Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. Sex Differences in the Susceptibility of Animals to the Seizure-Inducer PTZ and to the Delayed Consequences of a Seizure
2.2. Sex-Dependent Effects of Combined Administration of Vitamins B1 and B6 on the Severity of a Seizure and Its Delayed Consequences
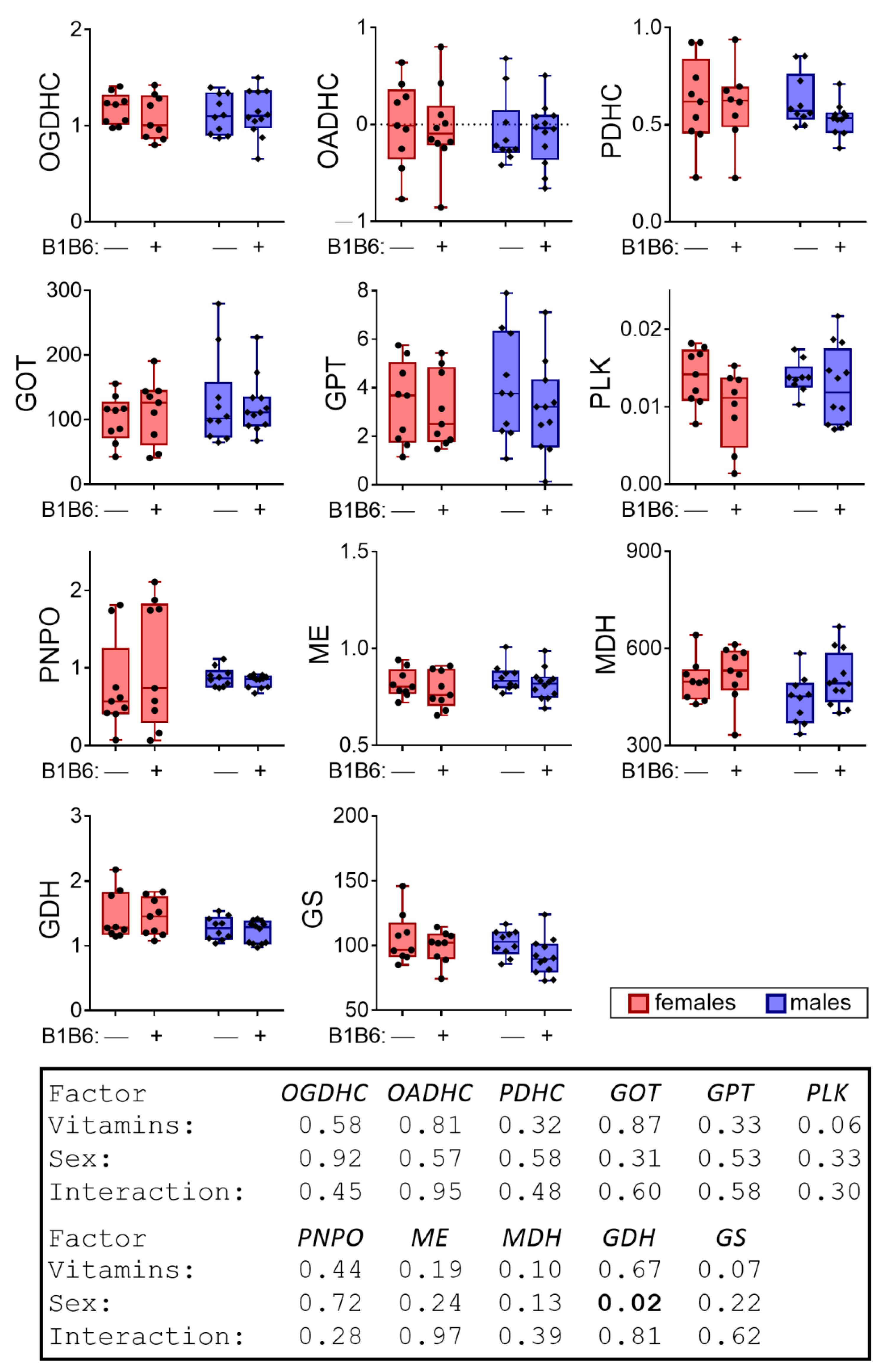
2.3. Effects of the Administration of Vitamins B1 and B6 to the Animals Not Exposed to PTZ
2.4. Interval between Administration of Vitamins B1/B6 and PTZ Influences the PTZ-Induced Seizure and Its Consequences in Females
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pentylenetetrazole Model of Seizures
4.3. Vitamin Administration
4.4. Animal Survival
4.5. Assessment of Physiological Parameters
- locomotor activity—the number of segments passed;
- rearing acts—the number of stands on the hind limbs;
- central activity—the number of the intersections of the small and central circles plus the number of movements from the walls intersecting the larger circle. In animals that did not move from the center (freezing), this indicator was equal to 0;
- total freezing time—time when the active behavior was lacking in all the sectors;
- grooming time;
- the number of acts of grooming;
- the number of defecation acts.
4.6. Preparation of Homogenates of the Rat Cerebral Cortex
4.7. Preparation of Tissue Extracts and Quantifications of Metabolites
4.8. Measurement of Enzymatic Activities
4.9. Statistical Analysis and Data Presentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTZ | pentylenetetrazole |
| OGDHC | 2-oxoglutarate dehydrogenase complex |
| OADHC | 2-oxoadipate dehydrogenase complex |
| PDHC | pyruvate dehydrogenase complex |
| GDH | glutamate dehydrogenase |
| MDH | malate dehydrogenase |
| PLK | pyridoxal kinase |
| PNPO | pyridoxal 5′-phosphate oxidase; |
| GS | glutamine synthetase |
| ME | malic enzyme |
| GOT | glutamate-oxaloacetate transaminase |
| GPT | glutamate-pyruvate transaminase |
| GABA | gamma-aminobutyric acid |
References
- Barile, A.; Nogués, I.; Di Salvo, M.L.; Bunik, V.; Contestabile, R.; Tramonti, A. Molecular characterization of pyridoxine 5′-phosphate oxidase and its pathogenic forms associated with neonatal epileptic encephalopathy. Sci. Rep. 2020, 10, 13621. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Mastrangelo, M.; Nogués, I.; Tolve, M.; Paiardini, A.; Carducci, C.; Mei, D.; Montomoli, M.; Tramonti, A.; Guerrini, R.; et al. Pyridoxine-5′-phosphate oxidase (Pnpo) deficiency: Clinical and biochemical alterations associated with the C.347g > A (P.·Arg116gln) mutation. Mol. Genet. Metab. 2017, 122, 135–142. [Google Scholar] [CrossRef]
- Plecko, B.; Zweier, M.; Begemann, A.; Mathis, D.; Schmitt, B.; Striano, P.; Baethmann, M.; Vari, M.S.; Beccaria, F.; Zara, F.; et al. Confirmation of mutations inPROSCas a novel cause of vitamin B6-dependent epilepsy. J. Med Genet. 2017, 54, 809–814. [Google Scholar] [CrossRef]
- Bugiardini, E.; Pope, S.; Feichtinger, R.G.; Poole, O.V.; Pittman, A.M.; Woodward, C.E.; Heales, S.; Quinlivan, R.; Houlden, H.; Mayr, J.A.; et al. Utility of Whole Blood Thiamine Pyrophosphate Evaluation in TPK1-Related Diseases. J. Clin. Med. 2019, 8, 991. [Google Scholar] [CrossRef] [Green Version]
- Tabarki, B. Thiamine Transporter Deficiency and Epilepsy. In Inherited Metabolic Epilepsies; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Treiman, D.M. GABAergic Mechanisms in Epilepsy. Epilepsia 2001, 42, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Baik, E.J. Glutamate Dehydrogenase as a Neuroprotective Target Against Neurodegeneration. Neurochem. Res. 2018, 44, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Tu, N.; Lee, T.-S.W.; Lai, J.C. Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem. Int. 2013, 63, 670–681. [Google Scholar] [CrossRef] [Green Version]
- Storici, P.; Capitani, G.; De Biase, D.; Moser, M.; John, R.A.; Jansonius, J.N.; Schirmer, T. Crystal Structure of GABA-Aminotransferase, a Target for Antiepileptic Drug Therapy. Biochemistry 1999, 38, 8628–8634. [Google Scholar] [CrossRef] [PubMed]
- Petrov, S.A.; Donesko, E.V. [Effect of thiamine and its metabolites on aspartate and alanine aminotransferase activity in the body of white rats and in donor blood]. Fiziologicheskii Zhurnal 1989, 35, 94–96. [Google Scholar]
- Mkrtchyan, G.; Aleshin, V.; Parkhomenko, Y.; Kaehne, T.; Di Salvo, M.L.; Parroni, A.; Contestabile, R.; Vovk, A.; Bettendorff, L.; Bunik, V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci. Rep. 2015, 5, 12583. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Liang, X.-J.; Li, W.-J.; Wu, D.; Liu, M.; Cao, B.-Y.; Chen, J.-J.; Qin, M.; Meng, X.; Gong, C.-X. Clinical and Molecular Spectrum of Glutamate Dehydrogenase Gene Defects in 26 Chinese Congenital Hyperinsulinemia Patients. J. Diabetes Res. 2018, 2018, 2802540. [Google Scholar] [CrossRef] [Green Version]
- Aleshin, V.A.; Mkrtchyan, G.V.; Bunik, V.I. Mechanisms of the non-coenzyme action of thiamin: Protein targets and medical significance. Biochem. Biokhimiia 2019, 84, 1051–1075. [Google Scholar] [CrossRef]
- Chiang, E.-P.; Smith, D.E.; Selhub, J.; Dallal, G.; Wang, Y.-C.; Roubenoff, R. Inflammation causes tissue-specific depletion of vitamin B. Arthritis Res. 2005, 7, R1254–R1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botez, M.; Botez, T.; Ross-Chouinard, A.; Lalonde, R. Thiamine and folate treatment of chronic epileptic patients: A controlled study with the Wechsler IQ scale. Epilepsy Res. 1993, 16, 157–163. [Google Scholar] [CrossRef]
- Vora, B.; Green, E.A.E.; Khuri, N.; Ballgren, F.; Sirota, M.; Giacomini, K.M. Drug-nutrient interactions: Aiscovering prescription drug inhibitors of the thiamine transporter ThTR-2 (SLC19A3). Am. J. Clin. Nutr. 2019, 111, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; MacLusky, N. Sex differences in the neurobiology of epilepsy: A preclinical perspective. Neurobiol. Dis. 2014, 72, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Velisek, L.; Kubová, H.; Pohl, M.; Staňková, L.; Mares, P.; Schickerova, R. Pentylenetetrazol-induced seizures in rats: An ontogenetic study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1992, 346, 588–591. [Google Scholar] [CrossRef]
- Squires, R.F.; Saederup, E.; Crawley, J.N.; Skolnick, P.; Paul, S.M. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984, 35, 1439–1444. [Google Scholar] [CrossRef]
- Huang, R.Q.; Bell-Horner, C.L.; Dibas, M.I.; Covey, D.F.; Drewe, J.A.; Dillon, G.H. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J. Pharmacol. Exp. Ther. 2001, 298, 986–995. [Google Scholar] [PubMed]
- Kalueff, A.V. Mapping convulsants’ binding to the GABA-A receptor chloride ionophore: A proposed model for channel binding sites. Neurochem. Int. 2007, 50, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Gachon, F.; Fonjallaz, P.; Damiola, F.; Gos, P.; Kodama, T.; Zakany, J.; Duboule, D.; Petit, B.; Tafti, M.; Schibler, U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004, 18, 1397–1412. [Google Scholar] [CrossRef] [Green Version]
- Tsepkova, P.M.; Artiukhov, A.; Boyko, A.I.; Aleshin, V.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine induces long-term changes in amino acid profiles and activities of 2-oxoglutarate and 2-oxoadipate dehydrogenases in rat brain. Biochemistry 2017, 82, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.; Tsepkova, P.; Aleshin, V.; Artiukhov, A.; Mkrtchyan, G.; Ksenofontov, A.; Baratova, L.; Ryabov, S.; Graf, A.; Bunik, V. Severe Spinal Cord Injury in Rats Induces Chronic Changes in the Spinal Cord and Cerebral Cortex Metabolism, Adjusted by Thiamine That Improves Locomotor Performance. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Trofimova, L.; Ksenofontov, A.; Baratova, L.; Bunik, V. Hypoxic Adaptation of Mitochondrial Metabolism in Rat Cerebellum Decreases in Pregnancy. Cells 2020, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Aleshin, V.A.; Zhou, X.; Krishnan, S.; Karlsson, A.; Bunik, V.I. Interplay Between Thiamine and p53/p21 Axes Affects Antiproliferative Action of Cisplatin in Lung Adenocarcinoma Cells by Changing Metabolism of 2-Oxoglutarate/Glutamate. Front. Genet. 2021, 12. [Google Scholar] [CrossRef]
- Terian, R.A.; Serdyuk, S.E.; Davtyan, K.V.; Drapkina, O.M. Electrocardiographic Presentation at Ictal and Postictal Periods of Epileptic Seizure. Russ. J. Cardiol. 2018, 92–102. [Google Scholar] [CrossRef]
- Woodruff, B.K.; Britton, J.W.; Tigaran, S.; Cascino, G.D.; Burritt, M.F.; McConnell, J.P.; Ravkilde, J.; Molgaard, H.; Andreasen, F.; Dam, M.; et al. Cardiac troponin levels following monitored epileptic seizures. Neurology 2003, 60, 1690–1692. [Google Scholar] [CrossRef]
- Nei, M.; Sperling, M.R.; Mintzer, S.; Ho, R.T. Long-term cardiac rhythm and repolarization abnormalities in refractory focal and generalized epilepsy. Epilepsia 2012, 53, e137–e140. [Google Scholar] [CrossRef]
- Reddy, D.S.; Thompson, W.; Calderara, G. Molecular mechanisms of sex differences in epilepsy and seizure susceptibility in chemical, genetic and acquired epileptogenesis. Neurosci. Lett. 2021, 750, 135753. [Google Scholar] [CrossRef]
- Lévesque, M.; Biagini, G.; Avoli, M. Neurosteroids and Focal Epileptic Disorders. Int. J. Mol. Sci. 2020, 21, 9391. [Google Scholar] [CrossRef]
- Reddy, D.S. Sex differences in the anticonvulsant activity of neurosteroids. J. Neurosci. Res. 2017, 95, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Trivisano, M.; Lucchi, C.; Rustichelli, C.; Terracciano, A.; Cusmai, R.; Ubertini, G.M.; Giannone, G.; Bertini, E.; Vigevano, F.; Gecz, J.; et al. Reduced steroidogenesis in patients with PCDH19-female limited epilepsy. Epilepsia 2017, 58, e91–e95. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Guillén-Ruiz, G.; Hernández-López, F.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav. Brain Res. 2021, 397, 112952. [Google Scholar] [CrossRef] [PubMed]
- Cordeira, J.; Kolluru, S.S.; Rosenblatt, H.; Kry, J.; Strecker, R.E.; McCarley, R.W. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav. Brain Res. 2018, 339, 124–129. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Vicente-Serna, J.; Rodríguez-Blanco, L.A.; Rovirosa-Hernández, M.D.J.; García-Orduña, F.; Carro-Juárez, M. Montanoa frutescensandMontanoa grandifloraExtracts Reduce Anxiety-Like Behavior during the Metestrus-Diestrus Phase of the Ovarian Cycle in Wistar Rats. BioMed Res. Int. 2014, 2014, 938060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, N.; Cabello, N.; Nicoleau, M.; Chroneos, Z.C.; Silveyra, P. Modulation of the lung inflammatory response to ozone by the estrous cycle. Physiol. Rep. 2019, 7, e14026. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Wang, Y.; Lu, Q.; Lian, Y.-N.; Anto, E.O.; Zhang, Y.; Wang, W. Chronic stress influences nociceptive sensitivity of female rats in an estrous cycle-dependent manner. Stress 2019, 23, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Martin-DeLeon, P.A. Uterosomes Exosomal cargo during the estrus cycle and interaction with sperm. Front. Biosci. 2016, 8, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillan, H.J.; Han, S.Y.; Cheong, I.; Herbison, A.E. GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology 2019, 160, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, E.; Leo, G.; Andreoli, N.; Fuxe, K.; Biagini, G.; Agnati, L.F. Homocysteine Potentiates Seizures and Cell Loss Induced by Pilocarpine Treatment. NeuroMolecular Med. 2010, 12, 248–259. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Wang, A.; Zhao, X. Association of Severity and Prognosis With Elevated Homocysteine Levels in Patients With Intracerebral Hemorrhage. Front. Neurol. 2020, 11, 571585. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Prasad, A.N. Inborn Errors of Metabolism and Epilepsy: Current Understanding, Diagnosis, and Treatment Approaches. Int. J. Mol. Sci. 2017, 18, 1384. [Google Scholar] [CrossRef] [Green Version]
- Badrinath, M.; John, S. Isoniazid Toxicity. In StatPearls; Treasure Island: Pinellas, FL, USA, 2021. [Google Scholar]
- Marcé-Grau, A.; Martí-Sánchez, L.; Baide-Mairena, H.; Ortigoza-Escobar, J.D.; Pérez-Dueñas, B. Genetic defects of thiamine transport and metabolism: A review of clinical phenotypes, genetics and functional studies. J. Inherit. Metab. Dis. 2019, 42, 581–597. [Google Scholar] [CrossRef]
- Ghatge, M.S.; Di Salvo, M.L.; Contestabile, R.; Eseonu, D.N.; Karve, S.; Schirch, V.; Safo, M.K. Molecular Defects of Vitamin B6 Metabolism Associated with Neonatal Epileptic Encephalopathy. In Miscellanea on Encephalopathies—A Second Look; IntechOpen: London, UK, 2012. [Google Scholar]
- Wilson, M.; Plecko, B.; Mills, P.B.; Clayton, P.T. Disorders affecting vitamin B 6 metabolism. J. Inherit. Metab. Dis. 2019, 42, 629–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimouni-Bloch, A.; Goldberg-Stern, H.; Strausberg, R.; Brezner, A.; Heyman, E.; Inbar, D.; Kivity, S.; Zvulunov, A.; Sztarkier, I.; Fogelman, R.; et al. Thiamine Deficiency in Infancy: Long-Term Follow-Up. Pediatr. Neurol. 2014, 51, 311–316. [Google Scholar] [CrossRef]
- Ebadi, M.; Metzler, D.; Christenson, W. Convulsant activity of pyridoxal sulphate and phosphonoethyl pyridoxal: Antagonism by GABA and its synthetic analogues. Neuropharmacology 1983, 22, 865–873. [Google Scholar] [CrossRef]
- Barile, A.; Tramonti, A.; di Salvo, M.L.; Nogués, I.; Nardella, C.; Malatesta, F.; Contestabile, R. Allosteric feedback inhibition of pyridoxine 5′-phosphate oxidase from Escherichia coli. J. Biol. Chem. 2019, 294, 15593–15603. [Google Scholar] [CrossRef]
- Belozertseva, I.; Merkulovs, D.; Vilitis, O.; Skryabin, B. Inctrumental Method for Determining the Estrous Cycle Stages in Small Laboratory Rodents. Lab. Zhivotnye dlya Nauchnych Issled. Lab. Anim. Sci. 2018, 1. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paccola, C.C.; Resende, C.G.; Stumpp, T.; Miraglia, S.M.; Cipriano, I. The rat estrous cycle revisited: A quantitative and qualitative analysis. Anim. Reprod. 2013, 10, 677–683. [Google Scholar]
- La Monaca, E.; Fodale, V. Effects of Anesthetics on Mitochondrial Signaling and Function. Curr. Drug Saf. 2012, 7, 126–139. [Google Scholar] [CrossRef]
- Greene, N.M. Anesthetics and Metabolism. Anesthesiology 1968, 29, 407. [Google Scholar] [CrossRef]
- Brunner, E.A.; Cheng, S.C.; Berman, M.L. Effects of Anesthesia on Intermediary Metabolism. Annu. Rev. Med. 1975, 26, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J. Effect of Anesthetics on Mitochondrial Function. Anesthesiologists 1973, 39, 153–164. [Google Scholar] [CrossRef]
- Kelz, M.B.; Mashour, G.A. The Biology of General Anesthesia from Paramecium to Primate. Curr. Biol. 2019, 29, R1199–R1210. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.P.; Pum, M.E.; Amato, D.; Schüttler, J.; Huston, J.P.; Silva, M.A.D.S. The in vivo neurochemistry of the brain during general anesthesia. J. Neurochem. 2011, 119, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Yu, D.-X.; Yin, B.-S.; Li, X.-R.; Li, L.-N.; Li, Y.-N.; Wang, Y.-X.; Chen, Y.; Liu, W.-H.; Gao, L. Xylazine Regulates the Release of Glycine and Aspartic Acid in Rat Brain. J. Veter.-Res. 2018, 62, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Karmarkar, S.W.; Bottum, K.M.; Tischkau, S.A. Considerations for the Use of Anesthetics in Neurotoxicity Studies. Comp. Med. 2010, 60, 256–262. [Google Scholar]
- Leary, S.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Pierozan, P.; Jernerén, F.; Ransome, Y.; Karlsson, O. The Choice of Euthanasia Method Affects Metabolic Serum Biomarkers. Basic Clin. Pharmacol. Toxicol. 2017, 121, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Aleshin, V.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration. J. Neurochem. 2020, 153, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Lüttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, e56573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zybina, A.; Anshakova, A.; Malinovskaya, J.; Melnikov, P.; Baklaushev, V.; Chekhonin, V.P.; Maksimenko, O.; Titov, S.; Balabanyan, V.; Kreuter, J.; et al. Nanoparticle-based delivery of carbamazepine: A promising approach for the treatment of refractory epilepsy. Int. J. Pharm. 2018, 547, 10–23. [Google Scholar] [CrossRef]
- Mkrtchyan, G.V.; Üçal, M.; Müllebner, A.; Dumitrescu, S.; Kames, M.; Moldzio, R.; Molcanyi, M.; Schaefer, S.; Weidinger, A.; Schaefer, U.; et al. Thiamine preserves mitochondrial function in a rat model of traumatic brain injury, preventing inactivation of the 2-oxoglutarate dehydrogenase complex. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 925–931. [Google Scholar] [CrossRef]
- Mkrtchyan, G.; Graf, A.; Bettendorff, L.; Bunik, V. Cellular thiamine status is coupled to function of mitochondrial 2-oxoglutarate dehydrogenase. Neurochem. Int. 2016, 101, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Pipkin, J.D.; Stella, V.J. Thiamine Whole Blood and Urinary Pharmacokinetics in Rats: Urethan-Induced Dose-Dependent Pharmacokinetics. J. Pharm. Sci. 1982, 71, 169–172. [Google Scholar] [CrossRef]
- Spinneker, A.; Sola, R.; Lemmen, V.; Castillo, M.J.; Pietrzik, K.; Gonzalez-Gross, M. Vitamin B6 status, deficiency and its consequences—An overview. Nutr. Hosp. 2007, 22, 7–24. [Google Scholar]
- Bettendorff, L.; Weekers, L.; Wins, P.; Schoffeniels, E. Injection of sulbutiamine induces an increase in thiamine triphosphate in rat tissues. Biochem. Pharmacol. 1990, 40, 2557–2560. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine Status in Humans and Content of Phosphorylated Thiamine Derivatives in Biopsies and Cultured Cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef] [Green Version]
- Hawk, P.B.; Oser, B.L.; Summerson, W.H. Practical physiological chemistry. By Philip B. Hawk, Bernard L. Oser, and William H. Summerson. The Blakiston Co., Inc. New York, 1954. 13th ed. xvi + 1439 pp. J. Am. Pharm. Assoc. 1955, 44, 62–63. [Google Scholar] [CrossRef]
- Galvin, R.; Bråthen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Vrolijk, M.F.; Hageman, G.J.; van de Koppel, S.; van Hunsel, F.; Bast, A. Inter-individual differences in pharmacokinetics of vitamin B6: A possible explanation of different sensitivity to its neuropathic effects. PharmaNutrition 2020, 12, 100188. [Google Scholar] [CrossRef]
- Schaumburg, H.; Kaplan, J.; Windebank, A.; Vick, N.; Rasmus, S.; Pleasure, D.; Brown, M.J. Sensory Neuropathy from Pyridoxine Abuse. N. Engl. J. Med. 1983, 309, 445–448. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebsch, G.; Montkowski, A.; Holsboer, F.; Landgraf, R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav. Brain Res. 1998, 94, 301–310. [Google Scholar] [CrossRef]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.; Mormède, P. Stress and emotionality: A multidimensional and genetic approach. Neurosci. Biobehav. Rev. 1997, 22, 33–57. [Google Scholar] [CrossRef]
- Estanislau, C.; Veloso, A.W.; Filgueiras, G.B.; Maio, T.P.; Dal-Cól, M.L.; Cunha, D.C.; Klein, R.; Carmona, L.F.; Fernández-Teruel, A. Rat self-grooming and its relationships with anxiety, dearousal and perseveration: Evidence for a self-grooming trait. Physiol. Behav. 2019, 209, 112585. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Morán, S.; Estanislau, C.; Cañete, T.; Blázquez, G.; Ráez, A.; Tobena, A.; Fernández-Teruel, A. Relationships of open-field behaviour with anxiety in the elevated zero-maze test: Focus on freezing and grooming. World J. Neurosci. 2014, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Baevsky, R.M.; Institute of Biomedical Problems of the Russian Academy of Sciences; Chernikova, A.G. Heart rate variability analysis: Physiological foundations and main methods. Cardiometry 2017, 10, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Graf, A.; Trofimova, L.; Loshinskaja, A.; Mkrtchyan, G.; Strokina, A.; Lovat, M.; Tylicky, A.; Strumilo, S.; Bettendorff, L.; Bunik, V.I. Up-regulation of 2-oxoglutarate dehydrogenase as a stress response. Int. J. Biochem. Cell Biol. 2013, 45, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, L.; Ksenofontov, A.; Mkrtchyan, G.; Graf, A.; Baratova, L.; Bunik, V. Quantification of Rat Brain Amino Acids: Analysis of the Data Consistency. Curr. Anal. Chem. 2016, 12, 349–356. [Google Scholar] [CrossRef]
- Schwab, M.A.; Kölker, S.; van den Heuvel, L.P.; Sauer, S.; Wolf, N.; Rating, D.; Hoffmann, G.F.; Smeitink, J.A.; Okun, J.G. Optimized Spectrophotometric Assay for the Completely Activated Pyruvate Dehydrogenase Complex in Fibroblasts. Clin. Chem. 2005, 51, 151–160. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Sussmane, S.; Koontz, J. A Fluorometric Assay for Pyridoxal Kinase Applicable to Crude Cell Extracts. Anal. Biochem. 1995, 225, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Krumschnabel, G.; Fontana-Ayoub, M.; Sumbalova, Z.; Heidler, J.; Gauper, K.; Fasching, M.; Gnaiger, E. Simultaneous High-Resolution Measurement of Mitochondrial Respiration and Hydrogen Peroxide Production. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin, Germany, 2015; Volume 1264, pp. 245–261. [Google Scholar]









| Score | Behavioral Manifestations of Seizures |
|---|---|
| 0 | Normal behavior, no abnormality |
| 1 | Immobilization, lying on belly |
| 2 | Head nodding, facial, forelimb or hindlimb myoclonus |
| 3 | Myoclonic twitches, continuous whole-body myoclonus, tail held up stiffly |
| 4 | Rearing, tonic seizure, falling down on a side |
| 5 | Tonic-clonic seizure, falling down on back, wild rushing and jumping |
| Animal Sex | Group | n | Survival, % |
|---|---|---|---|
| Females | PTZ | 9 | 100 |
| PTZ +B1,B6 (24 h) | 9 | 100 | |
| PTZ +B1,B6 (2 h) | 6 | 100 | |
| PTZ +B1,B6 (30 min) | 5 | 60 | |
| B1,B6 (24 h) | 8 | 100 | |
| Control | 9 | 100 | |
| Males | PTZ | 10 | 100 |
| PTZ +B1,B6 (24 h) | 12 | 100 | |
| B1,B6 (24 h) | 10 | 100 | |
| Control | 9 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleshin, V.A.; Graf, A.V.; Artiukhov, A.V.; Boyko, A.I.; Ksenofontov, A.L.; Maslova, M.V.; Nogués, I.; di Salvo, M.L.; Bunik, V.I. Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharmaceuticals 2021, 14, 737. https://doi.org/10.3390/ph14080737
Aleshin VA, Graf AV, Artiukhov AV, Boyko AI, Ksenofontov AL, Maslova MV, Nogués I, di Salvo ML, Bunik VI. Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharmaceuticals. 2021; 14(8):737. https://doi.org/10.3390/ph14080737
Chicago/Turabian StyleAleshin, Vasily A., Anastasia V. Graf, Artem V. Artiukhov, Alexandra I. Boyko, Alexander L. Ksenofontov, Maria V. Maslova, Isabel Nogués, Martino L. di Salvo, and Victoria I. Bunik. 2021. "Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model" Pharmaceuticals 14, no. 8: 737. https://doi.org/10.3390/ph14080737
APA StyleAleshin, V. A., Graf, A. V., Artiukhov, A. V., Boyko, A. I., Ksenofontov, A. L., Maslova, M. V., Nogués, I., di Salvo, M. L., & Bunik, V. I. (2021). Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharmaceuticals, 14(8), 737. https://doi.org/10.3390/ph14080737









