Labelling via [Al18F]2+ Using Precomplexed Al-NODA Moieties
Abstract
1. Introduction
2. Results
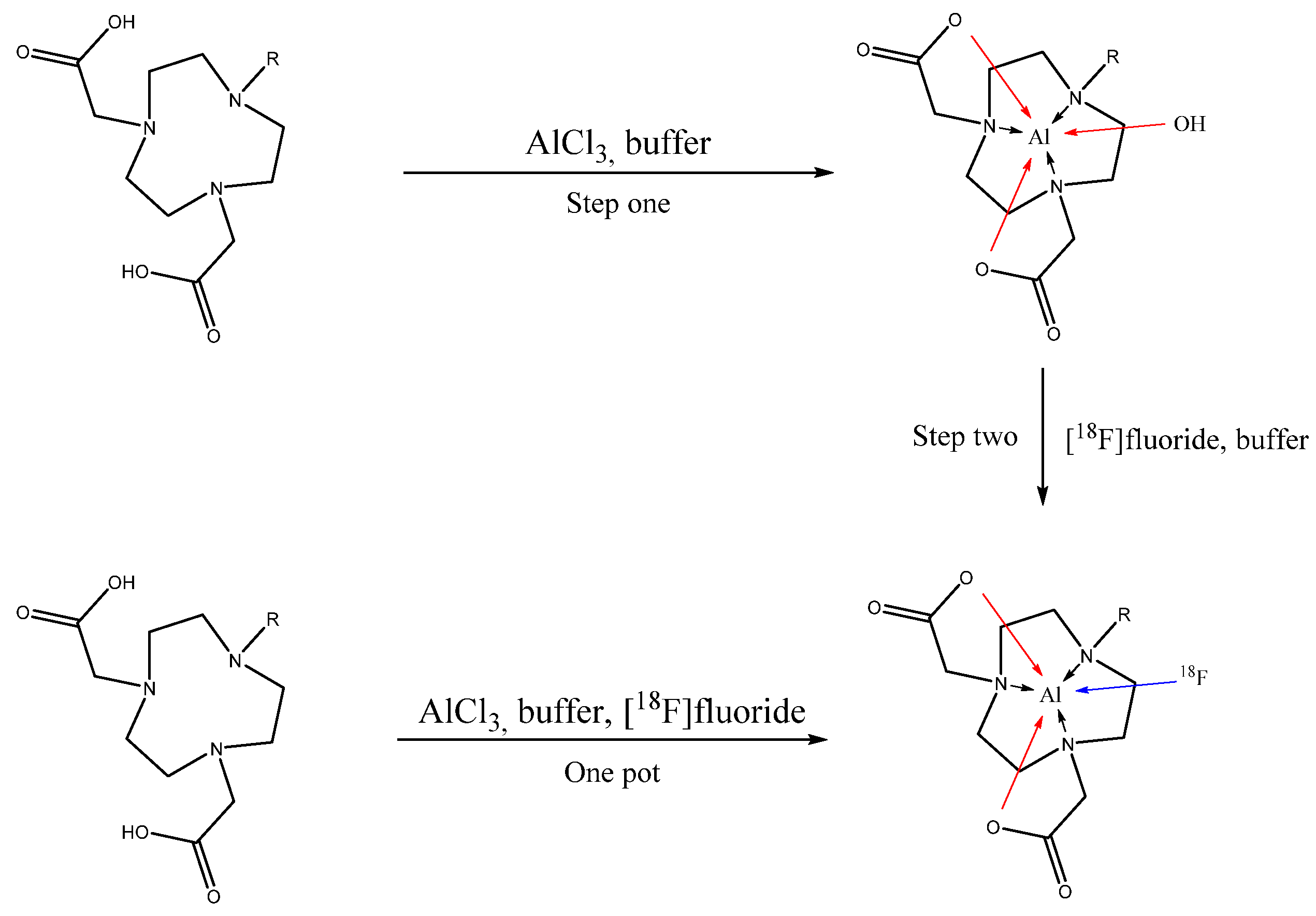
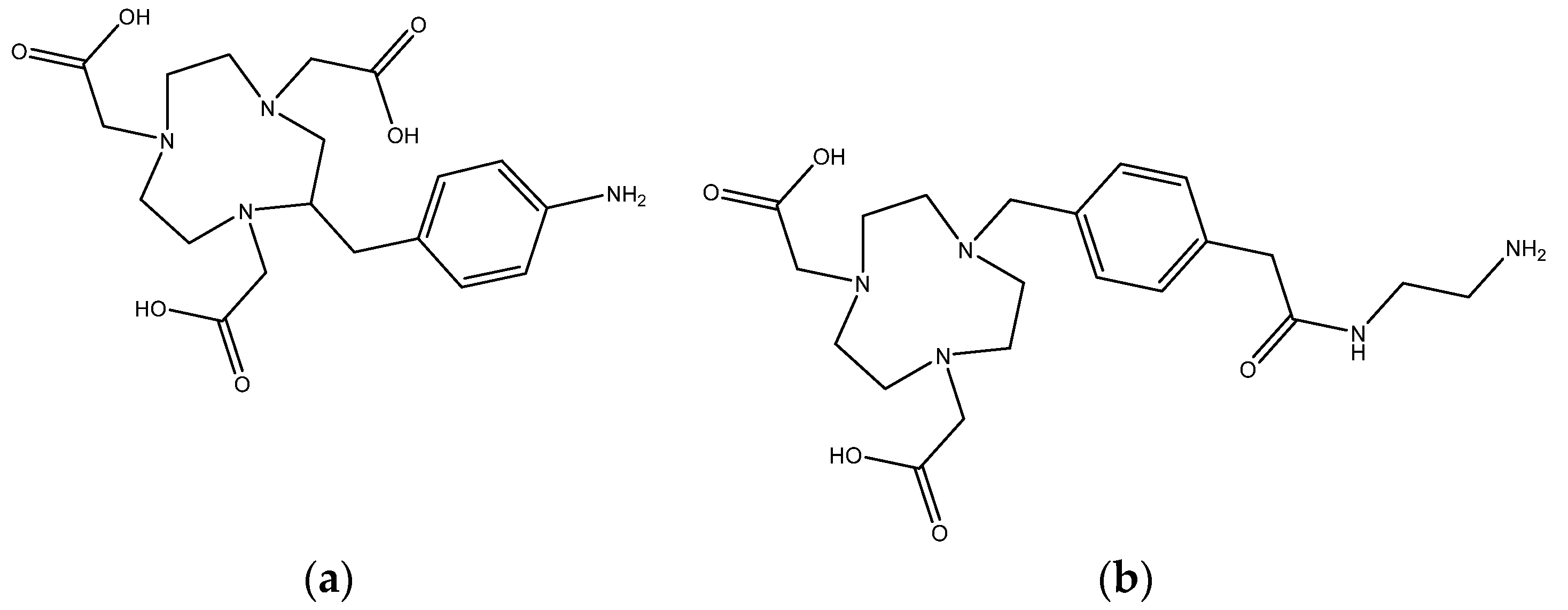
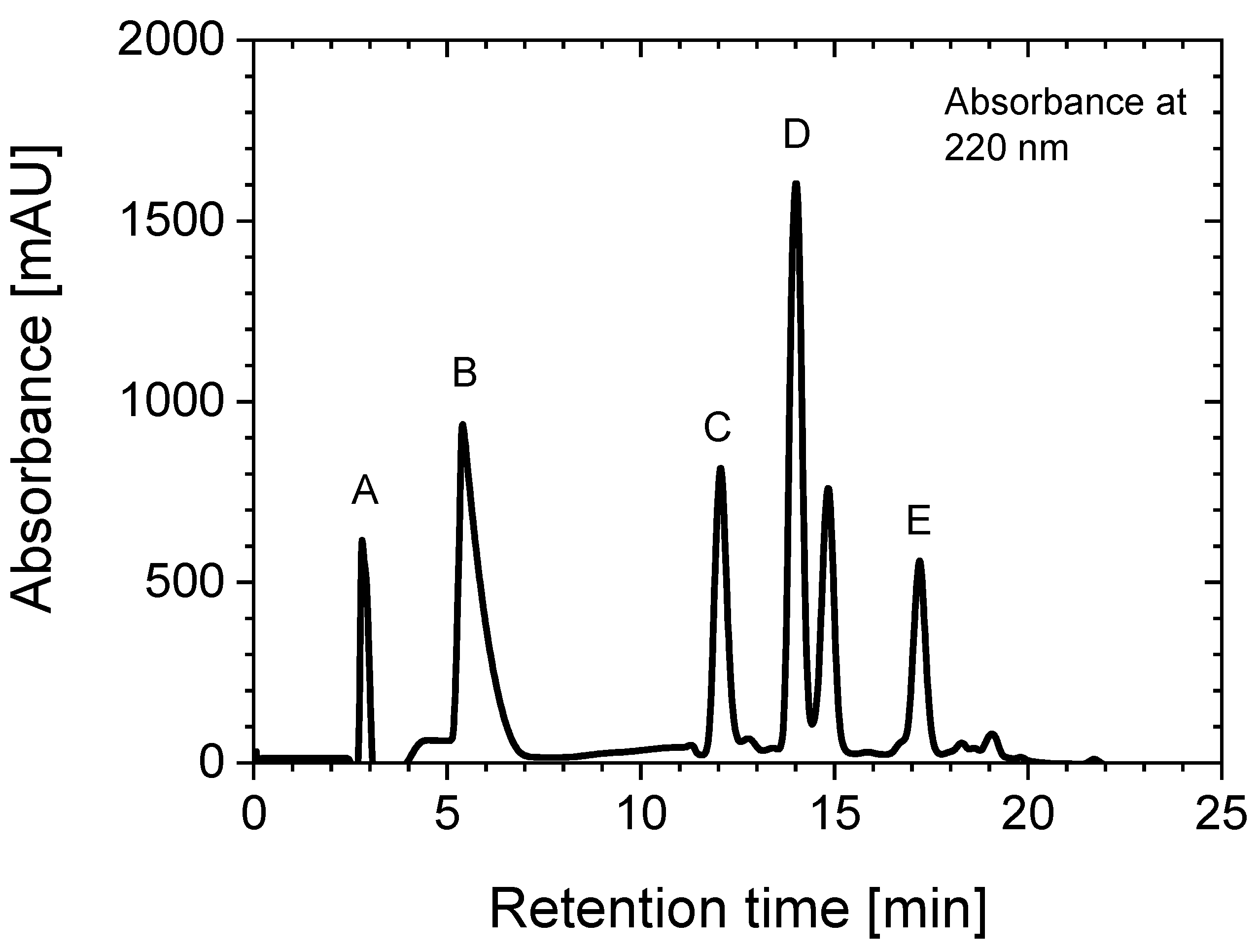
2.1. Aluminum Coordination
2.2. [18F] Fluoride Labelling
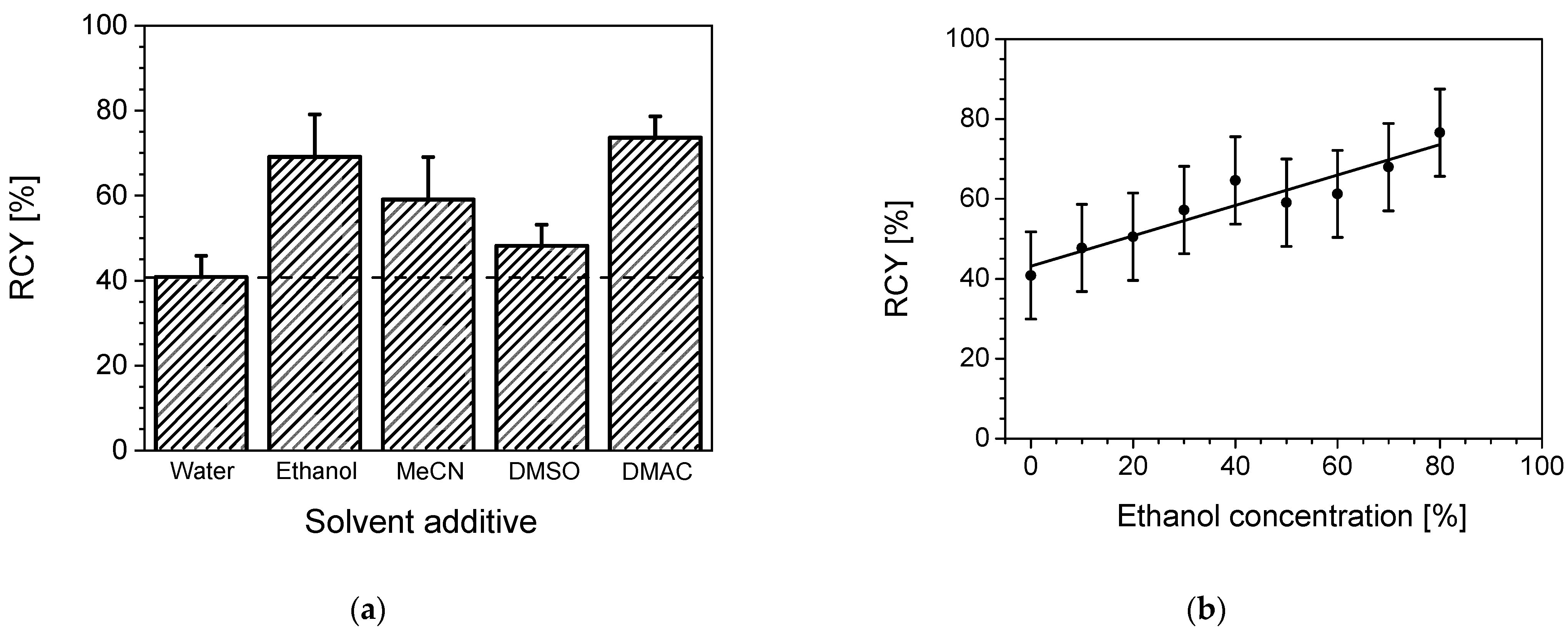
2.2.1. Solvent Dependency
2.2.2. Ethanol Concentration Dependency
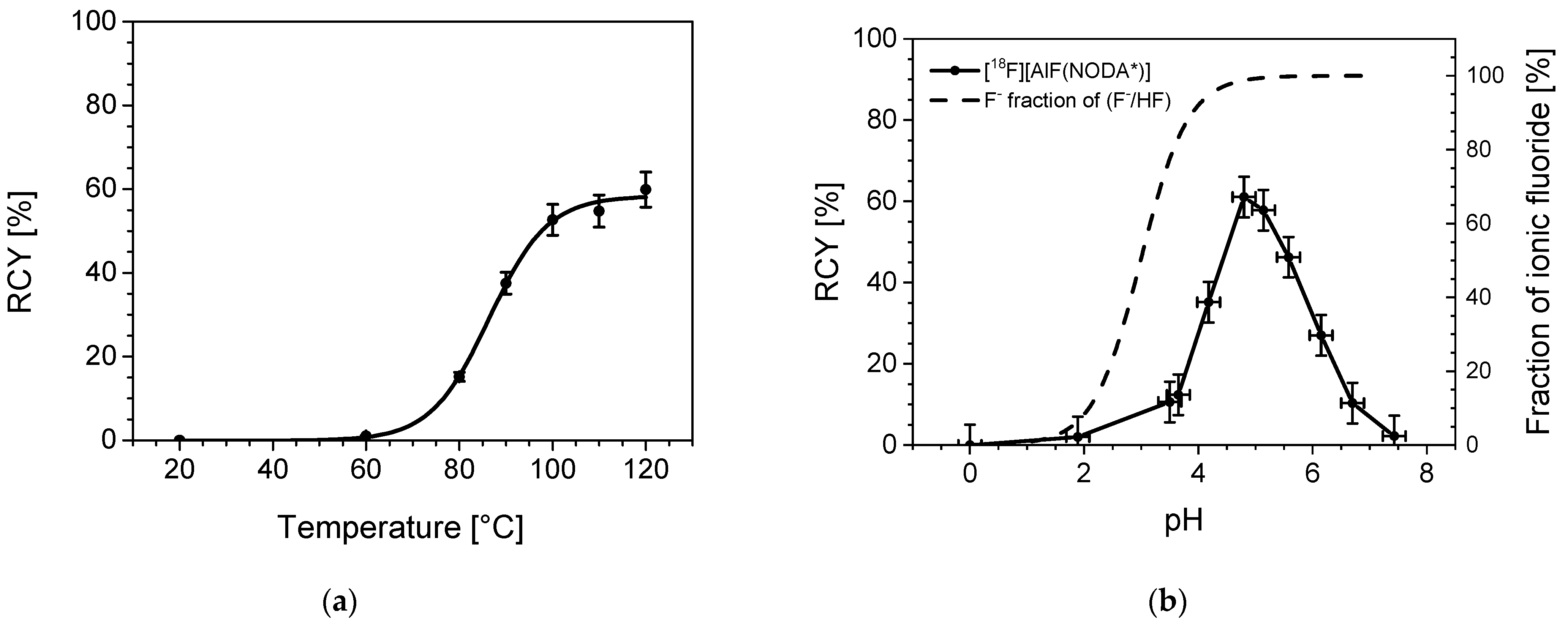
2.2.3. Temperature Dependency
2.2.4. pH Dependency
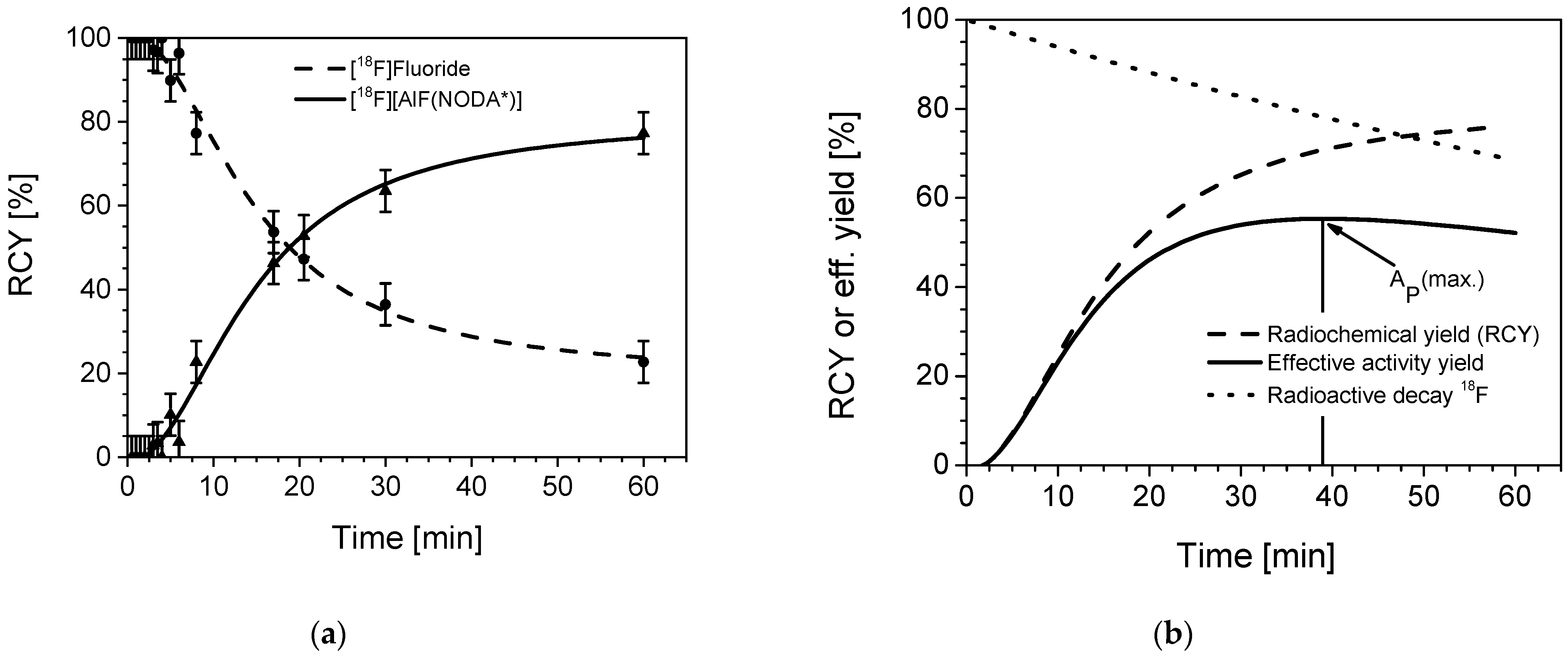
2.2.5. Time Dependency: Reaction Kinetics
3. Materials and Methods
3.1. Snythesis of Aluminum Chelates
3.1.1. Synthesis with the NOTA Chelator
3.1.2. Synthesis with the NODA Chelator
Time Dependency (Kinetics)
Production
3.2. Synthesis of [Al 18F]2+ Chelates
3.2.1. Solvent Dependency
3.2.2. Ethanol Concentration Dependency
3.2.3. Temperature Dependency
3.2.4. pH Dependency
3.2.5. Time Dependency (Kinetics)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, J.A.; Hungnes, I.N.; Ma, M.T.; Rivas, C. Bioconjugates of Chelators with Peptides and Proteins in Nuclear Medicine: Historical Importance, Current Innovations, and Future Challenges. Bioconjug. Chem. 2020, 31, 483–491. [Google Scholar]
- Opalinska, M.; Hubalewska-Dydejczyk, A.; Sowa-Staszczak, A. Radiolabeled peptides: Current and new perspectives. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 153–167. [Google Scholar] [CrossRef]
- Synowiecki, M.A.; Perk, L.R.; Nijsen, J.F.W. Production of novel diagnostic radionuclides in small medical cyclotrons. EJNMMI Radiopharm. Chem. 2018, 3, 3. [Google Scholar] [CrossRef]
- Vahidfar, N.; Eppard, E.; Farzanehfar, S.; Yordanova, A.; Fallahpoor, M.; Ahmadzadehfar, H. An Impressive Approach in Nuclear Medicine: Theranostics. PET Clin. 2021, 16, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef]
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef] [PubMed]
- uz Zaman, M.; Fatima, N.; Sajjad, Z.; Zaman, U.; Tahseen, R.; Zaman, A. 18FDG Synthesis and Supply: A Journey from Existing Centralized to Future Decentralized Models. Asian Pac. J. Cancer Prev. 2014, 15, 10057–10059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rösch, F. 68Ge/68Ga Generators and 68Ga Radiopharmaceutical Chemistry on Their Way into a New Century. J. Postgrad. Med. Edu. Res. 2013, 47, 18–25. [Google Scholar]
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Price, T.W.; Greenmana, J.; Stasiuk, G.J. Current advances in ligand design for inorganic positron emission tomography tracers 68Ga, 64Cu, 89Zr and 44Sc. Dalton Trans. 2016, 45, 15702–15724. [Google Scholar] [CrossRef]
- Uccelli, L.; Boschi, A..; Cittanti, C.; Martini, P.; Lodi, L.; Zappaterra, E..; Romani, S.; Zaccaria, S.; Cecconi, D.; Rambaldi, I.; et al. Automated Synthesis of 68Ga-DOTA-TOC with a Cationic Purification System: Evaluation of Methodological and Technical Aspects in Routine Preparations. Curr. Radiopharm. 2018, 11, 130–137. [Google Scholar] [CrossRef]
- Reverchon, J.; Khayi, F.; Roger, M.; Moreau, A.; Kryza, D. Optimization of the radiosynthesis of [68Ga]Ga-PSMA-11 using a Trasis MiniAiO synthesizer: Do we need to heat and purify? Nucl. Med. Commun. 2020, 41, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Sowa, A.R.; Jackson, I.M.; Desmond, T.J.; Alicea, J.; Mufarreh, A.J.; Pham, J.M.; Stauff, J.; Winton, W.P.; Fawaz, M.V.; Henderson, B.D.; et al. Futureproofing [18F]Fludeoxyglucose manufacture at an Academic Medical Center. EJNMMI Radiopharm. Chem. 2018, 3, 12. [Google Scholar] [CrossRef]
- Cai, L.; Lu, S.; Pike, V.W. Chemistry with [18F]Fluoride Ion. Eur. J. Org. Chem. 2008, 2008, 2853–2873. [Google Scholar] [CrossRef]
- Laverman, P.; McBride, W.J.; Sharkey, R.M.; Eek, A.; Joosten, L.; Oyen, W.J.G.; Goldenberg, D.M.; Boerman, O.C.A. A Novel Facile Method of Labeling Octreotide with 18F-fluorine. J. Nucl. Med. 2010, 51, 454–461. [Google Scholar] [CrossRef]
- D’Souza, C.A.; McBride, W.J.; Sharkey, R.M.; Todaro, L.J.; Goldenberg, D.M. High-Yielding Aqueous 18F-Labeling of Peptides via Al18F Chelation. Bioconjug. Chem. 2011, 22, 1793–1803. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, A. 18F-AlF Labeled Peptide and Protein Conjugates as Positron Emission Tomography Imaging Pharmaceuticals. Bioconjug. Chem. 2018, 29, 953–975. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Bouhlel, A.; Cantelli, C.; Garrigue, P.; Lisowski, V.; Guillet, B. A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling? Molecules 2019, 24, 2866. [Google Scholar] [CrossRef]
- Thisgaard, H.; Kumlin, J.; Langkjær, N.; Chua, J.; Hook, B.; Jensen, M.; Kassaian, A.; Zeisler, S.; Borjian, S.; Cross, M.; et al. Multi-curie production of gallium-68 on a biomedical cyclotron and automated radiolabelling of PSMA-11 and DOTATATE. EJNMMI Radiopharm. Chem. 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, C.M.; Stuparu, A.D.; van Dam, R.M.; Slavik, R. The Search for an Alternative to [68Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of 18F-labeled Somatostatin Analog Development. Theranostics 2019, 9, 1336–1347. [Google Scholar] [CrossRef]
- Olberg, D.E.; Hjelstuen, O.K. Labeling strategies of peptides with ¹⁸F for positron emission tomography. Curr. Top. Med. Chem. 2010, 10, 1669–1679. [Google Scholar] [CrossRef]
- McBride, W.J.; D’Souza, C.A.; Karacay, H.; Sharkey, R.M.; Goldenberg, D.M. New Lyophilized Kit for Rapid Radiofluorination of Peptides. Bioconjug. Chem. 2012, 23, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Cleeren, F.; Tshibangu, T.; Koole, M.; Serdons, K.; Dekerve, J.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. Al18F-NOTA-octreotide: First comparison with 68Ga-DOTATATE in a neuroendocrine tumor patient. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, X.; Shen, T.; Yao, Y.; Chen, M.; Li, Z.; Li, X.; Shen, J.; Kou, Y.; Chen, S.; et al. FAPI-04 PET/CT Using [18F]AlF Labeling Strategy: Automatic Synthesis, Quality Control, and In Vivo Assessment in Patient. Front. Oncol. 2021, 11, 649148. [Google Scholar] [CrossRef] [PubMed]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Lehrbuch der Anorganischen Chemie, 102nd ed.; Walter de Gruyter: Berlin, Germany, 2007; p. 449. [Google Scholar]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Open letter to journal editors on: International Consensus Radiochemistry Nomenclature Guideline. Ann. Nucl. Med. 2018, 32, 236–238. [Google Scholar] [CrossRef]
- Shetty, D.; Choi, S.Y.; Jeong, J.M.; Lee, J.Y.; Hoigebazar, L.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Lee, M.C.; Chung, Y.K. Stable aluminium fluoride chelates with triazacyclononane derivatives proved by X-ray crystallography and 18F-labeling study. Chem. Commun. 2011, 47, 9732–9734. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Simon, U.; Mottaghy, F.M.; Vogg, A.T.J. Labelling via [Al18F]2+ Using Precomplexed Al-NODA Moieties. Pharmaceuticals 2021, 14, 818. https://doi.org/10.3390/ph14080818
Kang D, Simon U, Mottaghy FM, Vogg ATJ. Labelling via [Al18F]2+ Using Precomplexed Al-NODA Moieties. Pharmaceuticals. 2021; 14(8):818. https://doi.org/10.3390/ph14080818
Chicago/Turabian StyleKang, Daniel, Ulrich Simon, Felix M. Mottaghy, and Andreas T. J. Vogg. 2021. "Labelling via [Al18F]2+ Using Precomplexed Al-NODA Moieties" Pharmaceuticals 14, no. 8: 818. https://doi.org/10.3390/ph14080818
APA StyleKang, D., Simon, U., Mottaghy, F. M., & Vogg, A. T. J. (2021). Labelling via [Al18F]2+ Using Precomplexed Al-NODA Moieties. Pharmaceuticals, 14(8), 818. https://doi.org/10.3390/ph14080818





