Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects
Abstract
1. Introduction
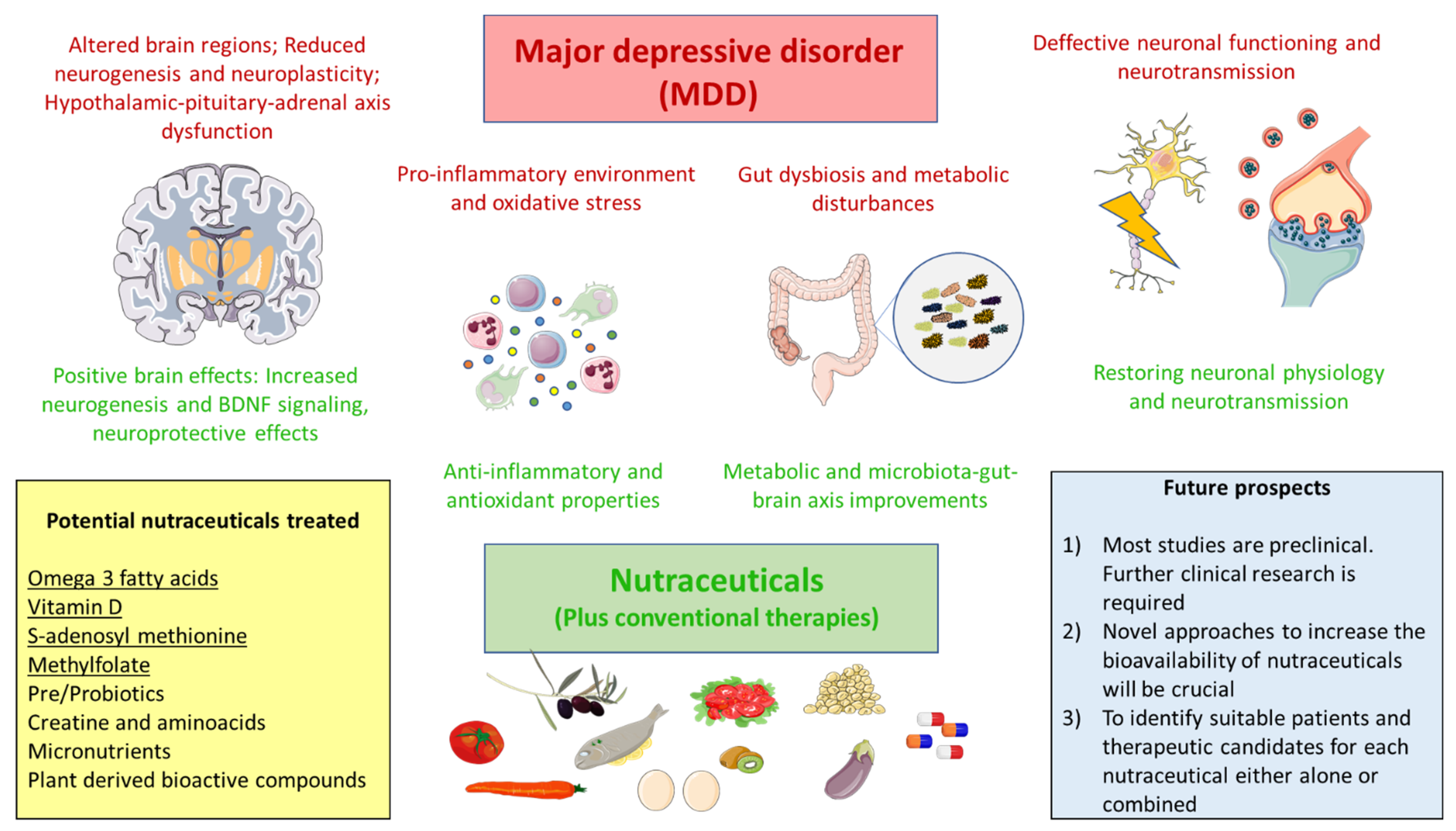
2. Pathophysiology of MDD
3. Nutraceuticals: Definition and Context
4. Nutraceuticals in MDD
4.1. Omega 3 Denosyl-Methionine
4.2. Vitamin D
4.3. S-Adenosyl Methionine
4.4. Methylfolate
4.5. Creatine and Aminoacids
4.6. Prebiotics and Probiotics
4.7. Micronutrients
4.8. Plant Derived Bioactive Compounds
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Somerville, A.J.; Baxter, A.J.; Norman, R.; Patten, S.B.; Vos, T.; Whiteford, H.A. Global Variation in the Prevalence and Incidence of Major Depressive Disorder: A Systematic Review of the Epidemiological Literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Lépine, J.P.; Briley, M. The Increasing Burden of Depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3–7. [Google Scholar] [CrossRef]
- Noble, R.E. Depression in women. Metabolism 2005, 54, 49–52. [Google Scholar] [CrossRef]
- Mckeever, A.; Agius, M.; Mohr, P. A review of the epidemiology of major depressive disorder and of its consequences for society and the individual. Psychiatr. Danub. 2017, 29, 222–231. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- Krause, J.S.; Reed, K.S.; McArdle, J.J. Factor Structure and Predictive Validity of Somatic and Nonsomatic Symptoms from the Patient Health Questionnaire-9: A Longitudinal Study after Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2010, 91, 1218–1224. [Google Scholar] [CrossRef]
- Elhai, J.D.; Contractor, A.A.; Tamburrino, M.; Fine, T.H.; Prescott, M.R.; Shirley, E.; Chan, P.K.; Slembarski, R.; Liberzon, I.; Galea, S.; et al. The Factor Structure of Major Depression Symptoms: A Test of Four Competing Models Using the Patient Health Questionnaire-9. Psychiatry Res. 2012, 199, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.A. Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity Classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Tolentino, J.C.; Schmidt, S.L. DSM-5 Criteria and Depression Severity: Implications for Clinical Practice. Front. Psychiatry 2018, 9, 450. [Google Scholar] [CrossRef]
- Davidson, J.R.T. Major Depressive Disorder Treatment Guidelines in America and Europe. J. Clin. Psychiatry 2010, 71, e04. [Google Scholar] [CrossRef]
- Katon, W.; Unützer, J.; Russo, J. Major Depression: The Importance of Clinical Characteristics and Treatment Response to Prognosis. Depress. Anxiety 2010, 27, 19–26. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Crain, L.A.; Solberg, L.I.; Unützer, J.; Maciosek, M.V.; Whitebird, R.R.; Rossom, R.C. The Effect of Depression Treatment on Work Productivity. Am. J. Manag. Care 2014, 20, e294–e301. [Google Scholar] [PubMed]
- Workplace Mental Health—Quantifying the Cost of Depression. Available online: https://www.workplacementalhealth.org/mental-health-topics/depression/quantifying-the-cost-of-depression (accessed on 22 June 2021).
- König, H.; König, H.H.; Konnopka, A. The Excess Costs of Depression: A Systematic Review and Meta-Analysis. Epidemiol. Psychiatr. Sci. 2019, 29, e30. [Google Scholar] [CrossRef]
- Fostick, L.; Silberman, A.; Beckman, M.; Spivak, B.; Amital, D. The Economic Impact of Depression: Resistance or Severity? Eur. Neuropsychopharmacol. 2010, 20, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, A.; Bicks, L.; Hasler, G. Social Functioning in Major Depressive Disorder. Neurosci. Biobehav. Rev. 2016, 69, 313–332. [Google Scholar] [CrossRef]
- Ishak, W.W.; Mirocha, J.; James, D.; Tobia, G.; Vilhauer, J.; Fakhry, H.; Pi, S.; Hanson, E.; Nashawati, R.; Peselow, E.D.; et al. Quality of Life in Major Depressive Disorder before/after Multiple Steps of Treatment and One-Year Follow-Up. Acta Psychiat. Scand. 2015, 131, 51–60. [Google Scholar] [CrossRef]
- Cuijpers, P.; Quero, S.; Dowrick, C.; Arroll, B. Psychological Treatment of Depression in Primary Care: Recent Developments. Curr. Psychiatry Rep. 2019, 21, 129. [Google Scholar] [CrossRef]
- Knapen, J.; Vancampfort, D.; Moriën, Y.; Marchal, Y. Exercise Therapy Improves Both Mental and Physical Health in Patients with Major Depression. Disabil. Rehabil. 2015, 37, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical Activity as an Imperative Support in Breast Cancer Management. Cancers 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the “SMILES” Trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 8, 2861. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, B.H. Depression as a Disease of Modernity: Explanations for Increasing Prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef]
- Lambert, K.G. Rising Rates of Depression in Today’s Society: Consideration of the Roles of Effort-Based Rewards and Enhanced Resilience in Day-to-Day Functioning. Neurosci. Biobehav. Rev. 2006, 30, 497–510. [Google Scholar] [CrossRef]
- Mullins, N.; Lewis, C.M. Genetics of Depression: Progress at Last. Curr. Psychiatry Rep. 2017, 19, 43. [Google Scholar] [CrossRef]
- Flint, J.; Kendler, K.S. The Genetics of Major Depression. Neuron 2014, 81, 484–503. [Google Scholar] [CrossRef]
- Fabbri, C.; Montgomery, S.; Lewis, C.M.; Serretti, A. Genetics and Major Depressive Disorder: Clinical Implications for Disease Risk, Prognosis and Treatment. Int. Clin. Psychopharmacol. 2020, 35, 233–242. [Google Scholar] [CrossRef]
- Lohoff, F.W. Overview of the genetics of major depressive disorder. Curr. Psychiatry Rep. 2010, 12, 539–546. [Google Scholar] [CrossRef]
- Shadrina, M.; Bondarenko, E.A.; Slominsky, P.A. Genetics Factors in Major Depression Disease. Front. Psychiatry 2018, 9, 334. [Google Scholar] [CrossRef]
- Lopizzo, N.; Chiavetto, L.B.; Cattane, N.; Plazzotta, G.; Tarazi, F.I.; Pariante, C.M.; Riva, M.A.; Cattaneo, A. Gene-Environment Interaction in Major Depression: Focus on Experience-Dependent Biological Systems. Front. Psychiatry 2015, 6, 68. [Google Scholar] [CrossRef]
- Jarett, R.B. Psychosocial Aspects of Depression and the Role of Psychotherapy. J. Clin. Psychiatry 1990, 51 (Suppl. 6), 26–35. [Google Scholar]
- Moosavi, A.; Ardekani, A.M. Role of Epigenetics in Biology and Human Diseases. Iran. Biomed. J. 2016, 20, 246–258. [Google Scholar]
- Lopez, J.P.; Kos, A.; Turecki, G. Major Depression and Its Treatment: MicroRNAs as Peripheral Biomarkers of Diagnosis and Treatment Response. Curr. Opin. Psychiatry 2018, 31, 7–16. [Google Scholar] [CrossRef]
- Hobara, T.; Uchida, S.; Otsuki, K.; Matsubara, T.; Funato, H.; Matsuo, K.; Suetsugi, M.; Watanabe, Y. Altered Gene Expression of Histone Deacetylases in Mood Disorder Patients. J. Psychiatr. Res. 2010, 44, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Penner-Goeke, S.; Binder, E.B. Epigenetics and Depression. Dialogues Clin. Neurosci. 2019, 21, 397–405. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar]
- Fakhoury, M. Revisiting the Serotonin Hypothesis: Implications for Major Depressive Disorders. Mol. Neurobiol. 2016, 53, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Moret, C.; Briley, M. The Importance of Norepinephrine in Depression. Neuropsychiatr. Dis. Treat. 2011, 7, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Rot, M.A.H.; Mathew, S.J.; Charney, D.S. Neurobiological Mechanisms in Major Depressive Disorder. Can. Med. Assoc. J. 2009, 180, 305–313. [Google Scholar]
- Racagni, G.; Popoli, M. Cellular and Molecular Mechanisms in the Long-Term Action of Antidepressants. Dialogues Clin. Neurosci. 2008, 10, 385–400. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Hansen, M.V.; Danielsen, A.K.; Hageman, I.; Rosenberg, J.; Gögenur, I. The Therapeutic or Prophylactic Effect of Exogenous Melatonin against Depression and Depressive Symptoms: A Systematic Review and Meta-Analysis. Eur. Neuropsychopharmacol. 2014, 24, 1719–1728. [Google Scholar] [CrossRef]
- Quera Salva, M.A.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian Rhythms, Melatonin and Depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Pandya, M.; Altinay, M.; Malone, D.A.; Anand, A. Where in the Brain Is Depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Pruessner, J.; Sousa, N.; Almeida, O.F.X.; van Dam, A.M.; Rajkowska, G.; Swaab, D.F.; Czéh, B. Neuropathology of Stress. Acta Neuropathol. 2014, 127, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Jaggar, M.; Fanibunda, S.E.; Ghosh, S.; Duman, R.S.; Vaidya, V.A. Chapter 6—The Neurotrophic Hypothesis of Depression Revisited: New Insights and Therapeutic Implications. In Neurobiology of Depression; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–62. ISBN 9780128133330. [Google Scholar]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Front. Psychiatry 2018, 8, 308. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the Pathophysiology of Depression: From Monoamines to the Neurogenesis Hypothesis Model—Are We There Yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Diaz, D.; Ortega, M.A.; Albillos, A.; Quintero, J.; Aubá, E.; Monserrat, J.; et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients with Major Depressive Disorder. J. Pers. Med. 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating Neuroimmune Systems in the Neurobiology of Depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- González-Díaz, S.N.; Arias-Cruz, A.; Elizondo-Villarreal, B.; Monge-Ortega, O.P. Psychoneuroimmunoendocrinology: Clinical Implications. World Allergy Org. J. 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Perez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls before and after Antidepressant Treatment: Results from a Meta-Analysis. J. Clin. Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative Stress and Major Depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for Depression: Recent Insights, Current Challenges and Future Prospects. Neuropsychiatr. Dis. Treat. 2017, 13, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Cryan, J.F.; O’riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota–Gut–Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef]
- Alvarez-Mon, M.A.; Gomez-Lahoz, A.M.; Orozco, A.; Lahera, G.; Sosa-Reina, M.D.; Diaz, D.; Albillos, A.; Quintero, J.; Molero, P.; Monserrat, J.; et al. Blunted Expansion of Regulatory T Lymphocytes Is Associated with Increased Bacterial Translocation in Patients with Major Depressive Disorder. Front. Psychiatry 2021, 11, 591962. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Gómez, A.M.; Orozco, A.; Lahera, G.; Sosa, M.D.; Diaz, D.; Auba, E.; Albillos, A.; Monserrat, J.; Alvarez-Mon, M. Abnormal Distribution and Function of Circulating Monocytes and Enhanced Bacterial Translocation in Major Depressive Disorder. Front. Psychiatry 2019, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of Nutraceuticals in Human Health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical—Definition and Introduction. AAPS PharmSci 2003, 5, 25. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘Nutraceuticals’: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The Nutraceutical Revolution: Its Impact on Food Industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Utilizing Food Matrix Effects to Enhance Nutraceutical Bioavailability: Increase of Curcumin Bioaccessibility Using Excipient Emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Brower, V.A. Nutraceutical a Day May Keep the Doctor Away. Consumers Are Turning Increasingly to Food Supplements to Improve Well-Being When Pharmaceuticals Fail. EMBO Rep. 2005, 6, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Srivastava, A.; Lall, R. Toxicity Potential of Nutraceuticals. Methods Mol. Biol. 2018, 1800, 367–394. [Google Scholar] [CrossRef]
- Télessy, I.G. Nutraceuticals. In The Role of Functional Food Security in Global Health; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–421. ISBN 9780128131480. [Google Scholar]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Williams, R.J.; Mohanakumar, K.P.; Beart, P.M. Neuro-Nutraceuticals: The Path to Brain Health via Nourishment is not so Distant. Neurochem. Int. 2015, 89, 1–6. [Google Scholar] [CrossRef]
- Kaner, G.; Soylu, M.; Yüksel, N.; Inanç, N.; Ongan, D.; Başmısırlı, E. Evaluation of Nutritional Status of Patients with Depression. BioMed Res. Int. 2015, 2015, 521481. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Burhani, M.D.; Rasenick, M.M. Fish Oil and Depression: The Skinny on Fats. J. Integr. Neurosci. 2017, 16, S115–S124. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Harmsen, I.; Assies, J.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Meta-Analysis and Meta-Regression of Omega-3 Polyunsaturated Fatty Acid Supplementation for Major Depressive Disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Ellis, S.P.; Geant, A.L.; Mann, J.J. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J. Clin. Psychiatry 2011, 72, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Salvati, S.; Natali, F.; Attorri, L.; Raggi, C.; Di Biase, A.; Sanchez, M. Stimulation of Myelin Proteolipid Protein Gene Expression by Eicosapentaenoic Acid in C6 Glioma Cells. Neurochem. Int. 2004, 44, 331–338. [Google Scholar] [CrossRef]
- Salvati, S.; Natali, F.; Attorri, L.; Di Benedetto, R.; Leonardi, F.; Di Biase, A.; Ferri, F.; Fortuna, S.; Lorenzini, P.; Sanchez, M.; et al. Eicosapentaenoic Acid Stimulates the Expression of Myelin Proteins in Rat Brain. J. Neurosci. Res. 2008, 86, 776–784. [Google Scholar] [CrossRef]
- Shail, M.S. Neuropsychiatry in Demyelination Disease: Using Depression as a Prodrome for Early Diagnosis and Treatment of Multiple Sclerosis. Cureus 2017, 9, e1813. [Google Scholar] [CrossRef]
- Williams, M.R.; Sharma, P.; Macdonald, C.; Pearce, R.; Hirsch, S.R.; Maier, M. Axonal Myelin Decrease in the Splenium in Major Depressive Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 387–395. [Google Scholar] [CrossRef]
- Sacchet, M.D.; Gotlib, I.H. Myelination of the Brain in Major Depressive Disorder: An in Vivo Quantitative Magnetic Resonance Imaging Study. Sci. Rep. 2017, 7, 2200. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Lai, W.; Jiang, W.; Liu, X.; Qian, L.; Zhang, Y.; Zhou, Z. Myelin Deficits in Patients with Recurrent Major Depressive Disorder: An Inhomogeneous Magnetization Transfer Study. Neurosci. Lett. 2021, 750, 135768. [Google Scholar] [CrossRef]
- Petursdottir, A.L.; Farr, S.A.; Morley, J.E.; Banks, W.A.; Skuladottir, G.V. Effect of Dietary N-3 Polyunsaturated Fatty Acids on Brain Lipid Fatty Acid Composition, Learning Ability, and Memory of Senescence-Accelerated Mouse. J. Gerontol. Ser. A 2008, 63, 1153–1160. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, Q.; You, Q.-L.; Li, Z.-L.; Hu, N.-Y.; Wang, Y.; Jin, Z.-L.; Li, S.-J.; Li, X.-W.; Yang, J.-M.; et al. Acute EPA-Induced Learning and Memory Impairment in Mice Is Prevented by DHA. Nat. Commun. 2020, 11, 5465. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.Á.; Peñaloza-Sancho, V.; Ahumada, J.; Fuenzalida, M.; Dagnino-Subiabre, A. N-3 Polyunsaturated Fatty Acid Supplementation Restored Impaired Memory and GABAergic Synaptic Efficacy in the Hippocampus of Stressed Rats. Nutr. Neurosci. 2017, 21, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O.; Hersberger, M.; Walitza, S.; Berger, G.E. Disentangling the Molecular Mechanisms of the Antidepressant Activity of Omega-3 Polyunsaturated Fatty Acid: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2021, 22, 4393. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging Class of Omega-3 Fatty Acid Endocannabinoids & their Derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-Inflammatory ω-3 Endocannabinoid Epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, E6034–E6043. [Google Scholar] [CrossRef]
- Chukaew, P.; Leow, A.; Saengsawang, W.; Rasenick, M.M. Potential Depression and Antidepressant-Response Biomarkers in Human Lymphoblast Cell Lines from Treatment-Responsive and Treatment-Resistant Subjects: Roles of SSRIs and Omega-3 Polyunsaturated Fatty Acids. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Bhat, S.A.; Ara, A. Omega-3 Fatty Acids and the Treatment of Depression: A Review of Scientific Evidence. Integr. Med. Res. 2015, 4, 132. [Google Scholar] [CrossRef]
- Baleztena, J.; Ruiz-Canela, M.; Sayon-Orea, C.; Pardo, M.; Añorbe, T.; Gost, J.I.; Gomez, C.; Ilarregui, B.; Bes-Rastrollo, M. Association between Cognitive Function and Supplementation with Omega-3 PUFAs and Other Nutrients in ≥75 Years Old Patients: A Randomized Multicenter Study. PLoS ONE 2018, 13, e0193568. [Google Scholar] [CrossRef]
- Wolters, M.; von der Haar, A.; Baalmann, A.-K.; Wellbrock, M.; Heise, T.L.; Rach, S. Effects of N-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the Skin: Focus on a Complex Relationship: A Review. J. Adv. Res. 2015, 6, 793. [Google Scholar] [CrossRef]
- Cuomo, A.; Giordano, N.; Goracci, A.; Fagiolini, A. Depression and Vitamin D Deficiency: Causality, Assessment, and Clinical Practice Implications. Neuropsychiatry 2017, 7, 606–614. [Google Scholar] [CrossRef]
- Filipovic, B.R.; Filipovic, B.F. Psychiatric Comorbidity in the Treatment of Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 3552. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28. [Google Scholar]
- Du, Y.; Gao, X.R.; Peng, L.; Ge, J.F. Crosstalk between the Microbiota-Gut-Brain Axis and Depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef] [PubMed]
- Lima-Ojeda, J.M.; Rupprecht, R.; Baghai, T.C. Gut Microbiota and Depression: Pathophysiology of Depression: Hypothalamic-Pituitary-Adrenal Axis and Microbiota-Gut-Brain Axis. Der Nervenarzt 2020, 91, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2021, 43, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Vitamin D and Microbiota: Two Sides of the Same Coin in the Immunomodulatory Aspects. Int. Immunopharmacol. 2020, 79. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, H.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and Intestinal Homeostasis: Barrier, Microbiota, and Immune Modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef]
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway. Front. Immunol. 2018, 9, 2484. [Google Scholar] [CrossRef]
- Huiberts, L.M.; Smolders, K.C.H.J. Effects of vitamin D on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep Med. Rev. 2021, 55, 101379. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and Seasonal Mitochondrial Protection: Unraveling Common Possible Mechanisms Involving Vitamin D and Melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.S.; Ahmad, H.S.; Bizhan, H.; Hosein, H.; Mohammad, A. The Effect of Vitamin D Supplement on the Score and Quality of Sleep in 20–50 Year-Old People with Sleep Disorders Compared with Control Group. Nutr. Neurosci. 2018, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D and the Omega-3 Fatty Acids Control Serotonin Synthesis and Action, Part 2: Relevance for ADHD, Bipolar Disorder, Schizophrenia, and Impulsive Behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D Hormone Regulates Serotonin Synthesis. Part 1: Relevance for Autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Sabir, M.S.; Haussler, M.R.; Mallick, S.; Kaneko, I.; Lucas, D.A.; Haussler, C.A.; Whitfield, G.K.; Jurutka, P.W. Optimal Vitamin D Spurs Serotonin: 1,25-Dihydroxyvitamin D Represses Serotonin Reuptake Transport (SERT) and Degradation (MAO-A) Gene Expression in Cultured Rat Serotonergic Neuronal Cell Lines. Genes Nutr. 2018, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Mazloum, S.R.; Maghsoudi, S.; Soleimani, D.; Khayyatzadeh, S.S.; Arekhi, S.; Arya, A.; Mirmoosavi, S.J.; Ferns, G.A.; Bahrami-Taghanaki, H.; et al. High Dose Vitamin D Supplementation Is Associated With a Reduction in Depression Score Among Adolescent Girls: A Nine-Week Follow-Up Study. J. Diet. Suppl. 2018, 15, 173–182. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. Psychometric Properties of the Beck Depression Inventory-II: A Comprehensive Review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Okereke, O.I.; Singh, A. The Role of Vitamin D in the Prevention of Late-Life Depression. J. Affect. Disord. 2016, 198, 1–14. [Google Scholar] [CrossRef]
- Lauinger, L.; Kaiser, P. Sensing and Signaling of Methionine Metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One Carbon Metabolism and Epigenetics: Understanding the Specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91. [Google Scholar] [CrossRef]
- Gao, J.; Cahill, C.M.; Huang, X.; Roffman, J.L.; Lamon-Fava, S.; Fava, M.; Mischoulon, D.; Rogers, J.T. S-Adenosyl Methionine and Transmethylation Pathways in Neuropsychiatric Diseases Throughout Life. Neurotherapeutics 2018, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- de Berardis, D.; Orsolini, L.; Serroni, N.; Girinelli, G.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Valchera, A.; Fornaro, M.; et al. A Comprehensive Review on the Efficacy of S-Adenosyl-L-Methionine in Major Depressive Disorder. CNS Neurol. Disord. Drug Targets 2016, 15, 35–44. [Google Scholar] [CrossRef]
- Sugden, C. One-Carbon Metabolism in Psychiatric Illness. Nutr. Res. Rev. 2006, 19, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Huang, Y.; Qiu, M.; Yin, C.; Ren, H.; Gan, H.; Li, H.; Zhou, Y.; Xia, J.; Li, W.; et al. Immunoassay of S-Adenosylmethionine and S-Adenosylhomocysteine: The Methylation Index as a Biomarker for Disease and Health Status. BMC Res. Notes 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Ikegame, T.; Bundo, M.; Murata, Y.; Kasai, K.; Kato, T.; Iwamoto, K. DNA Methylation of the BDNF Gene and Its Relevance to Psychiatric Disorders. J. Hum. Genet. 2013, 58, 434–438. [Google Scholar] [CrossRef]
- Vitetta, L.; Bambling, M.; Alford, H. The Gastrointestinal Tract Microbiome, Probiotics, and Mood. Inflammopharmacology 2014, 22, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-Adenosylmethionine (SAMe) Monotherapy for Depression: An 8-Week Double-Blind, Randomised, Controlled Trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-Adenosyl Methionine (SAMe) for Depression in Adults. Cochrane Database Syst. Rev. 2016, 10, CD011286. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Anheyer, D.; Cramer, H.; Dobos, G. Complementary Therapies for Clinical Depression: An Overview of Systematic Reviews. BMJ Open 2019, 9, e028527. [Google Scholar] [CrossRef]
- Mischoulon, D.; Alpert, J.E.; Arning, E.; Bottiglieri, T.; Fava, M.; Papakostas, G.I. Bioavailability of S-Adenosyl Methionine and Impact on Response in a Randomized Controlled Trial in Major Depressive Disorder. J. Clin. Psychiatry 2012, 73, 843. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, A.; Crescenzi, B.B.; Bolognesi, S.; Goracci, A.; Koukouna, D.; Rossi, R.; Fagiolini, A. S-Adenosylmethionine (SAMe) in Major Depressive Disorder (MDD): A Clinician-Oriented Systematic Review. Ann. Gener. Psychiatry 2020, 19, 50. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. Adjunctive S-Adenosylmethionine (SAMe) in Treating Non-Remittent Major Depressive Disorder: An 8-Week Double-Blind, Randomized, Controlled Trial. Eur. Neuropsychopharmacol. 2018, 28, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Price, L.H.; Carpenter, L.L.; Tyrka, A.R.; Papakostas, G.I.; Baer, L.; Dording, C.M.; Clain, A.J.; Durham, K.; Walker, R.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Trial of S-Adenosyl-L-Methionine (SAMe) versus Escitalopram in Major Depressive Disorder. J. Clin. Psychiatry 2014, 75, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Price, L.H.; Carpenter, L.L.; Tyrka, A.R.; Ng, C.H.; Papakostas, G.I.; Jaeger, A.; Fava, M.; Mischoulon, D. Is S-Adenosyl Methionine (SAMe) for Depression Only Effective in Males? A Re-Analysis of Data from a Randomized Clinical Trial. Pharmacopsychiatry 2015, 48, 141–144. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-Adenosyl Methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors for Antidepressant Nonresponders with Major Depressive Disorder: A Double-Blind, Randomized Clinical Trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P.; et al. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020, 22, 19M02578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cantley, L.C. Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Miller, A.L. The Methylation, Neurotransmitter, and Antioxidant Connections between Folate and Depression. Altern. Med. Rev. 2008, 13, 216–226. [Google Scholar]
- Subramaniapillai, M.; Carmona, N.E.; Rong, C.; McIntyre, R.S. Inflammation: Opportunities for Treatment Stratification among Individuals Diagnosed with Mood Disorders. Dialogues Clin. Neurosci. 2017, 19, 27. [Google Scholar] [CrossRef]
- Bender, A.; Hagan, K.E.; Kingston, N. The Association of Folate and Depression: A Meta-Analysis. J. Psychiatr. Res. 2017, 95, 9–18. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate Reductase and Psychiatric Diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Fava, M.; Mischoulon, D. Folate in Depression: Efficacy, Safety, Differences in Formulations, and Clinical Issues. J. Clin. Psychiatry 2009, 70 (Suppl. 5), 12–17. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, Folic Acid and 5-Methyltetrahydrofolate Are Not the Same Thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Stengler, M. The Role of Folate and MTHFR Polymorphisms in the Treatment of Depression. Altern. Ther. Health Med. 2021, 27, 53–57. [Google Scholar]
- Bedson, E.; Bell, D.; Carr, D.; Carter, B.; Hughes, D.; Jorgensen, A.; Lewis, H.; Lloyd, K.; McCaddon, A.; Moat, S.; et al. Folate Augmentation of Treatment—Evaluation for Depression (FolATED): Randomised Trial and Economic Evaluation. Health Technol. Assess. 2014, 18, 1–159. [Google Scholar] [CrossRef] [PubMed]
- Martone, G. Enhancement of Recovery from Mental Illness with L-Methylfolate Supplementation. Perspect. Psychiatr. Care 2018, 54, 331–334. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D.; et al. L-Methylfolate as Adjunctive Therapy for SSRI-Resistant Major Depression: Results of Two Randomized, Double-Blind, Parallel-Sequential Trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef]
- Jain, R.; Manning, S.; Cutler, A.J. Good, Better, Best: Clinical Scenarios for the Use of L-Methylfolate in Patients with MDD. CNS Spectr. 2020, 25, 750–764. [Google Scholar] [CrossRef]
- Shelton, R.C.; Pencina, M.J.; Barrentine, L.W.; Ruiz, J.A.; Fava, M.; Zajecka, J.M.; Papakostas, G.I. Association of Obesity and Inflammatory Marker Levels on Treatment Outcome: Results from a Double-Blind, Randomized Study of Adjunctive L-Methylfolate Calcium in Patients with MDD Who Are Inadequate Responders to SSRIs. J. Clin. Psychiatry 2015, 76, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Zajecka, J.M.; Fava, M.; Shelton, R.C.; Barrentine, L.W.; Young, P.; Papakostas, G.I. Long-Term Efficacy, Safety, and Tolerability of L-Methylfolate Calcium 15 Mg as Adjunctive Therapy with Selective Serotonin Reuptake Inhibitors: A 12-Month, Open-Label Study Following a Placebo-Controlled Acute Study. J. Clin. Psychiatry 2016, 77, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Savella, G.M.; Church, T.R.; Góez-Mogollón, L.; Sosinsky, A.Z.; Noe, O.B.; Kaimal, A.; Cohen, L.S. A Prenatal Supplement with Methylfolate for the Treatment and Prevention of Depression in Women Trying to Conceive and during Pregnancy. Ann. Clin. Psychiatry 2019, 31, 4. [Google Scholar]
- Dartois, L.L.; Stutzman, D.L.; Morrow, M. L-Methylfolate Augmentation to Antidepressants for Adolescents with Treatment-Resistant Depression: A Case Series. J. Child Adolesc. Psychopharmacol. 2019, 29, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Pitliuk, R.; da Costa Santos Fucci, T.P.P. L-Methylfolate, a New Option in Psychiatric Treatment, Would It Be Linked to Psoriasis Relapse? Einstein 2020, 18, eRC5522. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Kim, D.-H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The Metabolic Burden of Creatine Synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M.; Adriano, E. Beyond Sports: Efficacy and Safety of Creatine Supplementation in Pathological or Paraphysiological Conditions of Brain and Muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of Creatine Supplementation on Cognitive Function of Healthy Individuals: A Systematic Review of Randomized Controlled Trials. Exp. Gerontol. 2018, 108, 166. [Google Scholar] [CrossRef] [PubMed]
- Bakian, A.V.; Huber, R.S.; Scholl, L.; Renshaw, P.F.; Kondo, D. Dietary Creatine Intake and Depression Risk among U.S. Adults. Transl. Psychiatry 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Pazini, F.L.; Cunha, M.P.; Rodrigues, A.L.S. The Possible Beneficial Effects of Creatine for the Management of Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. [Google Scholar] [CrossRef]
- Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Creatine for the Treatment of Depression. Biomolecules 2019, 9, 406. [Google Scholar] [CrossRef]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Chronic Creatine Supplementation Alters Depression-like Behavior in Rodents in a Sex-Dependent Manner. Neuropsychopharmacology 2010, 35, 534. [Google Scholar] [CrossRef]
- Islam, M.R.; Ali, S.; Karmoker, J.R.; Kadir, M.F.; Ahmed, M.U.; Nahar, Z.; Islam, S.M.A.; Islam, M.S.; Hasnat, A.; Islam, M.S. Evaluation of Serum Amino Acids and Non-Enzymatic Antioxidants in Drug-Naïve First-Episode Major Depressive Disorder. BMC Psychiatry 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Vieira, K.F. Nutritional Therapies for Mental Disorders. Nutr. J. 2008, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of Inflammation-Associated Depression: Immune Influences on Tryptophan and Phenylalanine Metabolisms. Curr. Top. Behav. Neurosci. 2017, 31, 95–115. [Google Scholar] [CrossRef]
- Birkmayer, W.; Riederer, P.; Linauer, W.; Knoll, J. L-Deprenyl plus L-Phenylalanine in the Treatment of Depression. J. Neural Transm. 1984, 59, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Peselow, E.D.; Snyderman, S.; Gershon, S. D-Phenylalanine in Endogenous Depression. Am. J. Psychiatry 1980, 137, 1611–1612. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, T.; Granérus, A.K.; Linde, A.; Carlsson, A. “On-off” Phenomenon in Parkinson’s Disease: Relationship between Dopa and Other Large Neutral Amino Acids in Plasma. Neurology 1988, 38, 1245–1248. [Google Scholar] [CrossRef]
- Mosnik, D.M.; Spring, B.; Rogers, K.; Baruah, S. Tardive Dyskinesia Exacerbated after Ingestion of Phenylalanine by Schizophrenic Patients. Neuropsychopharmacology 1997, 16, 136–146. [Google Scholar] [CrossRef][Green Version]
- Levy, H.L.; Waisbren, S.E. Effects of Untreated Maternal Phenylketonuria and Hyperphenylalaninemia on the Fetus. N. Engl. J. Med. 1983, 309, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Gelenberg, A.J.; Wojcik, J.D.; Falk, W.E.; Baldessarini, R.J.; Zeisel, S.H.; Schoenfeld, D.; Mok, G.S. Tyrosine for Depression: A Double-Blind Trial. J. Affect. Disord. 1990, 19, 125–132. [Google Scholar] [CrossRef]
- Mouret, J.; Lemoine, P.; Minuit, M.P.; Robelin, N. L-Tyrosine Cures, Immediate and Long Term, Dopamine-Dependent Depressions. Clinical and Polygraphic Studies. Comptes Rendus Acad. Sci. Ser. III 1988, 306, 93–98. [Google Scholar]
- Parker, G.; Brotchie, H. Mood Effects of the Amino Acids Tryptophan and Tyrosine: “Food for Thought” III. Acta Psychiatr. Scand. 2011, 124, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a La Carte: Supplementation with the Serotonin Precursor 5-Hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef]
- Kikuchi, A.M.; Tanabe, A.; Iwahori, Y. A Systematic Review of the Effect of L-Tryptophan Supplementation on Mood and Emotional Functioning. J. Diet. Suppl. 2021, 18, 316–333. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102. [Google Scholar] [CrossRef]
- Gibson, E.L. Tryptophan Supplementation and Serotonin Function: Genetic Variations in Behavioural Effects. Proc. Nutr. Soc. 2018, 77, 174–188. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Martinez, P.; Tsai, A.C.; Muzoora, C.; Kembabazi, A.; Weiser, S.D.; Huang, Y.; Haberer, J.E.; Martin, J.N.; Bangsberg, D.R.; Hunt, P.W. Reversal of the Kynurenine Pathway of Tryptophan Catabolism May Improve Depression in ART-Treated HIV-Infected Ugandans. J. Acquir. Immune Defic. Syndr. 2014, 65, 456–462. [Google Scholar] [CrossRef]
- Lombardi, V.C.; De Meirleir, K.L.; Subramanian, K.; Nourani, S.M.; Dagda, R.K.; Delaney, S.L.; Palotás, A. Nutritional Modulation of the Intestinal Microbiota; Future Opportunities for the Prevention and Treatment of Neuroimmune and Neuroinflammatory Disease. J. Nutr. Biochem. 2018, 61, 1–16. [Google Scholar] [CrossRef]
- Gawlik-Kotelnicka, O.; Strzelecki, D. Probiotics as a Treatment for “Metabolic Depression”? A Rationale for Future Studies. Pharmaceuticals 2021, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, I.; Toniato, E.; Robuffo, I. Psychobiome Feeding Mind: Polyphenolics in Depression and Anxiety. Curr. Top. Med. Chem. 2018, 18, 2108–2115. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of Serotonin Signaling/Metabolism by Akkermansia Muciniphila and Its Extracellular Vesicles through the Gut-Brain Axis in Mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia Spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia Muciniphila Subtype Alleviates High-Fat Diet-Induced Metabolic Disorders and Inhibits the Neurodegenerative Process in Mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Roesch, L.; Thiago, P.; Russell, J.T.; Pepine, C.J.; Holbert, R.C.; Raizada, M.K.; Triplett, E.W. Depression Phenotype Identified by Using Single Nucleotide Exact Amplicon Sequence Variants of the Human Gut Microbiome. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.H.; Hoang, L.B.; Trinh, T.O.; Tran, T.; Dao, V.L. Psychobiotics for Patients with Chronic Gastrointestinal Disorders Having Anxiety or Depression Symptoms. J. Multidiscipl. Healthc. 2021, 14, 1395–1402. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The Role of Prebiotics in Cognition, Anxiety, and Depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The Effects of Vitamin B on the Immune/Cytokine Network and Their Involvement in Depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Lewis, J.E.; Tiozzo, E.; Melillo, A.B.; Leonard, S.; Chen, L.; Mendez, A.; Woolger, J.M.; Konefal, J. The Effect of Methylated Vitamin B Complex on Depressive and Anxiety Symptoms and Quality of Life in Adults with Depression. ISRN Psychiatry 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and “At-Risk” Individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of Personalized Diet and Probiotic Supplementation on Inflammation, Nutritional Parameters and Intestinal Microbiota—The “RISTOMED Project”: Randomized Controlled Trial in Healthy Older People. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression-A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Al-Fartusie, F.S.; Al-Bairmani, H.K.; Al-Garawi, Z.S.; Yousif, A.H. Evaluation of Some Trace Elements and Vitamins in Major Depressive Disorder Patients: A Case–Control Study. Biol. Trace Elem. Res. 2018, 189, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Bansal, Y. Antidepressant-like Activity of Beta-Carotene in Unstressed and Chronic Unpredictable Mild Stressed Mice. J. Funct. Foods 2014, 7, 425–434. [Google Scholar] [CrossRef]
- Manosso, L.M.; Camargo, A.; Dafre, A.L.; Rodrigues, A. Vitamin E for the Management of Major Depressive Disorder: Possible Role of the Anti-Inflammatory and Antioxidant Systems. Nutr. Neurosci. 2020, 1–15. [Google Scholar] [CrossRef]
- LaChance, L.R.; Ramsey, D. Antidepressant Foods: An Evidence-Based Nutrient Profiling System for Depression. World J. Psychiatry 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S. Vitamins, Minerals, and Mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef]
- Tako, E. Dietary Trace Minerals. Nutrients 2019, 11, 2823. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.; Islam, M.S.; Hasnat, A. Alterations of Serum Macro-Minerals and Trace Elements Are Associated with Major Depressive Disorder: A Case-Control Study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Wu, M.N.; He, F.; Tang, Q.R.; Chen, J.; Gu, X.; Zhai, Y.J.; Li, F.D.; Zhang, T.; Wang, X.Y.; Lin, J.F. Association between Depressive Symptoms and Supplemental Intake of Calcium and Vitamin D in Older Adults. J. Nutr. Health Aging 2020, 24, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hoane, M.R. The Role of Magnesium Therapy in Learning and Memory. Magn. Cent. Nerv. Syst. 2011, 115–124. [Google Scholar]
- Serefko, A.; Szopa, A.; Wlaź, P.; Nowak, G.; Radziwoń-Zaleska, M.; Skalski, M.; Poleszak, E. Magnesium in Depression. Pharmacol. Rep. 2013, 65, 547–554. [Google Scholar] [CrossRef]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of Magnesium Supplementation on Depression Status in Depressed Patients with Magnesium Deficiency: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of Magnesium Supplementation in the Treatment of Depression: A Randomized Clinical Trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef]
- Eby, G.A.; Eby, K.L. Rapid Recovery from Major Depression Using Magnesium Treatment. Med. Hypotheses 2006, 67, 362–370. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.; de Santana, M.; Costa, P.; Pereira, E.M.; Nepomuceno, C.; Queiroz, V.; de Oliveira, L.; Machado, M.; de Sena, E.P. Zinc Supplementation Combined with Antidepressant Drugs for Treatment of Patients with Depression: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Szopa, A.; Serefko, A.; Poleszak, E.; Nowak, G. The Role of Magnesium and Zinc in Depression: Similarities and Differences. Magn. Res. 2018, 31, 78–89. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, S.; Chen, W.; Yan, L.; Chen, Y.; Wen, H.; Liu, D.; Rosenblat, J.D.; Wang, J.; Cao, B. Trace Elements Differences in the Depression Sensitive and Resilient Rat Models. Biochem. Biophys. Res. Commun. 2020, 529, 204–209. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and Mechanisms of Emotional Behavior. J. Nutr. Biochem. 2014, 25, 1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chao, H.H.; Huang, W.T.; Chen, S.C.; Yang, H.Y. Psychiatric Disorders Risk in Patients with Iron Deficiency Anemia and Association with Iron Supplementation Medications: A Nationwide Database Analysis. BMC Psychiatry 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Shayganfard, M. Are Essential Trace Elements Effective in Modulation of Mental Disorders? Update and Perspectives. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Bschor, T. Lithium in the Treatment of Major Depressive Disorder. Drugs 2014, 74, 855–862. [Google Scholar] [CrossRef]
- Undurraga, J.; Sim, K.; Tondo, L.; Gorodischer, A.; Azua, E.; Tay, K.H.; Tan, D.; Baldessarini, R.J. Lithium Treatment for Unipolar Major Depressive Disorder: Systematic Review. J. Psychopharmacol. 2019, 33, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174. [Google Scholar] [CrossRef]
- Gónzalez, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2020, 12, 348. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive Substances of Plant Origin. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–35. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Bioactive Products from Fungi. Food Bioact. 2017, 59–87. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Kim, S.K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Characteristics of Selected Antioxidative and Bioactive Compounds in Meat and Animal Origin Products. Antioxidants 2019, 8, 335. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and Caffeine Consumption and Depression: A Meta-Analysis of Observational Studies. Austr. New Zealand J. Psychiatry 2016, 50, 228–242. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, Tea, Caffeine and Risk of Depression: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Liu, Q.S.; Deng, R.; Fan, Y.; Li, K.; Meng, F.; Li, X.; Liu, R. Low Dose of Caffeine Enhances the Efficacy of Antidepressants in Major Depressive Disorder and the Underlying Neural Substrates. Mol. Nutr. Food Res. 2017, 61, 1600910. [Google Scholar] [CrossRef]
- López-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and Selective Adenosine Receptor Antagonists as New Therapeutic Tools for the Motivational Symptoms of Depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Rusconi, A.C.; Valeriani, G.; Carluccio, G.M.; Majorana, M.; Carlone, C.; Raimondo, P.; Ripà, S.; Marino, P.; Coccanari de Fornari, M.A.; Biondi, M. Coffee Consumption in Depressive Disorders: It’s not one size fits all. Riv. Psichiatr. 2014, 49, 164–171. [Google Scholar] [CrossRef]
- Cova, I.; Leta, V.; Mariani, C.; Pantoni, L.; Pomati, S. Exploring Cocoa Properties: Is Theobromine a Cognitive Modulator? Psychopharmacology 2019, 236, 561–572. [Google Scholar] [CrossRef]
- Baggott, M.J.; Childs, E.; Hart, A.B.; de Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of Theobromine in Healthy Volunteers. Psychopharmacology 2013, 228, 109. [Google Scholar] [CrossRef]
- Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The Relevance of Theobromine for the Beneficial Effects of Cocoa Consumption. Front. Pharmacol. 2015, 6, 30. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; Sousa, D.P. De Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef]
- German-Ponciano, L.J.; Rosas-Sánchez, G.U.; Rivadeneyra-Domínguez, E.; Rodríguez-Landa, J.F. Advances in the Preclinical Study of Some Flavonoids as Potential Antidepressant Agents. Scientifica 2018, 2018, 2963565. [Google Scholar] [CrossRef]
- Trebatická, J.; Ďuračková, Z. Psychiatric Disorders and Polyphenols: Can They Be Helpful in Therapy? Oxid. Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for Depression: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, L.; Yin, H.; Shen, L.; Zheng, W.; Zhang, K.; Zeng, J.; Hu, C.; Liu, Y. Hydroxytyrosol Alleviates Oxidative Stress and Neuroinflammation and Enhances Hippocampal Neurotrophic Signaling to Improve Stress-Induced Depressive Behaviors in Mice. Food Funct. 2021, 12, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [PubMed]
- Shayganfard, M. Molecular and Biological Functions of Resveratrol in Psychiatric Disorders: A Review of Recent Evidence. Cell Biosci. 2020, 10, 128. [Google Scholar] [CrossRef]
- di Pierro, F. A Nutraceutical Role for Cannabidiol. Why Not? A Review of Its Pharmacological Properties and Clinical Applications. Nutrafoods 2015, 14, 111–117. [Google Scholar] [CrossRef]
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, Cannabinoids, and Health. Dialogues Clin. Neurosci. 2017, 19, 309. [Google Scholar] [CrossRef]
- Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Emerging Evidence for the Antidepressant Effect of Cannabidiol and the Underlying Molecular Mechanisms. J. Chem. Neuroanat. 2019, 98, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Calapai, G.; Mannucci, C.; Chinou, I.; Cardia, L.; Calapai, F.; Sorbara, E.E.; Firenzuoli, B.; Ricca, V.; Gensini, G.F.; Firenzuoli, F. Preclinical and Clinical Evidence Supporting Use of Cannabidiol in Psychiatry. Evid.-Based Complement. Altern. Med. 2019, 2019, 2509129. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; et al. Nutraceuticals for Major Depressive Disorder- More Is Not Merrier: An 8-Week Double-Blind, Randomised, Controlled Trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef]
- Moughan, P.J. Holistic Properties of Foods: A Changing Paradigm in Human Nutrition. J. Sci. Food Agric. 2020, 100, 5056–5063. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Bertram, H.C.; Bonjour, J.-P.; de Groot, L.; Dupont, D.; Feeney, E.; Ipsen, R.; Lecerf, J.M.; Mackie, A.; McKinley, M.C.; et al. Whole Dairy Matrix or Single Nutrients in Assessment of Health Effects: Current Evidence and Knowledge Gaps. Am. J. Clin. Nutr. 2017, 105, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of Nutraceuticals: Role of the Food Matrix, Processing Conditions, the Gastrointestinal Tract, and Nanodelivery Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
| Nutraceutical | Main Dietary Sources | Probable Antidepressant Effects | Clinical Evidence | Side Effects or Limitations | References |
|---|---|---|---|---|---|
| Omega 3 | Oily fish, nuts, seeds | Targeting of lipid rafts and G coupled protein receptors; Influencing neurotransmission; Stimulation of myelin proteins; Improved cognitive functioning and neuronal cytoarchitecture; Positive influence in the endocannabinoid system and BDNF activity; Anti-inflammatory effects | Major clinical efficacy appears at combined doses of EPA (>60%) + DHA | Long-term results; More effective in patients with mild to moderate depressive symptoms | [81,82,83,99,100] |
| Vitamin D | Oily fish, dairy products, eggs, seafood | Immune and microbial homeostasis; Serotonin synthesis; Circadian clock; Increased BDNF activity | Vitamin D3 at a dose of 50,000 IU/week show possible antidepressant effects also improving sleep quality vitamin D deficiency as a possible risk factor for late-life depression | Further clinical evidence is warranted | [114,115,116,118,120] |
| SAMe | Endogenously synthesized or supplements | Influence monoamine synthesis and activity; Improved methylation status and BDNF activity; Anti-inflammatory effects and targeting microbiota–gut–brain axis. | Combination of SAMe with standard antidepressants but not alone have demonstrated the safety, efficacy and tolerability of this component. One study also shows improved action when combined with probiotics | Possible sex-dependent effects; | [129,130,131,132,133,134,135,136,137,138] |
| Methylfolate | Endogenously synthesized or supplements | Improved monoamine synthesis and activity; Anti-inflammatory effects; Restoring SAMe levels in the organism | More benefits than supplementation with folate are obtained; 15 mg of methylfolate but not 7.5 exert possible antidepressant effects with standard therapies; High BMI, inflammatory mediators and leptin levels appears to act as predictive; Pregnant women or individuals with reduced MTHFR activity may be potential candidates for this nutraceutical | One case report study described a relapse of psoriasis in a 61 years old woman after 15 mg/day of methylfolate; Further evidence is warranted | [149,150,151,152,153,154] |
| Prebiotics | Fruits, vegetables, whole grains, legumes | Growing of beneficial bacteria | Some studies have found mild efficacy of prebiotic in MDD, but more prominently with probiotics | Prebiotics alone may not have any positive action for patients with MDD | [195,196,197] |
| Probiotics | Yogurt, kefir, kombucha, tempeh, miso | Growing of beneficial bacteria in the gut | Certain probiotic species alone or in combination improves clinical parameters in patients <65 years | Elderly people appear to be less sensitive to probiotics | [190,191,192,193,194] |
| Carnitine | Fish, meats, dairy products | Anti-inflammatory effects; Antioxidant; Improved metabolic profile; Cognitive enhancement; Neuroplastic effects; Increased BDNF activity; Probable effects in neurotransmitter functioning | 2 to 6 g per day of creatine supplementation appears to be well-tolerated and effective in patients with MDD | In patients with bipolar depression, it may increase the risk of suffering from hypomania/mania; Most studies are conducted in women (Sex-dependent effect); Further evidence is warranted | [160,161,162,163] |
| Tyrosine, | Poultry, dairy products, avocado, nuts, pumpkin/sesame seeds | Involved in dopamine and norepinephrine synthesis; Anti-inflammatory effects | Combined use of 100 mg/kg tyrosine plus imipramine show no conclusive antidepressant activity in comparison to placebo; Patients with low levels of dopamine may beneficiate from this nutraceutical | Available data is still controversial | [176,177,178] |
| Phenylalanine | Red meats, fish, eggs, dairy products, soy, nuts. | Involved in dopamine and norepinephrine synthesis; Anti-inflammatory effects | Some clinical studies described some favorable effects of phenylalanine in MDD | There are no recent studies conducted in the use of this nutraceutical; it may be related to important adverse effects in patients with Parkinson Disease and pregnant women | [171,172,173,174,175] |
| Tryptophan | Soy, fish, poultry, eggs, dairy products, cocoa | Involved in serotonin synthesis | 0.14–3 g of tryptophan per day in a context of a healthy diet may favorably influence patient’s mood | Tryptophan may be converted to quinolinic acid in patients with MDD (Neurotoxicity) | [179,180,181,182,183] |
| Vitamin B | Whole grains, meats, eggs, dairy products, seeds, nuts, dark leafy vegetables, fruits | Anti-inflammatory and antioxidant properties | Most trials are negative. However, dual supplementation of probiotics plus vitamin B8 obtained some clinical improvements | Including a varied diet with high vitamin content is much more effective than supplementation according to available scientific data | [198,199,200,201,204] |
| Vitamin A | Fruits and vegetables, meats, fish and dairy products | Serum levels of this component are reduced in patients with MDD. | [205,206,207] | ||
| Vitamin E | Dark leafy vegetables, nuts, seeds, vegetable oils | Serum levels of this component are reduced in patients with MDD | [205,206,207] | ||
| Calcium | Dairy products, fish, dark leafy vegetables | Neuronal gene expression, energy production, membrane excitability, synaptogenesis, synaptic transmission and cognitive functions | One study obtained favorable results from calcium plus vitamin D supplementation, but not alone | This nutraceutical has not provided too much interest | [212,213] |
| Magnesium | Dairy products, fish, dark leafy vegetables, legumes, nuts, seeds | Involved in complex cognitive processes | Daily consumption of 500 mg magnesium oxide per day improved depression status and hypomagnesemia; A 6-weeks intervention trial with magnesium in comparison to 6 weeks without any supplement in patients with mild and moderate MDD, regardless of age, gender, baseline severity of depression, baseline magnesium level, or use of antidepressant treatments; Magnesium exerts rapid actions (1–2 weeks) | Little evidence available | [216,217,218] |
| Zinc | Dairy products, fish, dark leafy vegetables, legumes, nuts, seeds, fish, red meat, poultry. | Pleiotropic effects | Zinc combined with antidepressants maximize clinical outcomes even in non-responsive patients | [220,221] | |
| Trace elements (Iron, selenium, manganese) | Meat, fish, cereals, milk and dairy foods, vegetables and nuts | Targeting oxidative stress, monoaminergic system, systemic and local inflammation, GABAergic system, sleep regulation and neuroprotective effects mediated by BDNF | Iron supplementation might provide prophylactic effects in patients with anemia | Further evidence is warranted | [222,223,224,225,226] |
| Ultra-trace elements (Lithium) | Potato, vegetables, fish and seafood | Involved in complex cognitive processes | Some studies reported positive outcomes from long-term prophylaxis for non-responder patients, as well as in the prevention of suicidal thoughts | Most studies are conducted in patients with bipolar disorder | [227,228,229] |
| Alkaloids (Caffeine and theobromine) | Coffee, cocoa | Pleiotropic effects (Non selective antagonist of adenosine receptors) | Different studies have demonstrated the antidepressant effects of caffeine and probably of theobromine | High doses of both components are associated with increased anxiety, depressive and negative symptoms | [238,239,240,241,242,243,244,245] |
| Flavonoids polyphenols | Vegetables and fruits | Improved functioning of the monoaminergic system, GABAergic transmission, BDNF activity and amelioration of the neuroinflammatory response in the brain | No clinical studies have been conducted | [248,249] | |
| Nonflavonoids polyphenols | Curcumin (Curcuma), resveratrol (grapes) Hydroxytyrosol (Olive oil) | Improving BDNF activity, serotoninergic and dopamine transmission, antioxidant and anti-inflammatory properties | Some studies have proven antidepressant benefits from curcumin in Asian patients with MDD | Further evidence is warranted | [250,251,252,253,254,255,256] |
| CBD | Only supplements | Influences serotoninergic, glutamatergic and GABAergic transmission, as well as the endocannabinoid system; Targeting of multiple cellular and molecular components, increasing the levels of BDNF, neurogenesis and neuroplasticity | - | There are no clinical studies conducted probably by its social perception | [257,258,259,260,261] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Monserrat, J.; Lahera, G.; Mora, F.; Rodriguez-Quiroga, A.; Fernandez-Rojo, S.; Quintero, J.; et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals 2021, 14, 821. https://doi.org/10.3390/ph14080821
Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G, Mora F, Rodriguez-Quiroga A, Fernandez-Rojo S, Quintero J, et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals. 2021; 14(8):821. https://doi.org/10.3390/ph14080821
Chicago/Turabian StyleAlvarez-Mon, Miguel A., Miguel A. Ortega, Cielo García-Montero, Oscar Fraile-Martinez, Jorge Monserrat, Guillermo Lahera, Fernando Mora, Alberto Rodriguez-Quiroga, Sonia Fernandez-Rojo, Javier Quintero, and et al. 2021. "Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects" Pharmaceuticals 14, no. 8: 821. https://doi.org/10.3390/ph14080821
APA StyleAlvarez-Mon, M. A., Ortega, M. A., García-Montero, C., Fraile-Martinez, O., Monserrat, J., Lahera, G., Mora, F., Rodriguez-Quiroga, A., Fernandez-Rojo, S., Quintero, J., & Alvarez-Mon, M. (2021). Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals, 14(8), 821. https://doi.org/10.3390/ph14080821











