Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxicity Assays
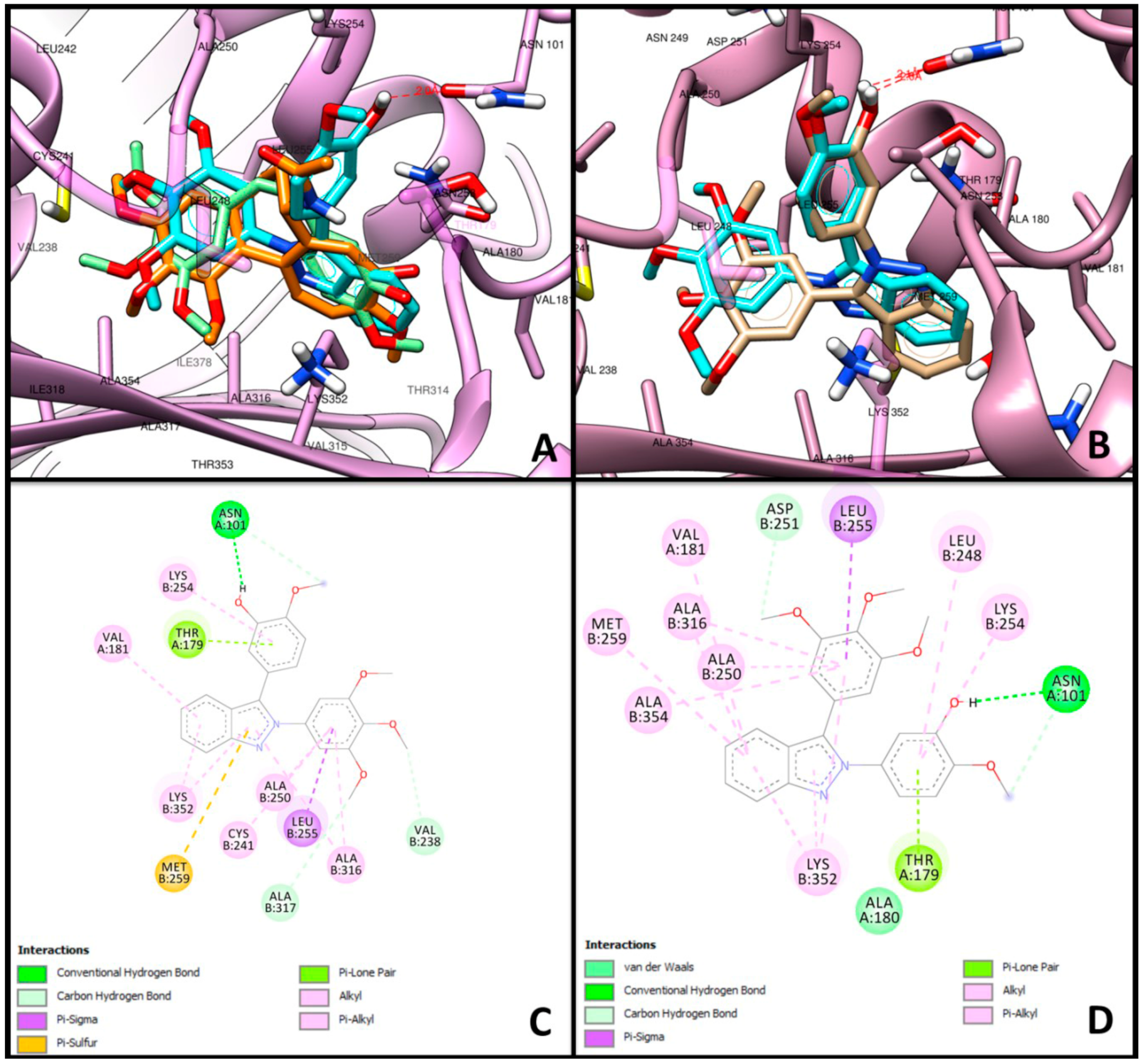
2.3. Molecular Docking
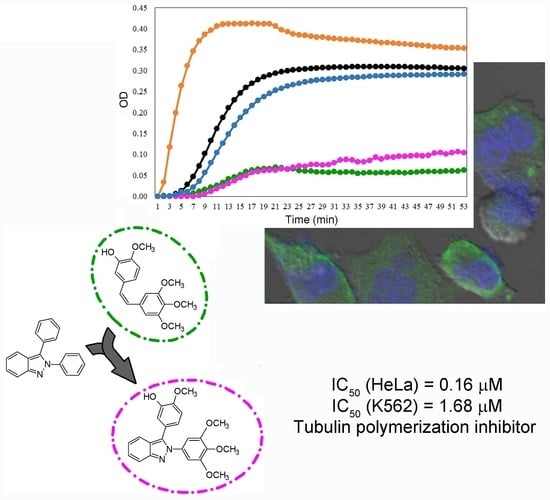
2.4. In Vitro Tubulin Polymerization Assays
2.5. Indirect Immunofluorescence (IFI)
2.6. Effect of Compound 5, PTX, and CA-4 on Soluble and Polymerized Tubulin
3. Materials and Methods
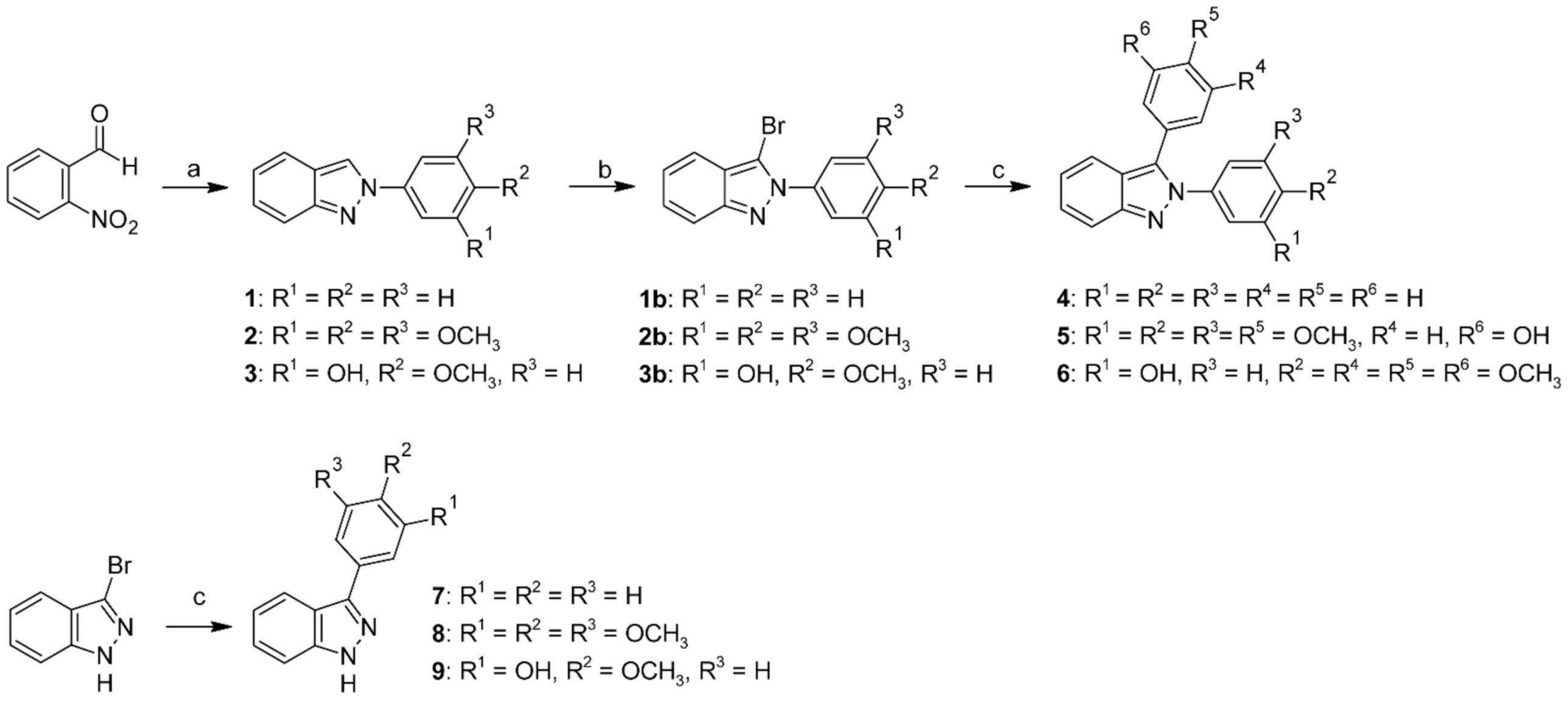
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of 2-Phenyl-2H-indazole Derivatives
2-Phenyl-2H-indazole (1)
2-(3,4,5-Trimethoxyphenyl)-2H-indazole (2)
5-(2H-Indazol-2-yl)-2-methoxyphenol (3)
3.1.2. General Procedure for the Synthesis of 3-Bromo-2-phenyl-2H-indazole Derivatives
3-Bromo-2-phenyl-2H-indazole (1b)
3-Bromo-2-(3,4,5-trimethoxyphenyl)-2H-indazole (2b)
3-Bromo-2-(3,4,5-trimethoxyphenyl)-2H-indazole (3b)
3.1.3. General Procedure for Palladium-Catalyzed Coupling Reactions
2,3-Diphenyl-2H-indazole (4)
2-Methoxy-5-(2-(3,4,5-trimethoxyphenyl)-2H-indazol-3-yl)phenol (5)
2-Methoxy-5-(3-(3,4,5-trimethoxyphenyl)-2H-indazol-2-yl)phenol (6)
3-Phenyl-1H-indazole (7)
3-(3,4,5-Trimethoxyphenyl)-1H-indazole (8)
5-(1H-Indazol-3-yl)-2-methoxyphenol (9)
3.2. Cytotoxicity Assays in Human Cells
3.3. Molecular Docking
3.4. Tubulin Polymerization Assays
3.5. Indirect Immunofluorescence Assays
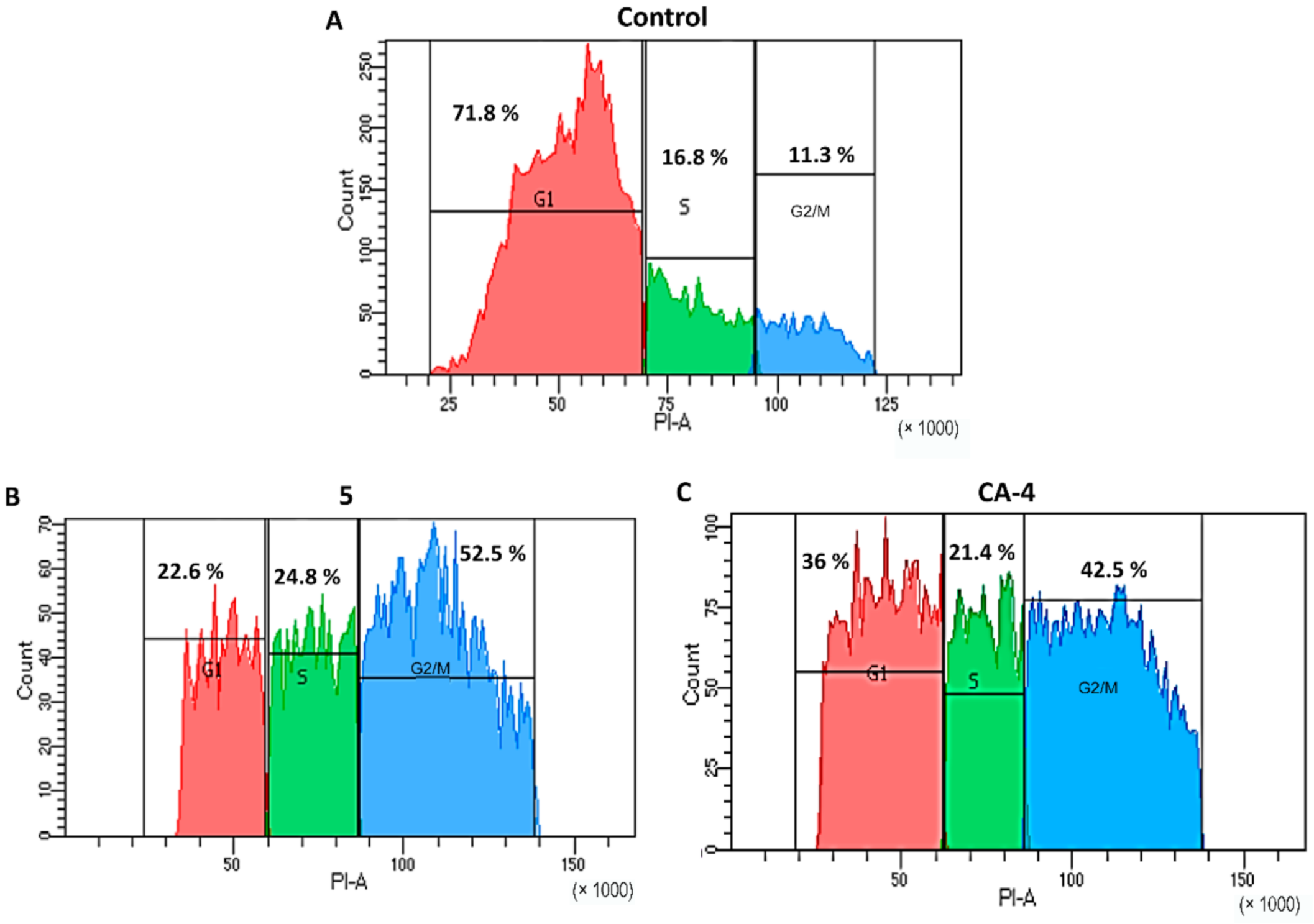
3.6. Cell Cycle Analysis
3.7. Western Blot Analysis of Soluble and Polymerized Tubulin in HeLa cells
3.8. Antioxidant Evaluation
3.8.1. DPPH Assay
3.8.2. ABTS Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. What Is Cancer? Available online: https://training.seer.cancer.gov/disease/cancer/review.html (accessed on 26 March 2021).
- WHO (World Health Organization). Available online: http://www.who.int/cancer/en/ (accessed on 7 November 2020).
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2011, 21, 1509–1523. [Google Scholar] [CrossRef]
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, Q.; Wang, Z.; Huang, G.; Li, S. Recent Advances in the Development of Indazole-based Anticancer Agents. ChemMedChem 2018, 13, 1490–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-G.; Liang, C.-G.; Zhang, W.-H. Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Villanueva, J.; Yépez-Mulia, L.; González-Sánchez, I.; Palacios-Espinosa, J.F.; Soria-Arteche, O.; Sainz-Espuñes, T.D.R.; Cerbón, M.A.; Rodríguez-Villar, K.; Rodríguez-Vicente, A.K.; Cortés-Gines, M.; et al. Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents. Molecules 2017, 22, 1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, A.S.; Genazzani, A. Medicinal Chemistry of Combretastatin A4: Present and Future Directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef]
- Cadogan, J.I.G.; Mackie, R.K. 2-Phenylindazole. Org. Synth. 1968, 48, 113. [Google Scholar]
- Waalwijk, P.S.; Cohen-Fernandes, P.; Habraken, C.L. Indazole studies. 3. The bromination of 2-phenyl-2H-indazole. Formation and structure determination of mono-, di-, and tribromo-2-phenyl-2H-indazoles. J. Org. Chem. 1984, 49, 3401–3403. [Google Scholar] [CrossRef]
- Huff, B.E.; Koenig, T.M.; Mitchell, D.; Staszak, M.A. Synthesis of unsymmetrical biaryls using a modified Suzuki cross-coupling: 4-Biphenylcarboxaldehyde. ([1,1′-Biphenyl]-4-carboxaldehyde). Org. Synth. 2004, 10, 102. [Google Scholar]
- Wehbe, H.; Kearney, C.M.; Pinney, K.G. Combretastatin A-4 resistance in H460 human lung carcinoma demonstrates distinctive alterations in beta-tubulin isotype expression. Anticancer. Res. 2005, 25, 3865–3870. [Google Scholar] [PubMed]
- Duan, Y.-T.; Man, R.-J.; Tang, D.-J.; Yao, Y.-F.; Tao, X.-X.; Yu, C.; Liang, X.-Y.; Makawana, J.A.; Zou, M.-J.; Wang, Z.-C.; et al. Design, Synthesis and Antitumor Activity of Novel link-bridge and B-Ring Modified Combretastatin A-4 (CA-4) Analogues as Potent Antitubulin Agents. Sci. Rep. 2016, 6, 25387. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Sun, L.; Lou, H.; Ji, M. Synthesis and biological evaluation of Combretastatin A-4 derivatives containing a 3’-O-substituted carbonic ether moiety as potential antitumor agents. Chem. Central J. 2013, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Greene, L.M.; Nathwani, S.M.; Bright, S.A.; Fayne, D.; Croke, A.; Gagliardi, M.; McElligott, A.; O’Connor, L.; Carr, M.; Keely, N.O.; et al. The Vascular Targeting Agent Combretastatin-A4 and a Novel cis-Restricted β-Lactam Analogue, CA-432, Induce Apoptosis in Human Chronic Myeloid Leukemia Cells and Ex Vivo Patient Samples Including Those Displaying Multidrug Resistance. J. Pharmacol. Exp. Ther. 2010, 335, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Seddigi, Z.S.; Malik, M.S.; Saraswati, A.P.; Ahmed, S.A.; Babalghith, A.O.; Lamfon, H.A.; Kamal, A. Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents. MedChemComm 2017, 8, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moen, M.D.; McKeage, K.; Plosker, G.L.; Siddiqui, M.A. Imatinib: A review of its use in chronic myeloid leukaemia. Drugs 2007, 67, 299–320. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.; Bento, A.P.S.F.F.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2011, 40, D1100–D1107. [Google Scholar] [CrossRef] [Green Version]
- Bruggisser, R.; von Daeniken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of Plant Extracts, Phytoestrogens and Antioxidants with the MTT Tetrazolium Assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Morris, G.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Gaspari, R.; Prota, A.E.; Bargsten, K.; Cavalli, A.; Steinmetz, M.O. Structural Basis of cis- and trans-Combretastatin Binding to Tubulin. Chem 2017, 2, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Cytoskeleton Inc. Tubulin Polymerization Assay Using >99% Pure Tubulin, od Based—Porcine (BK006P). Manual: Tubulin Polymerization Assay Kit. Available online: https://www.cytoskeleton.com/bk006p (accessed on 26 March 2021).
- Shelanski, M.L.; Gaskin, F.; Cantor, C.R. Microtubule Assembly in the Absence of Added Nucleotides. Proc. Natl. Acad. Sci. USA 1973, 70, 765–768. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Timasheff, S.N. In Vitro Reconstitution of Calf Brain Microtubules: Effects of Solution Variables. Biochemistry 1977, 16, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Brancale, A.; Ricci, A.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. Convergent synthesis and biological evaluation of 2-amino-4-(3′,4′,5′-trimethoxyphenyl)-5-aryl thiazoles as microtubule targeting agents. J. Med. Chem. 2011, 54, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lai, Y.; Zhu, M.; Huang, S.; Feng, W.; Gu, X. Combretastatin A4 Regulates Proliferation, Migration, Invasion, and Apoptosis of Thyroid Cancer Cells via PI3K/Akt Signaling Pathway. Med Sci. Monit. 2016, 22, 4911–4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Zhang, J.; Xue, N.; Hu, Y.; Yang, B.; He, Q. Novel combretastatin A-4 derivative XN0502 induces cell cycle arrest and apoptosis in A549 cells. Investig. N. Drugs 2010, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Kanthou, C.; Greco, O.; Stratford, A.; Cook, I.; Knight, R.; Benzakour, O.; Tozer, G. The Tubulin-Binding Agent Combretastatin A-4-Phosphate Arrests Endothelial Cells in Mitosis and Induces Mitotic Cell Death. Am. J. Pathol. 2004, 165, 1401–1411. [Google Scholar] [CrossRef]
- Zacharaki, P.; Stephanou, G.; Demopoulos, N.A. Comparison of the aneugenic properties of nocodazole, paclitaxel and griseofulvinin vitro. Centrosome defects and alterations in protein expression profiles. J. Appl. Toxicol. 2012, 33, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, X.; Chang, H. Proliferation inhibition and apoptosis of breast cancer MCF-7 cells under the influence of colchicine. J. BUON Off. J. Balk. Union Oncol. 2016, 21, 570–575. [Google Scholar]
- Ibrahim, T.S.; Hawwas, M.M.; Malebari, A.M.; Taher, E.S.; Omar, A.M.; Neamatallah, T.; Abdel-Samii, Z.K.; Safo, M.K.; Elshaier, Y.A.M.M. Discovery of novel quinoline-based analogues of combretastatin A-4 as tubulin polymerisation inhibitors with apoptosis inducing activity and potent anticancer effect. J. Enzym. Inhib. Med. Chem. 2021, 36, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, C.; LaRock, R.C.; Shi, F. Synthesis of 2H-Indazoles by the [3 + 2] Dipolar Cycloaddition of Sydnones with Arynes. J. Org. Chem. 2011, 76, 8840–8851. [Google Scholar] [CrossRef] [Green Version]
- Vidyacharan, S.; Sagar, A.; Chaitra, N.C.; Sharada, D.S. A facile synthesis of 2H-indazoles under neat conditions and further transformation into aza-γ-carboline alkaloid analogues in a tandem one-pot fashion. RSC Adv. 2014, 4, 34232–34236. [Google Scholar] [CrossRef]
- Ohnmacht, S.A.; Culshaw, A.J.; Greaney, M.F. Direct Arylations of 2H-Indazoles On Water. Org. Lett. 2009, 12, 224–226. [Google Scholar] [CrossRef]
- Croce, P.D.; La Rosa, C. A Convenient synthesis of indazoles. Synthesis 1984, 1984, 982–983. [Google Scholar] [CrossRef]
- Jin, T.; Yamamoto, Y. An Efficient, Facile, and General Synthesis of 1H-Indazoles by 1,3-Dipolar Cycloaddition of Arynes with Diazomethane Derivatives. Angew. Chem. Int. Ed. 2007, 46, 3323–3325. [Google Scholar] [CrossRef]
- Quintero, A.; Pelcastre, A.; Solano, J.D. Antitumoral activity of new pyrimidine derivatives of sesquiterpene lactones. J. Pharm. Sci. 1999, 2, 108–112. [Google Scholar]
- Loza-Mejía, M.A.; Olvera-Vázquez, S.; Maldonado-Hernández, K.; Guadarrama-Salgado, T.; González-Sánchez, I.; Rodríguez-Hernández, F.; Solano, J.D.; Rodríguez-Sotres, R.; Lira-Rocha, A. Synthesis, cytotoxic activity, DNA topoisomerase-II inhibition, molecular modeling and structure–activity relationship of 9-anilinothiazolo[5,4-b]quinoline derivatives. Bioorg. Med. Chem. 2009, 17, 3266–3277. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.D.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, J.; Shoichet, B.K. ZINC—A Free Database of Commercially Available Compounds for Virtual Screening. J. Chem. Inf. Model. 2004, 45, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.T.G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision E.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dassault Systèmes BIOVIA Discovery Studio; Version 17.2.0.16349; Dassault Systemes: San Diego, CA, USA, 2016.
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, A.; Shaik, A.B.; Polepalli, S.; Reddy, V.S.; Kumar, G.B.; Gupta, S.; Krishna, K.V.S.R.; Nagabhushana, A.; Mishra, R.K.; Jain, N. Pyrazole–Oxadiazole conjugates: Synthesis, antiproliferative activity and inhibition of tubulin polymerization. Org. Biomol. Chem. 2014, 12, 7993–8007. [Google Scholar] [CrossRef]
- Palacios-Espinosa, J.F.; Arroyo-García, O.; García-Valencia, G.; Linares, E.; Bye, R.; Romero, I. Evidence of the anti-Helicobacter pylori, gastroprotective and anti-inflammatory activities of Cuphea aequipetala infusion. J. Ethnopharmacol. 2014, 151, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
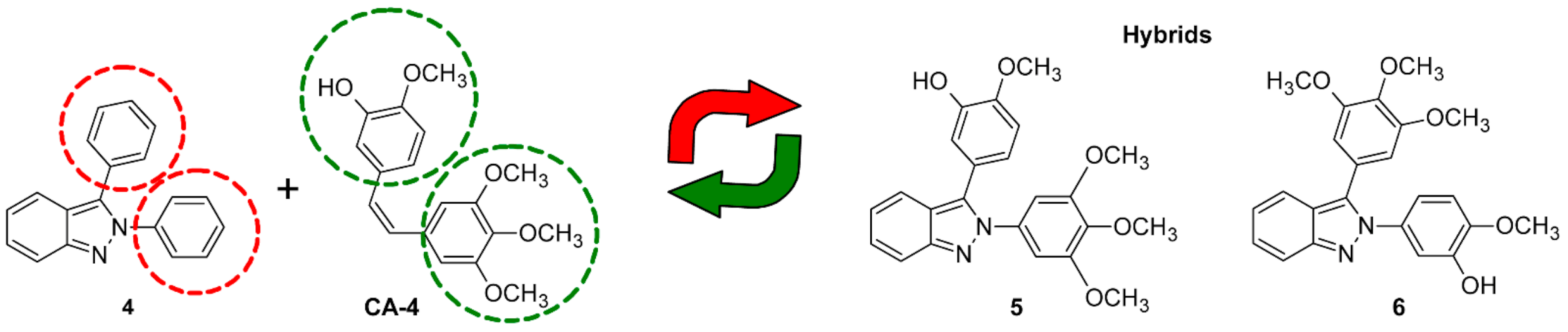



| Compound | HeLa | SK-LU-1 | K562 |
|---|---|---|---|
| 4 | 125.8 ± 14.8 1 | 96.43 ± 8.65 | > 120 |
| 5 | 0.16 ± 0.01 | 6.63 ± 0.52 | 1.68 ± 0.27 |
| 6 | 38.6 ± 7.51 | 25.21 ± 9.06 | 18.59 ± 1.56 |
| Cisplatin | 18.5 ± 3.0 | 12.0 ± 2.5 | - |
| Imatinib | - | - | 2.37 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Villanueva, J.; Matadamas-Martínez, F.; Yépez-Mulia, L.; Pérez-Koldenkova, V.; Leyte-Lugo, M.; Rodríguez-Villar, K.; Cortés-Benítez, F.; Macías-Jiménez, A.P.; González-Sánchez, I.; Romero-Velásquez, A.; et al. Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids. Pharmaceuticals 2021, 14, 815. https://doi.org/10.3390/ph14080815
Pérez-Villanueva J, Matadamas-Martínez F, Yépez-Mulia L, Pérez-Koldenkova V, Leyte-Lugo M, Rodríguez-Villar K, Cortés-Benítez F, Macías-Jiménez AP, González-Sánchez I, Romero-Velásquez A, et al. Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids. Pharmaceuticals. 2021; 14(8):815. https://doi.org/10.3390/ph14080815
Chicago/Turabian StylePérez-Villanueva, Jaime, Félix Matadamas-Martínez, Lilián Yépez-Mulia, Vadim Pérez-Koldenkova, Martha Leyte-Lugo, Karen Rodríguez-Villar, Francisco Cortés-Benítez, Ana Perla Macías-Jiménez, Ignacio González-Sánchez, Ariana Romero-Velásquez, and et al. 2021. "Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids" Pharmaceuticals 14, no. 8: 815. https://doi.org/10.3390/ph14080815
APA StylePérez-Villanueva, J., Matadamas-Martínez, F., Yépez-Mulia, L., Pérez-Koldenkova, V., Leyte-Lugo, M., Rodríguez-Villar, K., Cortés-Benítez, F., Macías-Jiménez, A. P., González-Sánchez, I., Romero-Velásquez, A., Palacios-Espinosa, J. F., & Soria-Arteche, O. (2021). Synthesis and Cytotoxic Activity of Combretastatin A-4 and 2,3-Diphenyl-2H-indazole Hybrids. Pharmaceuticals, 14(8), 815. https://doi.org/10.3390/ph14080815