vernal keratoconjunctivitis and other eosinophil-mediated external eye diseases amongst Palestinians. Ophthalmic Epidemiol. 2000, 7, 149–157. [Google Scholar] [CrossRef]
- Saraclar, Y.; Yigit, S.; Adalioglu, G.; Tuncer, A.; Tuncbilek, E. Prevalence of Allergic Diseases and Influencing Factors in Primary-School Children in the Ankara Region of Turkey. J. Asthma 1997, 34, 23–30. [Google Scholar] [CrossRef]
- La Rosa, M.; Lionetti, E.; Reibaldi, M.; Russo, A.; Longo, A.; Leonardi, S.; Tomarchio, S.; Avitabile, T.; Reibaldi, A. Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 2013, 39, 18. [Google Scholar] [CrossRef] [Green Version]
- Bremond-Gignac, D.; Donadieu, J.; Leonardi, A.; Pouliquen, P.; Doan, S.; Chiambarretta, F.; Montan, P.; Milazzo, S.; Hoang-Xuan, T.; Baudouin, C.; et al. Prevalence of vernal keratoconjunctivitis: A rare disease? Br. J. Ophthalmol. 2008, 92, 1097–1102. [Google Scholar] [CrossRef]
- Vichyanond, P.; Pacharn, P.; Pleyer, U.; Leonardi, A. Vernal keratoconjunctivitis: A severe allergic eye disease with remodeling changes. Pediatr. Allergy Immunol. 2014, 25, 314–322. [Google Scholar] [CrossRef]
- Zicari, A.M.; Capata, G.; Nebbioso, M.; De Castro, G.; Midulla, F.; Leonardi, L.; Loffredo, L.; Spalice, A.; Perri, L.; Duse, M. Vernal Keratoconjunctivitis: An update focused on clinical grading system. Ital. J. Pediatr. 2019, 45, 64. [Google Scholar] [CrossRef]
- Leonardi, A.; Lazzarini, D.; Motterle, L.; Bortolotti, M.; Deligianni, V.; Curnow, J.; Bonini, S.; Fregona, I.A. Vernal Keratoconjunctivitis-like Disease in Adults. Am. J. Ophthalmol. 2013, 155, 796–803. [Google Scholar] [CrossRef]
- Singhal, D.; Sahay, P.; Maharana, P.K.; Raj, N.; Sharma, N.; Titiyal, J.S. Vernal Keratoconjunctivitis. Surv. Ophthalmol. 2019, 64, 289–311. [Google Scholar] [CrossRef]
- Kawuma, M. The Clinical Picture of Vernal Kerato-Conjunctivitis in Uganda. Community Eye Health 2001, 14, 66–67. [Google Scholar]
- De Smedt, S.K.; Nkurikiye, J.; Fonteyne, Y.S.; Tuft, S.J.; Gilbert, C.E.; Kestelyn, P. Vernal Keratoconjunctivitis in School Children in Rwanda: Clinical Presentation, Impact on School Attendance, and Access to Medical Care. Ophthalmology 2012, 119, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Ajaiyeoba, A. Prevalence of atopic diseases in Nigerian children with vernal kerato-conjunctivitis. West Afr. J. Med. 2004, 22, 15–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayilu, D.; Legesse, K.; Lakachew, N.; Asferaw, M. Prevalence and associated factors of vernal keratoconjunctivitis among children in Gondar city, Northwest Ethiopia. BMC Ophthalmol. 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanathanee, O.; Bhoomibunchoo, C.; Suwan-Apichon, O. Treatment of asymmetrical vernal keratoconjunctivitis with supratarsal corticosteroid injection. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- De Smedt, S.; Wildner, G.; Kestelyn, P. Vernal keratoconjunctivitis: An update. Br. J. Ophthalmol. 2012, 97, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, M.; Baiardini, I.; Lambiase, A.; Aronni, S.; Fassio, O.; Gramiccioni, C.; Bonini, S.; Bonini, S. Development and Testing of the Quality of Life in Children with Vernal Keratoconjunctivitis Questionnaire. Am. J. Ophthalmol. 2007, 144, 557–563.e2. [Google Scholar] [CrossRef]
- Leonardi, A.; Doan, S.; Amrane, M.; Ismail, D.; Montero, J.; Németh, J.; Aragona, P.; Bremond-Gignac, D. A Randomized, Controlled Trial of Cyclosporine A Cationic Emulsion in Pediatric Vernal Keratoconjunctivitis: The VEKTIS Study. Ophthalmology 2019, 126, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Al-Akily, S.A.; Bamashmus, M.A. Ocular complications of severe vernal keratoconjunctivitis (VKC) in Yemen. Saudi J. Ophthalmol. 2011, 25, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Alemayehu, A.M.; Yibekal, B.T.; Fekadu, S.A. Prevalence of vernal keratoconjunctivitis and its associated factors among children in Gambella town, southwest Ethiopia, June 2018. PLoS ONE 2019, 14, e0215528. [Google Scholar] [CrossRef] [Green Version]
- Magaña, D.; Aguilar, G.; Linares, M.; Ayala-Balboa, J.; Santacruz, C.; Chávez, R.; Estrada-Parra, S.; Garfias, Y.; Lascurain, R.; Jiménez-Martínez, M.C. Intracellular IL-4, IL-5, and IFN-gamma as the main characteristic of CD4+CD30+ T cells after allergen stimulation in patients with vernal keratoconjunctivitis. Mol. Vis. 2015, 21, 443–450. [Google Scholar]
- Cavet, M.E.; Volhejn, S.; Harrington, K.L.; Zhang, J.Z. Anti-allergic effects of mapracorat, a novel selective glucocorticoid receptor agonist, in human conjuncti-val fibroblasts and epithelial cells. Mol. Vis. 2013, 19, 1515–1525. [Google Scholar]
- Solomon, A. Corneal complications of vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 489–494. [Google Scholar] [CrossRef]
- Irkeç, M.; Bozkurt, B. Epithelial cells in ocular allergy. Curr. Allergy Asthma Rep. 2003, 3, 352–357. [Google Scholar] [CrossRef]
- Liu, M.; Gao, H.; Wang, T.; Wang, S.; Li, S.; Shi, W. An essential role for dendritic cells in vernal keratoconjunctivitis: Analysis by laser scanning confocal microscopy. Clin. Exp. Allergy 2014, 44, 362–370. [Google Scholar] [CrossRef]
- Asano-Kato, N.; Fukagawa, K.; Okada, N.; Kawakita, T.; Takano, Y.; Dogru, M.; Tsubota, K.; Fujishima, H. TGF-beta1, IL-1beta, and Th2 cytokines stimulate vascular endothelial growth factor production from conjunctival fibroblasts. Exp. Eye Res. 2005, 80, 555–560. [Google Scholar] [CrossRef]
- Uchio, E.; Ono, S.Y.; Ikezawa, Z.; Ohno, S. Tear levels of interferon-gamma, interleukin (IL)-2, IL-4 and IL-5 in patients with vernal keratoconjunctivi-tis, atopic keratoconjunctivitis and allergic conjunctivitis. Clin. Exp. Allergy 2000, 30, 103–109. [Google Scholar] [CrossRef]
- Chigbu, D.I. Immunology Relevant to Allergic Ocular Surface Diseases. In Allergic Disorders of the Ocular Surface; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 53–74. [Google Scholar]
- Murphy, K.P.; Travers, P.; Walport, M. The Induced Response of Innate Immunity. In Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2012; pp. 75–125. [Google Scholar]
- Zhan, H.; Smith, L.; Calder, V.; Buckley, R.; Lightman, S. Clinical and Immunological Features of Atopic Keratoconjunctivitis. Int. Ophthalmol. Clin. 2003, 43, 59–71. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Jain, P.; Khan, Z.K. Immune Mechanisms, Pathology, and Management of Allergic Ocular Diseases. In Advanced Concepts in Human Immunology: Prospects for Disease Control; Springer: Cham, Switzerland, 2020; pp. 229–277. [Google Scholar]
- Chigbu, D.I.; Minhas, B.K. Immunopathology of allergic conjunctivitis. Eur. Med. J. 2018, 3, 76–83. [Google Scholar]
- Kumagai, N.; Fukuda, K.; Fujitsu, Y.; Yamamoto, K.; Nishida, T. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog. Retin. Eye Res. 2006, 25, 165–187. [Google Scholar] [CrossRef]
- Enríquez-De-Salamanca, A.; Castellanos, E.; Stern, M.E.; Fernández, I.; Carreno, E.; García-Vázquez, C.; Herreras, J.M.; Calonge, M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis. 2010, 16, 862–873. [Google Scholar]
- Yi, S.; Zhai, J.; Niu, R.; Zhu, G.; Wang, M.; Liu, J.; Huang, H.; Wang, Y.; Jing, X.; Kang, L.; et al. Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nat. Commun. 2018, 24, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Parham, P. IgE-Mediated Immunity and Allergy. In The Immune System; Garland Science: New York, NY, USA, 2015; pp. 401–431. [Google Scholar]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2007, 38, 4–18. [Google Scholar] [CrossRef]
- Murphy, K.P.; Travers, P.; Walport, M. Allergy and Allergic Diseases. In Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2012; pp. 571–610. [Google Scholar]
- Fujishima, H.; Fukagawa, K.; Okada, N.; Takano, Y.; Tsubota, K.; Hirai, H.; Nagata, K.; Matsumoto, K.; Saito, H. Prostaglandin D2 Induces Chemotaxis in Eosinophils via Its Receptor CRTH2 and Eosinophils May Cause Severe Ocular Inflammation in Patients with Allergic Conjunctivitis. Cornea 2005, 24, S66–S70. [Google Scholar] [CrossRef]
- Zinchuk, O.; Fukushima, A.; Zinchuk, V.; Fukata, K.; Ueno, H. Direct action of platelet activating factor (PAF) induces eosinophil accumulation and enhances expression of PAF receptors in conjunctivitis. Mol. Vis. 2005, 11, 114–123. [Google Scholar]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Riol-Blanco, L.; Sánchez-Sánchez, N.; Torres, A.; Tejedor, A.; Narumiya, S.; Corbí, A.L.; Sánchez-Mateos, P.; Fernandez, J.L.R. The Chemokine Receptor CCR7 Activates in Dendritic Cells Two Signaling Modules That Independently Regulate Chemotaxis and Migratory Speed. J. Immunol. 2005, 174, 4070–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, P.; de Paiva, C.; Cunningham, M.A.; Hwang, C.S.; Pflugfelder, S.C.; Li, D.-Q. TSLP and Downstream Molecules in Experimental Mouse Allergic Conjunctivitis. Investig. Opthalmol. Vis. Sci. 2010, 51, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Takai, T. TSLP Expression: Cellular Sources, Triggers, and Regulatory Mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, A.; Radice, M.; Fregona, I.A.; Plebani, M.; Abatangelo, G.; Secchi, A.G. Histamine Effects on Conjunctival Fibroblasts from Patients with Vernal Conjunctivitis. Exp. Eye Res. 1999, 68, 739–746. [Google Scholar] [CrossRef]
- Fujitsu, Y.; Fukuda, K.; Kumagai, N.; Nishida, T. IL-4-induced cell proliferation and production of extracellular matrix proteins in human conjunctival fibroblasts. Exp. Eye Res. 2003, 76, 107–114. [Google Scholar] [CrossRef]
- Leonardi, A.; Cortivo, R.; Fregona, I.; Plebani, M.; Secchi, A.G.; Abatangelo, G. Effects of Th2 Cytokines on Expression of Collagen, MMP-1, and TIMP-1 in Conjunctival Fibroblasts. Investig. Opthalmol. Vis. Sci. 2003, 44, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Kumagai, N.; Fujitsu, Y.; Nishida, T. Fibroblasts as Local Immune Modulators in Ocular Allergic Disease. Allergol. Int. 2006, 55, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Andrew, D.P.; Ruffing, N.; Kim, C.H.; Miao, W.; Heath, H.; Li, Y.; Murphy, K.; Campbell, J.; Butcher, E.C.; Wu, L. C-C Chemokine Receptor 4 Expression Defines a Major Subset of Circulating Nonintestinal Memory T Cells of Both Th1 and Th2 Potential. J. Immunol. 2001, 166, 103–111. [Google Scholar] [CrossRef]
- Ochkur, S.I.; Jacobsen, E.A.; Lacy, P. Eosinophil Shape Change and Secretion. Methods Mol. Biol. 2021, 2241, 199–219. [Google Scholar] [CrossRef]
- Leonardi, A.; Brun, P.; Tavolato, M.; Plebani, M.; Abatangelo, G.; Secchi, A.G. Tumor necrosis factor-alpha (TNF-α) in seasonal allergic conjunctivitis and vernal keratoconjunctivitis. Eur. J. Ophthalmol. 2003, 13, 606–610. [Google Scholar] [CrossRef]
- El-Asrar, A.M.A.; Geboes, K.; Al-Kharashi, S.; Tabbara, K.F.; Missotten, L.; Desmet, V. Adhesion molecules in vernal keratoconjunctivitis. Br. J. Ophthalmol. 1997, 81, 1099–1106. [Google Scholar] [CrossRef]
- Inada, N.; Ishimori, A.; Shoji, J. CCL20/MIP-3 alpha mRNA expression in the conjunctival epithelium of normal indi-viduals and patients with vernal keratoconjunctivitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1977–1984. [Google Scholar] [CrossRef] [Green Version]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Chigbu, D.G.I. The pathophysiology of ocular allergy: A review. Contact Lens Anterior Eye 2009, 32, 3–15. [Google Scholar] [CrossRef]
- Kumagai, N.; Yamamoto, K.; Fukuda, K.; Nakamura, Y.; Fujitsu, Y.; Nuno, Y.; Nishida, T. Active matrix metalloproteinases in the tear fluid of individuals with vernal keratoconjunctivitis. J. Allergy Clin. Immunol. 2002, 110, 489–491. [Google Scholar] [CrossRef]
- Tsubota, K.; Takamura, E.; Hasegawa, T.; Kobayashi, T.K. Detection by Brush Cytology of Mast Cells and Eosinophils in Allergic and Vernal Conjunctivitis. Cornea 1991, 10, 525–531. [Google Scholar] [CrossRef]
- Bruschi, G.; Ghiglioni, D.G.; Osnaghi, S.; Rosazza, C.; Marafon, D.P.; Landi, M.; Marchisio, P.G. Role of ocular cytology in vernal keratoconjunctivitis. Immun. Inflamm. Dis. 2020, 8, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A. Vernal keratoconjunctivitis: Pathogenesis and treatment. Prog. Retin. Eye Res. 2002, 21, 319–339. [Google Scholar] [CrossRef]
- Shahriari, M.; Hosseini, S.B.; Aliakbar-Navahi, R.; Javadi, M.A.; Abrishami, Y. Allergic Conjunctival Granuloma Presenting the Splendore-Hoeppli Phenomenon; Report of Two Cases and Review of Literature. J. Ophthalmic Vis. Res. 2015, 10, 481–483. [Google Scholar] [CrossRef] [Green Version]
- Chigbu, D.I. Vernal Keratoconjunctivitis. In Allergic Disorders of the Ocular Surface; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 109–126. [Google Scholar]
- Leonardi, A.; Bogacka, E.; Fauquert, J.L.; Kowalski, M.L.; Groblewska, A.; Jedrzejczak-Czechowicz, M.; Doan, S.; Marmouz, F.; Demoly, P.; Delgado, L. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy 2012, 67, 1327–1337. [Google Scholar] [CrossRef]
- Cameron, J.A.; Mullaney, P.B. Amblyopia resulting from shield ulcers and plaques of the cornea in vernal keratoconjunc-tivitis. J. Pediatr. Ophthalmol. Strabismus 1997, 34, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology 1995, 102, 985–993. [Google Scholar] [CrossRef]
- Sridhar, M.S.; Gopinathan, U.; Rao, G.N. Fungal Keratitis Associated with Vernal Keratoconjunctivitis. Cornea 2003, 22, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L. Allergic and immunologic disorders of the eye. Part II: Ocular allergy. J. Allergy Clin. Immunol. 2000, 106, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Fior, G.; Mori, A.; Osnaghi, S.; Ghiglioni, D. An Update on the Therapeutic Approach to Vernal Keratoconjunctivitis. Pediatr. Drugs 2016, 18, 347–355. [Google Scholar] [CrossRef]
- Collum, L.M.T. Vernal Keratoconjunctivitis. Acta Ophthalmol. Scand. 1999, 77, 14–16. [Google Scholar] [CrossRef]
- Çorum, I.; Yeniad, B.; Bilgin, L.K.; Ilhan, R. Efficiency of Olopatadine Hydrochloride 0.1% in the Treatment of Vernal Keratoconjunctivitis and Goblet Cell Density. J. Ocul. Pharmacol. Ther. 2005, 21, 400–405. [Google Scholar] [CrossRef]
- Keklikci, U.; Soker, S.I.; Sakalar, Y.B.; Unlu, K.; Özekinci, S.; Tunik, S. Efficacy of topical cyclosporin A 0.05% in conjunctival impression cytology specimens and clinical findings of severe vernal keratoconjunctivitis in children. Jpn. J. Ophthalmol. 2008, 52, 357–362. [Google Scholar] [CrossRef]
- Rao, S.K.; Meenakshi, S.; Srinivasan, B.; Baluswamy, S. Perilimbal bulbar conjunctival pigmentation in vernal conjunctivitis: Prospective evaluation of a new clinical sign in an Indian population. Cornea 2004, 23, 356–359. [Google Scholar] [CrossRef]
- Read, S.A.; Swann, P.G. Unilateral pseudogerontoxon. Clin. Exp. Optom. 2009, 92, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Pattnaik, L.; Acharya, L. A comprehensive review on vernal keratoconjunctivitis with emphasis on proteomics. Life Sci. 2015, 128, 47–54. [Google Scholar] [CrossRef]
- Ohbayashi, M.; Manzouri, B.; Morohoshi, K.; Fukuda, K.; Ono, S.J. The role of histamine in ocular allergy. Adv. Exp. Med. Biol. 2010, 709, 43–52. [Google Scholar] [CrossRef]
- Gary, R.K.; Woodward, D.F.; Nieves, A.L.; Williams, L.S.; Gleason, J.G.; Wasserman, M.A. Characterization of the conjunctival vasopermeability response to leukotrienes and their involvement in immediate hypersensitivity. Investig. Ophthalmol. Vis. Sci. 1988, 29, 119–126. [Google Scholar]
- Woodward, D.F.; Ledgard, S.E. Effect of LTD4 on conjunctival vasopermeability and blood-aqueous barrier integrity. Investig. Ophthalmol. Vis. Sci. 1985, 26, 481–485. [Google Scholar]
- Dartt, D.A.; Hodges, R.R.; Li, D.; Shatos, M.A.; Lashkari, K.; Serhan, C.N. Conjunctival Goblet Cell Secretion Stimulated by Leukotrienes Is Reduced by Resolvins D1 and E1 To Promote Resolution of Inflammation. J. Immunol. 2011, 186, 4455–4466. [Google Scholar] [CrossRef] [Green Version]
- Woodward, D.F.; Hawley, S.B.; Williams, L.S.; Ralston, T.R.; Protzman, C.E.; Spada, C.S.; Nieves, A.L. Studies on the ocular pharmacology of prostaglandin D2. Investig. Ophthalmol. Vis. Sci. 1990, 31, 138–146. [Google Scholar]
- Meng, Q.; Ying, S.; Corrigan, C.J.; Wakelin, M.; Assoufi, B.; Moqbel, R.; Kay, A.B. Effects of rapamycin, cyclosporin A, and dexamethasone on interleukin 5-induced eosinophil degranulation and prolonged survival. Allergy 1997, 52, 1095–1101. [Google Scholar] [CrossRef]
- Henriksson, J.T.; Coursey, T.G.; Corry, D.B.; De Paiva, C.S.; Pflugfelder, S.C. IL-13 Stimulates Proliferation and Expression of Mucin and Immunomodulatory Genes in Cul-tured Conjunctival Goblet Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4186–4197. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Takahashi, Y.; Inoue, S. Histamine Induces Melanogenesis and Morphologic Changes by Protein Kinase a Activation via H2 Receptors in Human Normal Melanocytes. J. Investig. Dermatol. 2000, 114, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Niwano, T.; Terazawa, S.; Nakajima, H.; Imokawa, G. The stem cell factor-stimulated melanogenesis in human melanocytes can be abrogated by interrupting the phosphorylation of MSK1: Evidence for involvement of the p38/MSK1/CREB/MITF axis. Arch. Dermatol. Res. 2018, 310, 187–196. [Google Scholar] [CrossRef]
- Hogaboam, C.; Kunkel, S.L.; Strieter, R.M.; Taub, D.D.; Lincoln, P.; Standiford, T.J.; Lukacs, N.W. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J. Immunol. 1998, 160, 6166–6171. [Google Scholar]
- Luk, F.O.J.; Wong, V.W.Y.; Rao, S.K.; Lam, D.S.C. Perilimbal conjunctival pigmentation in Chinese patients with vernal keratoconjunctivitis. Eye 2007, 22, 1011–1014. [Google Scholar] [CrossRef]
- Saboo, U.S.; Basu, S.; Tiwari, S.; Mohamed, A.; Vemuganti, G.K.; Sangwan, V.S. Clinical and Cytologic Evidence of Limbal Stem Cell Deficiency in Eyes with Long-Standing Vernal Keratoconjunctivitis. Asia Pac. J. Ophthalmol. 2013, 2, 88–93. [Google Scholar] [CrossRef]
- Ghiglioni, D.G.; Zicari, A.M.; Parisi, G.F.; Marchese, G.; Indolfi, C.; Diaferio, L.; Brindisi, G.; Ciprandi, G.; Marseglia, G.L.; Miraglia del Giudice, M. Vernal keratoconjunctivitis: An update. Eur. J. Ophthalmol. 2021, 31. [Google Scholar] [CrossRef]
- Jeng, B.H.; Whitcher, J.P.; Margolis, T.P. Pseudogerontoxon. Clin. Exp. Ophthalmol. 2004, 32, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Bielory, B.; Bielory, L. Atopic Dermatitis and Keratoconjunctivitis. Immunol. Allergy Clin. N. Am. 2010, 30, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, M.H. Ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Irkec, M.T.; Bozkurt, B. Molecular immunology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.; Basu, S.; Saboo, U.S.; Murthy, S.I.; Vaddavalli, P.K.; Sangwan, V.S. Management, Clinical Outcomes, and Complications of Shield Ulcers in Vernal Keratoconjunctivitis. Am. J. Ophthalmol. 2013, 155, 550–559.e1. [Google Scholar] [CrossRef]
- Solomon, A.; Zamir, E.; Levartovsky, S.; Frucht-Pery, J. Surgical management of corneal plaques in vernal keratoconjunctivitis: A clinicopathologic study. Cornea 2004, 23, 608–612. [Google Scholar] [CrossRef]
- Ozbek, Z.; Burakgazi, A.Z.; Rapuano, C.J. Rapid healing of vernal shield ulcer after surgical debridement: A case report. Cornea 2006, 25, 472–473. [Google Scholar] [CrossRef]
- Pelegrin, L.; Gris, O.; Adán, A.; Plazas, A. Superficial keratectomy and amniotic membrane patch in the treatment of corneal plaque of vernal keratoconjunctivitis. Eur. J. Ophthalmol. 2008, 18, 131–133. [Google Scholar] [CrossRef]
- Luo, J.Y.; Chen, Z.; Guo, J.-J.; Guo, Z.-J.; Lan, X.; Sun, B.-Q. Efficacy of air purifier therapy in allergic rhiniti. Asian Pac. J. Allergy Immunol. 2018, 36, 217–221. [Google Scholar]
- Bielory, L.; Meltzer, E.O.; Nichols, K.K.; Melton, R.; Thomas, R.K.; Bartlett, J.D. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc. 2013, 34, 408–420. [Google Scholar] [CrossRef]
- Dupuis, P.; Prokopich, C.L.; Hynes, A.; Kim, H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin. Immunol. 2020, 16, 1–18. [Google Scholar] [CrossRef]
- Gokhale, N.S. Systematic approach to managing vernal keratoconjunctivitis in clinical practice: Severity grading system and a treatment algorithm. Indian J. Ophthalmol. 2016, 64, 145–148. [Google Scholar] [CrossRef]
- Bonini, S.; Barney, N.P.; Schiavone, M.; Centofanti, M.; Berruto, A.; Bonini, S. Effectiveness of nedocromil sodium 2% eyedrops on clinical symptoms and tear fluid cytology of patients with vernal conjunctivitis. Eye 1992, 6 Pt 6, 648–652. [Google Scholar] [CrossRef]
- Caldwell, D.R.; Verin, P.; Hartwich-Young, R.; Meyer, S.M.; Drake, M.M. Efficacy and safety of lodoxamide 0.1% vs cromolyn sodium 4% in patients with vernal keratoconjunctivitis. Am. J. Ophthalmol. 1992, 113, 632–637. [Google Scholar] [CrossRef]
- Tabbara, K.F.; Arafat, N.T. Cromolyn Effects on Vernal Keratoconjunctivitis in Children. Arch. Ophthalmol. 1977, 95, 2184–2186. [Google Scholar] [CrossRef]
- Kjellman, N.-I.M.; Stevens, M.T. Clinical Experience with Tilavist: An Overview of Efficacy and Safety. Allergy 1995, 50, 14–22. [Google Scholar] [CrossRef]
- Leonardi, A. Effect of lodoxamide and disodium cromoglycate on tear eosinophil cationic protein in vernal keratoconjunctivitis. Br. J. Ophthalmol. 1997, 81, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Mantelli, F.; Santos, M.S.; Petitti, T.; Sgrulletta, R.; Cortes, M.; Lambiase, A.; Bonini, S. Systematic review and meta-analysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br. J. Ophthalmol. 2007, 91, 1656–1661. [Google Scholar] [CrossRef]
- Secchi, A.; Ciprandi, G.; Leonardi, A.; Deschenes, J.; Abelson, M.B.; The Emadine Study Group. Safety and Efficacy Comparison of Emedastine 0.05% Ophthalmic Solution Compared to Levocabastine 0.05% Ophthalmic Suspension in Pediatric Subjects with Allergic Conjunctivitis. Acta Ophthalmol. Scand. 2000, 78, 42–47. [Google Scholar] [CrossRef]
- Verin, P.; Easty, D.; Secchi, A.; Ciprandi, G.; Partouche, P.; Nemeth-Wasmer, G.; Brancato, R.; Harrisberg, C.; Estivin-Ebrardt, C.; Coster, D.; et al. Clinical evaluation of twice-daily emedastine 0.05% eye drops (emadine eye drops) versus levocabastine 0.05% eye drops in patients with allergic conjunctivitis. Am. J. Ophthalmol. 2001, 131, 691–698. [Google Scholar] [CrossRef]
- Yanni, J.M.; Weimer, L.K.; Sharif, N.A.; Xu, S.X.; Gamache, D.A.; Spellman, J.M. Inhibition of Histamine-Induced Human Conjunctival Epithelial Cell Responses by Ocular Allergy Drugs. Arch. Ophthalmol. 1999, 117, 643–647. [Google Scholar] [CrossRef]
- Guidera, A.C.; Luchs, J.I.; Udell, I.J. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology 2001, 108, 936–944. [Google Scholar] [CrossRef]
- Costa, A.; Gomes, J.Á.P.; Marculino, L.G.C.; Liendo, V.L.; Barreiro, T.P.; Dos Santos, M.S. Supratarsal injection of triamcinolone for severe vernal keratoconjunctivitis in children. Arq. Bras. Oftalmol. 2017, 80, 186–188. [Google Scholar] [CrossRef]
- Holsclaw, D.S.; Whitcher, J.P.; Wong, I.G.; Margolis, T.P. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am. J. Ophthalmol. 1996, 121, 243–249. [Google Scholar] [CrossRef]
- Singh, S.; Pal, V.; Dhull, C. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J. Ophthalmol. 2001, 49, 241–245. [Google Scholar]
- Zaouali, S.; Kahloun, R.; Attia, S.; Jelliti, B.; Trigui, M.; Ben Yahia, S.; Messaoud, R.; Khairallah, M. Supratarsal injection of triamcinolone acetonide and childhood allergic keratoconjunctivitis. Int. Ophthalmol. 2012, 32, 99–106. [Google Scholar] [CrossRef]
- Elliott, J.F.; Lin, Y.; Mizel, S.B.; Bleackley, R.C.; Harnish, D.G.; Paetkau, V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science 1984, 226, 1439–1441. [Google Scholar] [CrossRef]
- Whitcup, S.M.; Chan, C.C.; Luyo, D.A.; Bo, P.; Li, Q. Topical cyclosporine inhibits mast cell-mediated conjunctivitis. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2686–2693. [Google Scholar]
- Pucci, N.; Caputo, R.; Mori, F.; De Libero, C.; Di Grande, L.; Massai, C.; Bernardini, R.; Novembre, E. Long-Term Safety and Efficacy of Topical Cyclosporine in 156 Children with Vernal Keratoconjunctivitis. Int. J. Immunopathol. Pharmacol. 2010, 23, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Benezra, D.; Pe’Er, J.; Brodsky, M.; Cohen, E. Cyclosporine Eyedrops for the Treatment of Severe Vernal Keratoconjunctivitis. Am. J. Ophthalmol. 1986, 101, 278–282. [Google Scholar] [CrossRef]
- Lambiase, A.; Leonardi, A.; Sacchetti, M.; Deligianni, V.; Sposato, S.; Bonini, S. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J. Allergy Clin. Immunol. 2011, 128, 896–897.e9. [Google Scholar] [CrossRef] [PubMed]
- Secchi, A.G.; Tognon, M.S.; Leonardi, A. Topical Use of Cyclosporine in the Treatment of Vernal Keratoconjunctivitis. Am. J. Ophthalmol. 1990, 110, 641–645. [Google Scholar] [CrossRef]
- Oray, M.; Toker, E. Tear cytokine levels in vernal keratoconjunctivitis: The effect of topical 0.05% cyclosporine a therapy. Cornea 2013, 32, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Yücel, O.E.; Ulus, N.D. Efficacy and safety of topical cyclosporine A 0.05% in vernal keratoconjunctivitis. Singap. Med. J. 2016, 57, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Gokhale, N.S.; Samant, R.; Sharma, V. Oral cyclosporine therapy for refractory severe vernal keratoconjunctivitis. Indian J. Ophthalmol. 2012, 60, 220–223. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Zavareh, M.K.; Farzbod, F.; Mahbod, M.; Behrouz, M.J. Topical 0.005% tacrolimus eye drop for refractory vernal keratoconjunctivitis. Eye 2011, 25, 872–880. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ebihara, N.; Fujishima, H.; Fukushima, A.; Kumagai, N.; Nakagawa, Y.; Namba, K.; Okamoto, S.; Shoji, J.; Takamura, E.; et al. A Randomized, Placebo-Controlled Clinical Trial of Tacrolimus Ophthalmic Suspension 0.1% in Severe Allergic Conjunctivitis. J. Ocul. Pharmacol. Ther. 2010, 26, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Shoughy, S.S.; Jaroudi, M.O.; Tabbara, K.F. Efficacy and safety of low-dose topical tacrolimus in vernal keratoconjunctivitis. Clin. Ophthalmol. 2016, 10, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Vichyanond, P.; Kosrirukvongs, P. Use of Cyclosporine A and Tacrolimus in Treatment of Vernal Keratoconjunctivitis. Curr. Allergy Asthma Rep. 2013, 13, 308–314. [Google Scholar] [CrossRef]
- Vichyanond, P.; Tantimongkolsuk, C.; Dumrongkigchaiporn, P.; Jirapongsananuruk, O.; Visitsunthorn, N.; Kosrirukvongs, P. Vernal keratoconjunctivitisResult of a novel therapy with 0.1% topical ophthalmic FK-506 ointment. J. Allergy Clin. Immunol. 2004, 113, 355–358. [Google Scholar] [CrossRef]
- Labcharoenwongs, P.; Jirapongsananuruk, O.; Visitsunthorn, N.; Kosrirukvongs, P.; Saengin, P.; Vichyanond, P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac. J. Allergy Immunol. 2012, 30, 177–184. [Google Scholar]
- Sánchez-Hernández, M.C.; Montero, J.; Rondon, C.; Del Castillo, J.M.B.; Velázquez, E.; Herreras, J.M.; Fernández-Parra, B.; Merayo-Lloves, J.; Del Cuvillo, A.; Vega, F.; et al. Consensus document on allergic conjunctivitis (DECA). J. Investig. Allergol. Clin. Immunol. 2015, 25, 94–106. [Google Scholar]
- Cox, L.; Nelson, H.; Lockey, R.; Calabria, C.; Chacko, T.; Finegold, I.; Nelson, M.; Weber, R.; Bernstein, D.I.; Blessing-Moore, J.; et al. Allergen immunotherapy: A practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, S1–S55. [Google Scholar] [CrossRef]
- Leonardi, A.; Silva, D.; Formigo, D.P.; Bozkurt, B.; Sharma, V.; Allegri, P.; Rondon, C.; Calder, V.; Ryan, D.; Kowalski, M.L.; et al. Management of ocular allergy. Allergy 2019, 74, 1611–1630. [Google Scholar] [CrossRef] [Green Version]
- Roberts, G.; Pfaar, O.; Akdis, C.A.; Ansotegui, I.J.; Durham, S.R.; Van Wijk, R.G.; Halken, S.; Linnemann, D.L.; Pawankar, R.; Pitsios, C.; et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy 2017, 73, 765–798. [Google Scholar] [CrossRef] [Green Version]
- Derriman, L.; Nguyen, D.Q.; Ramanan, A.V.; Dick, A.D.; Tole, D.M. Intravenous immunoglobulin (IVIg) in the management of severe refractory vernal keratoconjunctivitis. Br. J. Ophthalmol. 2010, 94, 667–669. [Google Scholar] [CrossRef]
- Tworek, D.; Bochenska-Marciniak, M.; Kuprys-Lipinska, I.; Kupczyk, M.; Kuna, P. Perennial is More Effective than Preseasonal Subcutaneous Immunotherapy in the Treatment of Seasonal Allergic Rhinoconjunctivitis. Am. J. Rhinol. Allergy 2013, 27, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Calderon, M.A.; Penagos, M.; Sheikh, A.; Canonica, G.W.; Durham, S.R. Sublingual immunotherapy for allergic conjunctivitis: Cochrane systematic review and meta-analysis. Clin. Exp. Allergy 2011, 41, 1263–1272. [Google Scholar] [CrossRef]
- Eifan, A.O.; Shamji, M.H.; Durham, S.R. Long-term clinical and immunological effects of allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 586–593. [Google Scholar] [CrossRef]
- Broide, D.H. Immunomodulation of Allergic Disease. Annu. Rev. Med. 2009, 60, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.Y.; Erekosima, N.; Suarez-Cuervo, C.; Ramanathan, M.; Kim, J.M.; Ward, D.; Chelladurai, Y.; Segal, J.B. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013. [Google Scholar]
- Brunn, G.J.; Hudson, C.C.; Sekulić, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C.; Abraham, R.T. Phosphorylation of the Translational Repressor PHAS-I by the Mammalian Target of Rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef]
- Bryk, B.; Hahn, K.; Cohen, S.M.; Teleman, A.A. MAP4K3 regulates body size and metabolism in Drosophila. Dev. Biol. 2010, 344, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budanov, A.V.; Karin, M. p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Lee, J.H.; Lee, H.J.; Chang, S.Y.; Chung, S.-H. Rapamycin attenuates Th2-driven experimental allergic conjunctivitis. Clin. Immunol. 2018, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, Y.; Ma, L.; Wang, X.; Chi, H.; Zhang, S.; Liu, T.; Li, Z.; Xiang, D.; Dong, Y.; et al. Rapamycin Nano-Micelle Ophthalmic Solution Reduces Corneal Allograft Rejection by Potentiating Myeloid-Derived Suppressor Cells’ Function. Front. Immunol. 2018, 9, 2283. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.J.; Puy, R.; Weiner, J.M. Injection allergen immunotherapy for asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001186. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.J.; Puy, R.M.; Weiner, J.M. Is allergen immunotherapy effective in asthma? A meta-analysis of randomized controlled trials. Am. J. Respir. Crit. Care Med. 1995, 151, 969–974. [Google Scholar] [CrossRef]
- Walker, S.; Durham, S.R.; Till, S.; Roberts, G.; Corrigan, C.; Leech, S.C.; Krishna, M.T.; Rajakulasingham, R.K.; Williams, A.; Chantrell, J.; et al. Immunotherapy for allergic rhinitis. Clin. Exp. Allergy 2011, 41, 1177–1200. [Google Scholar] [CrossRef]
- Ross, R.N.; Nelson, H.S.; Finegold, I. Effectiveness of specific immunotherapy in the treatment of asthma: A meta-analysis of prospective, randomized, double-blind, placebo-controlled studies. Clin. Ther. 2000, 22, 329–341. [Google Scholar] [CrossRef]
- Frew, A.J. Allergen immunotherapy. J. Allergy Clin. Immunol. 2010, 125, S306–S313. [Google Scholar] [CrossRef]
- Moote, W.; Kim, H.; Ellis, A.K. Allergen-specific immunotherapy. Allergy Asthma Clin. Immunol. 2018, 14, 53. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Yao, J.; Li, B. Expression of TSLP and Downstream Molecules IL-4, IL-5, and IL-13 on the Eye Surface of Patients with Various Types of Allergic Conjunctivitis. J. Ophthalmol. 2016, 2016, 5072781. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Huang, G.; Hu, B.; Song, Y.; Shi, Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin. Exp. Immunol. 2011, 164, 256–264. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast Cells Enhance T Cell Activation: Importance of Mast Cell Costimulatory Molecules and Secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef] [Green Version]
- Seshasayee, D.; Lee, W.P.; Zhou, M.; Shu, J.; Suto, E.; Zhang, J.; Diehl, L.; Austin, C.D.; Meng, Y.G.; Tan, M.; et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Investig. 2007, 117, 3868–3878. [Google Scholar] [CrossRef] [Green Version]
- Reinach, P.S.; Mergler, S.; Okada, Y.; Saika, S. Ocular transient receptor potential channel function in health and disease. BMC Ophthalmol. 2015, 15, 153. [Google Scholar] [CrossRef] [Green Version]
- Shim, W.-S.; Tak, M.-H.; Lee, M.-H.; Kim, M.; Koo, J.-Y.; Lee, C.-H.; Oh, U. TRPV1 Mediates Histamine-Induced Itching via the Activation of Phospholipase A2 and 12-Lipoxygenase. J. Neurosci. 2007, 27, 2331–2337. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Lee, H.S.; Joo, C.-K. TRPV1 Antagonist Suppresses Allergic Conjunctivitis in a Murine Model. Ocul. Immunol. Inflamm. 2016, 26, 440–448. [Google Scholar] [CrossRef]
- Samivel, R.; Kim, D.W.; Son, H.R.; Rhee, Y.-H.; Kim, E.H.; Kim, J.H.; Bae, J.-S.; Chung, Y.-J.; Chung, P.-S.; Raz, E.; et al. The role of TRPV1 in the CD4+ T cell-mediated inflammatory response of allergic rhinitis. Oncotarget 2016, 7, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Kim, Y.S.; Olson, W.P.; Li, F.; Guo, C.; Luo, W.; Huang, A.J.; Liu, Q. A histamine-independent itch pathway is required for allergic ocular itch. J. Allergy Clin. Immunol. 2016, 137, 1267–1270.e6. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.-H.; Chang, S.Y.; Lee, H.J.; Choi, S.H. The C-C chemokine receptor 6 (CCR6) is crucial for Th2-driven allergic conjunctivitis. Clin. Immunol. 2015, 161, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, S.; Lee, H.S.; Khandelwal, P.; Saban, D.R. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am. J. Pathol. 2012, 180, 2351–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquet, J.; DeVillier, P.; Anto, J.M.; Bewick, M.; Haahtela, T.; Arnavielhe, S.; Bedbrook, A.; Murray, R.; Van Eerd, M.; Fonseca, J.A.; et al. Daily allergic multimorbidity in rhinitis using mobile technology: A novel concept of the MASK study. Allergy 2018, 73, 1622–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario, N.; Bielory, L. Epidemiology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 471–476. [Google Scholar] [CrossRef]
- Maio, S.; Baldacci, S.; Bresciani, M.; Simoni, M.; Latorre, M.; Murgia, N.; Spinozzi, F.; Braschi, M.; Antonicelli, L.; Brunetto, B.; et al. RItA: The Italian severe/uncontrolled asthma registry. Allergy 2017, 73, 683–695. [Google Scholar] [CrossRef]
- Lemmetyinen, R.E.; Karjalainen, J.V.; But, A.; Renkonen, R.L.; Pekkanen, J.R.; Toppila-Salmi, S.K.; Haukka, J. Higher mortality of adults with asthma: A 15-year follow-up of a population-based cohort. Allergy 2018, 73, 1479–1488. [Google Scholar] [CrossRef]
- Vehof, J.; Kozareva, D.; Hysi, P.G.; Hammond, C. Prevalence and risk factors of dry eye disease in a British female cohort. Br. J. Ophthalmol. 2014, 98, 1712–1717. [Google Scholar] [CrossRef]
- Kim, M.; Oh, J.-H.; Park, C.Y.; Lee, S.W. Dry Eye Disease and Allergic Conditions: A Korean Nationwide Population-Based Study. Am. J. Rhinol. Allergy 2016, 30, 397–401. [Google Scholar] [CrossRef]
- Cingi, C.; Gevaert, P.; Mösges, R.; Rondon, C.; Hox, V.; Rudenko, M.; Muluk, N.B.; Scadding, G.; Manole, F.; Hupin, C.; et al. Multi-morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology Task Force Report. Clin. Transl. Allergy 2017, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Michailopoulos, P.; Almaliotis, D.; Georgiadou, I.; Papakosta, D.; Gougoulias, K.; Giouleka, P.; Gioulekas, D.; Siempis, T.; Karampatakis, V. Allergic Conjunctivitis in Patients with Respiratory Allergic Symptoms; a Retrospective Study in Greece. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2017, 6, 3–9. [Google Scholar]
- Wang, X.; Shi, X.-D.; Li, L.-F.; Zhou, P.; Shen, Y.-W.; Song, Q.-K. Prevalence and clinical features of adult atopic dermatitis in tertiary hospitals of China. Medicine 2017, 96, e6317. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Akinlade, B.; Guttman-Yassky, E.; De Bruin-Weller, M.; Simpson, E.; Blauvelt, A.; Cork, M.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.; Ebihara, N.; Kishimoto, T.; Fukushima, A. Amelioration of conjunctival giant papillae by dupilumab in patients with atopic keratoconjunctivitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1152–1155. [Google Scholar] [CrossRef]
- Utine, C.A.; Li, G.; Asbell, P.; Pflugfelder, S.; Akpek, E. Ocular surface disease associated with dupilumab treatment for atopic diseases. Ocul. Surf. 2021, 19, 151–156. [Google Scholar] [CrossRef]
- Vingopoulos, F.; Lazzaro, D.R. Dupilumab-Associated Blepharoconjunctivitis with Giant Papillae. Int. Med. Case Rep. J. 2020, 13, 303–305. [Google Scholar] [CrossRef]
- Kimura, A.; Takeda, A.; Ikebukuro, T.; Hori, J. Serum IgE reduction and paradoxical eosinophilia associated with allergic conjunctivitis after dupilumab therapy. J. Ophthalmic Inflamm. Infect. 2021, 11, 1–4. [Google Scholar] [CrossRef]
- Bansal, A.; Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Blauvelt, A.; de Bruin-Weller, M.; Corren, J.; Sher, L.; Guttman-Yassky, E.; Chen, Z.; et al. Conjunctivitis in Dupilumab Clinical Trials for Adolescents with Atopic Dermatitis or Asthma. Am. J. Clin. Dermatol. 2021, 22, 101–115. [Google Scholar] [CrossRef]
- Kopp, M.V.; Hamelmann, E.; Zielen, S.; Kamin, W.; Bergmann, K.-C.; Sieder, C.; Stenglein, S.; Seyfried, S.; Wahn, U.; The DUAL Study Group. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin. Exp. Allergy 2009, 39, 271–279. [Google Scholar] [CrossRef]
- Doan, S.; Amat, F.; Gabison, E.; Saf, S.; Cochereau, I.; Just, J. Omalizumab in Severe Refractory Vernal Keratoconjunctivitis in Children: Case Series and Review of the Literature. Ophthalmol. Ther. 2017, 6, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.A.; Marcotte, G.V.; MacGlashan, D.; Togias, A.; Saini, S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J. Allergy Clin. Immunol. 2004, 114, 527–530. [Google Scholar] [CrossRef]
- Taillé, C.; Doan, S.; Neukirch, C.; Aubier, M. Omalizumab for severe atopic keratoconjunctivitis. BMJ Case Rep. 2010, 2010. [Google Scholar] [CrossRef]
- Heffler, E.; Picardi, G.; Liuzzo, M.T.; Pistorio, M.P.; Crimi, N. Omalizumab Treatment of Vernal Keratoconjunctivitis. JAMA Ophthalmol. 2016, 134, 461–463. [Google Scholar] [CrossRef]
- De Klerk, T.A.; Biswas, S.; Sharma, V.; Arkwright, P.D. Severe vernal keratoconjunctivitis successfully treated with subcutaneous omalizumab. J. AAPOS 2013, 17, 305–306. [Google Scholar] [CrossRef]
- Simpson, R.S.; Lee, J.K. Omalizumab as single-dose therapy for vernal keratoconjunctivitis. Ann. Allergy Asthma Immunol. 2019, 122, 119–120. [Google Scholar] [CrossRef]
- Leonardi, A.; Motterle, L.; Bortolotti, M. Allergy and the eye. Clin. Exp. Immunol. 2008, 153, 17–21. [Google Scholar] [CrossRef]
- Ono, S.J. Vernal keratoconjunctivitis: Evidence for immunoglobulin E-dependent and immunoglobulin E-independent eosinophilia. Clin. Exp. Allergy 2003, 33, 279–281. [Google Scholar] [CrossRef]
- Tanaka, M.; Dogru, M.; Takano, Y.; Miyake-Kashima, M.; Asano-Kato, N.; Fukagawa, K.; Tsubota, K.; Fujishima, H. The Relation of Conjunctival and Corneal Findings in Severe Ocular Allergies. Cornea 2004, 23, 464–467. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Hogan, S.P. The Eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Pouliquen, I.J.; Kornmann, O.; Barton, S.V.; Price, J.A.; Ortega, H.G. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int. J. Clin. Pharmacol. Ther. 2015, 53, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.L.; Villanueva, J.M.; Buckmeier, B.K.; Yamada, Y.; Filipovich, A.H.; Assa’Ad, A.H.; Rothenberg, M.E. Anti–IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J. Allergy Clin. Immunol. 2008, 121, 1473–1483.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markham, A. Reslizumab: First Global Approval. Drugs 2016, 76, 907–911. [Google Scholar] [CrossRef]
- Galo, A.P.; Labor, M.; Tiotiu, A.; Baiardini, I.; Scichilone, N.; Braido, F. Impact of reslizumab on outcomes of severe asthmatic patients: Current perspectives. Patient Relat. Outcome Meas. 2018, 9, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Klion, A.D.; Law, M.A.; Noel, P.; Kim, Y.-J.; Haverty, T.P.; Nutman, T.B. Safety and efficacy of the monoclonal anti–interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood 2004, 103, 2939–2941. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Katial, R.; Gossage, D.; Sari, S.; Wang, B.; Kolbeck, R.; Coyle, A.J.; Koike, M.; Spitalny, G.L.; Kiener, P.A.; et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti–IL-5 receptor α antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 2010, 125, 1237–1244.e2. [Google Scholar] [CrossRef]
- Nowak, R.M.; Parker, J.M.; Silverman, R.A.; Rowe, B.H.; Smithline, H.; Khan, F.; Fiening, J.P.; Kim, K.; Molfino, N.A. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am. J. Emerg. Med. 2015, 33, 14–20. [Google Scholar] [CrossRef]

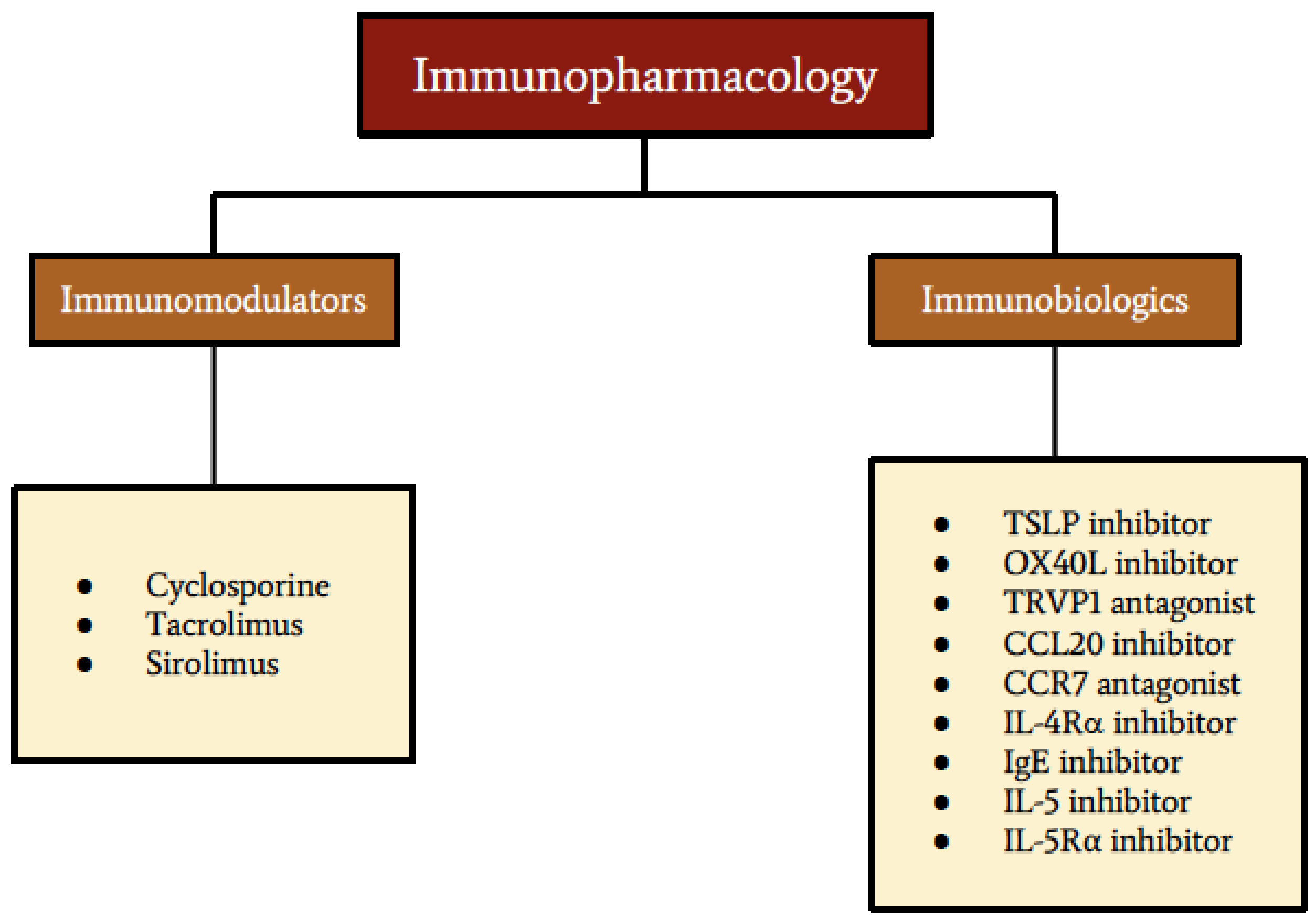

| Immunomodulator | Mechanism | Studied Ocular Therapeutic Effects |
|---|---|---|
| Cyclosporine | Calcineurin inhibitor | Decreases levels of IL-4, IL-5, IL-17A, TNF-alpha, interferon gamma, and eotaxin in the tear film, as well as reduces density of inflammatory cells within the conjunctiva, thereby effectively treating VKC [79,92,139]. |
| Tacrolimus | Calcineurin inhibitor | Effective in refractory cases of VKC [140,141,142,147]. |
| Sirolimus (rapamycin) | Inhibits mTORC1 | Blocks IL-2 and inflammatory effects on the ocular surface, but it is not yet established in VKC treatment [158,161]. |
| Immunobiologic Target | Mechanism of Therapy |
|---|---|
| TSLP inhibitor | Inhibition of TSLP expression by conjunctival epithelial cells in patients with VKC can downregulate the expression of costimulatory molecules (CD40, CD80, and CD86) on DC. This attenuates the Th2-mediated immune response in VKC [169,170]. |
| OX40 inhibitor | Inhibition of OX40L expression by myeloid DC and mast cells can reduce the infiltration of eosinophils and Th2 cells into the conjunctiva. This attenuates the Th2-mediated inflammation in VKC [171,172]. |
| TRVP1 antagonist | TRPV1 antagonist has an inhibitory effect on TCR signaling pathways and activation of CD4+T cells [176]. Blocking the expression of TRVP1 by conjunctival epithelial cells attenuates histamine-dependent pruritus in allergic conjunctivitis [177]. |
| CCL20 inhibitor | Blocking CCR6-CCL20 interaction via inhibition of CCL20 expression can ameliorate Th2-driven allergic inflammation in the conjunctiva of patients with VKC [73]. |
| CCR7 antagonist | CCR7 antagonists inhibit the migration of mature DC to the lymph node, resulting in amelioration of Th2-driven immunopathological mechanisms [179]. |
| IL-4Rα inhibitor | Dupilumab is a monoclonal antibody against interleukin-4 receptor alpha, which can inhibit the signaling of IL-4 and IL-13 [189,190]. |
| IgE inhibitor | Omalizumab is a monoclonal antibody that reduces the availability of free IgE and induces the downregulation of FcεRI expression on mast cells [197,198,199]. |
| IL-5 inhibitor | Mepolizumab is an IgG1-type monoclonal antibody that binds to soluble IL-5, blocking IL5 from binding to IL-5Rα expressed on eosinophils. This results in reduced activation and recruitment of eosinophils [208,209,210]. |
| IL-5 inhibitor | Reslizumab is an IgG4-type monoclonal antibody against IL-5, which results in a reduced level of eosinophils [210,211,212,213]. |
| IL-5Rα inhibitor | Benralizumab is an IgG1-type monoclonal antibody against IL-5Rα on eosinophils that inhibits IL-5 from binding to its receptors on eosinophils, resulting in decreased activation and recruitment of eosinophils [214,215]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chigbu, D.I.; Labib, B.A. Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives. Pharmaceuticals 2021, 14, 658. https://doi.org/10.3390/ph14070658
Chigbu DI, Labib BA. Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives. Pharmaceuticals. 2021; 14(7):658. https://doi.org/10.3390/ph14070658
Chicago/Turabian StyleChigbu, DeGaulle I., and Bisant A. Labib. 2021. "Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives" Pharmaceuticals 14, no. 7: 658. https://doi.org/10.3390/ph14070658
APA StyleChigbu, D. I., & Labib, B. A. (2021). Immunopharmacology in Vernal Keratoconjunctivitis: Current and Future Perspectives. Pharmaceuticals, 14(7), 658. https://doi.org/10.3390/ph14070658





