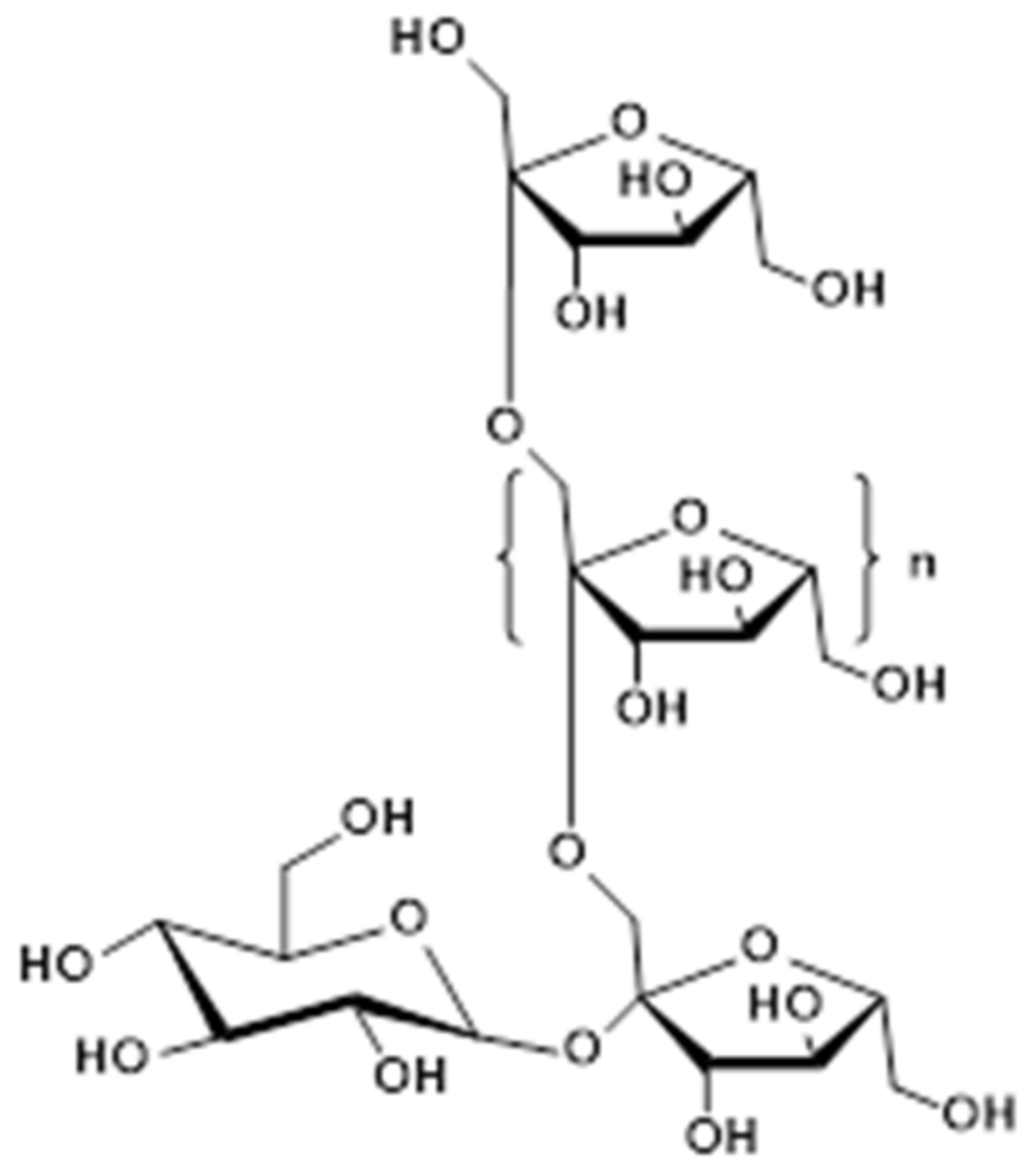
Inulin and Its Application in Drug Delivery
Abstract
:1. Introduction
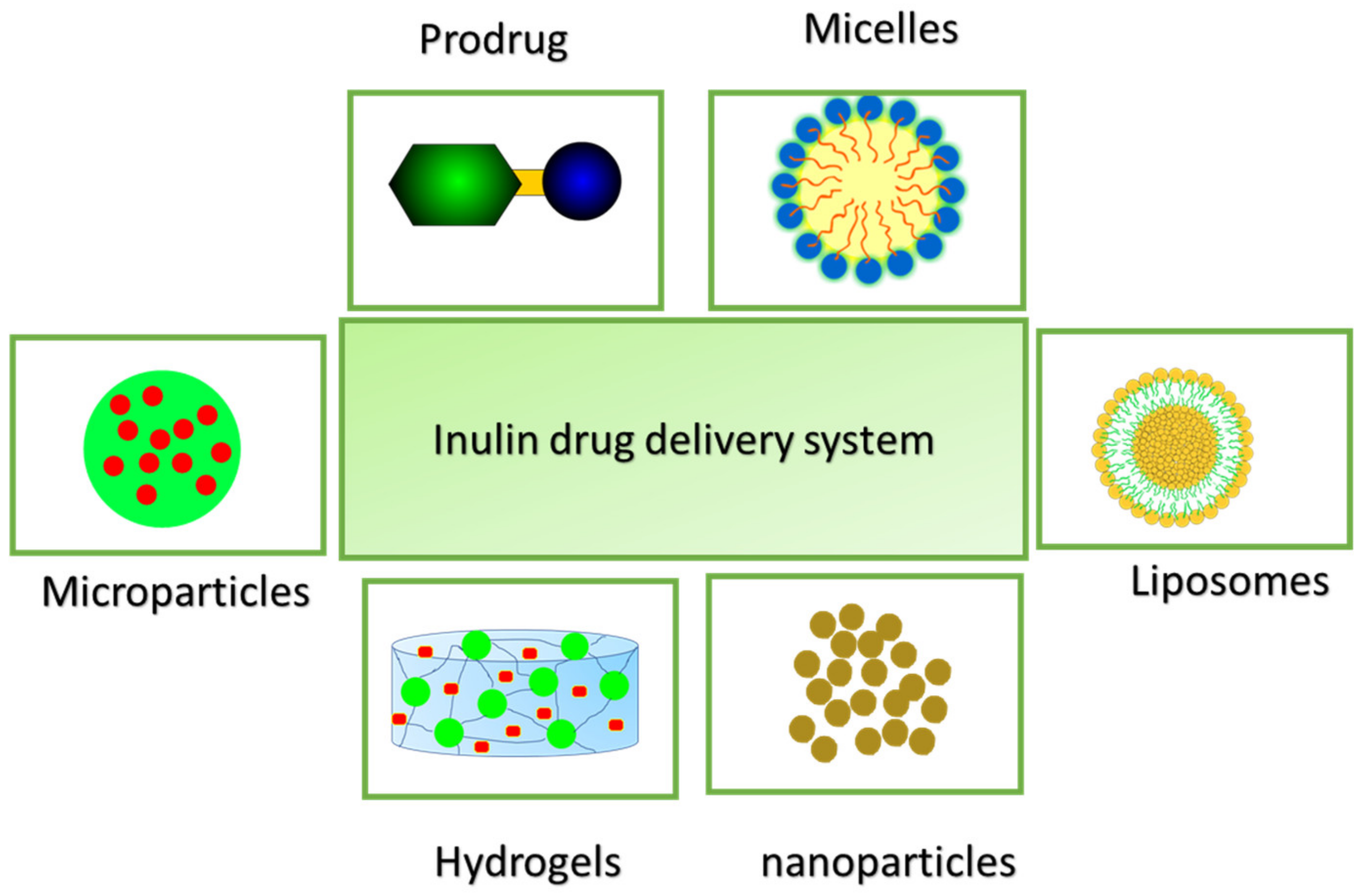
2. Inulin Drug Delivery Systems
- Hydrogel
- Micelles
- Liposomes
- Prodrugs/Conjugates
- Inulin complex/chelating
- Microparticles
- Nanoparticles
- Solid dispersion
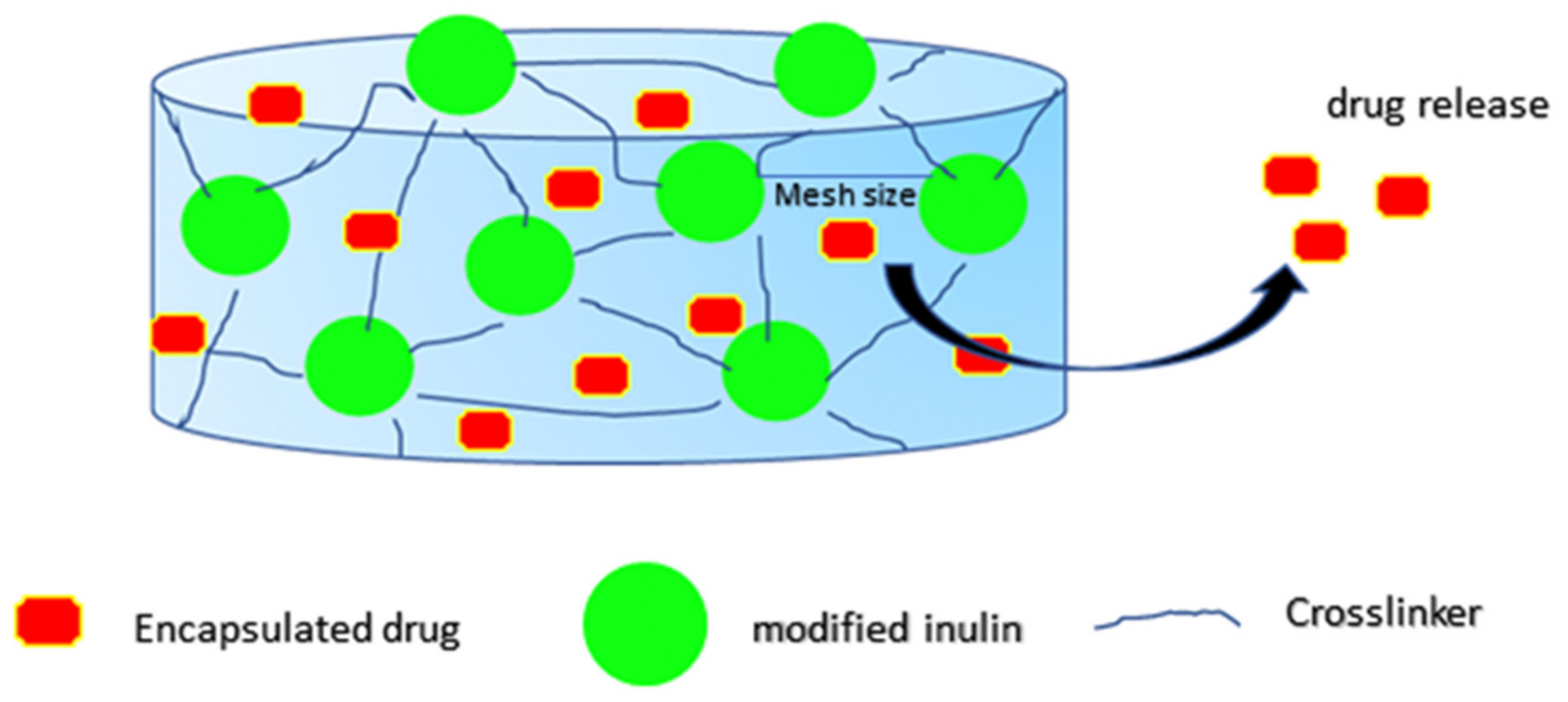
2.1. Inulin Hydrogels
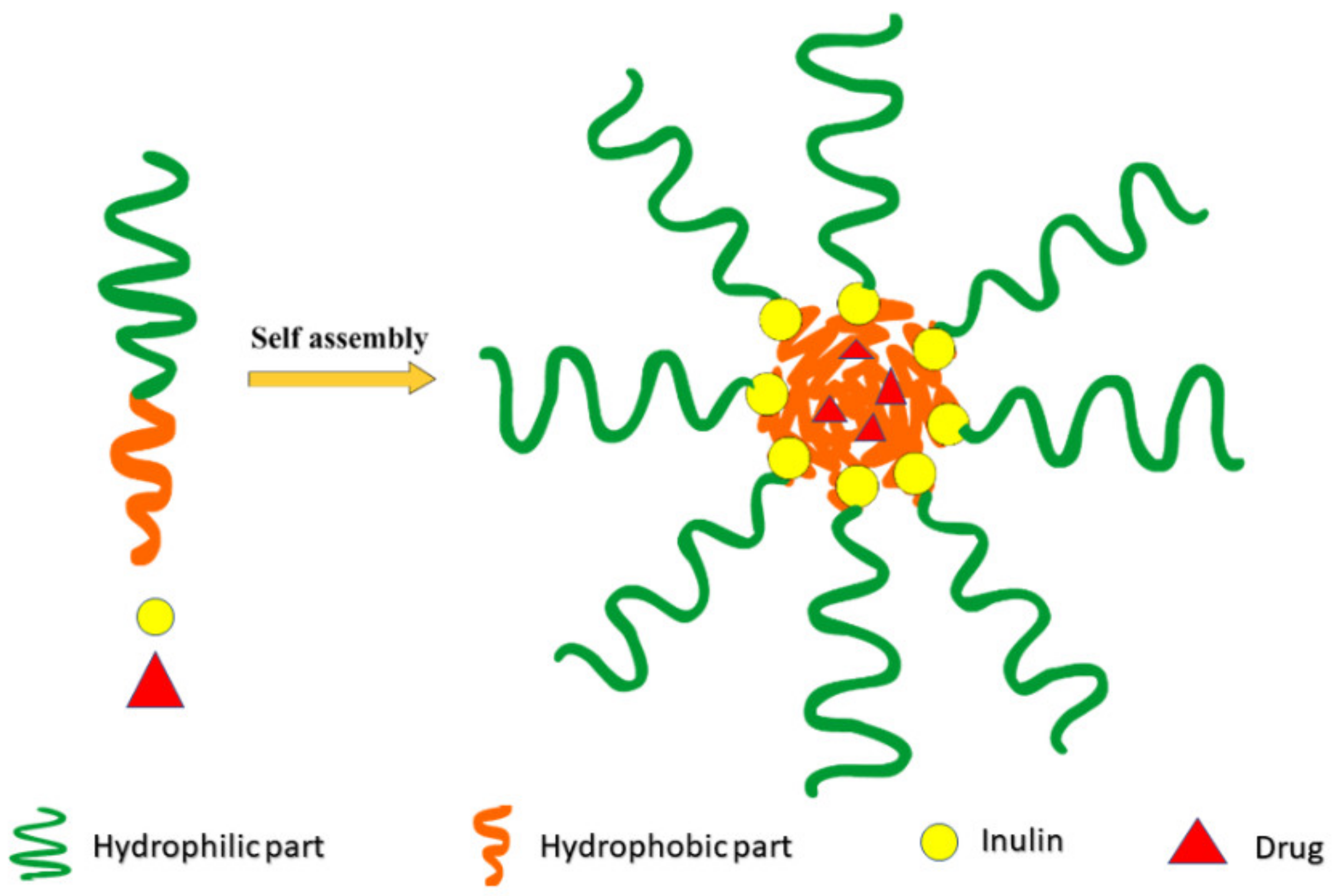
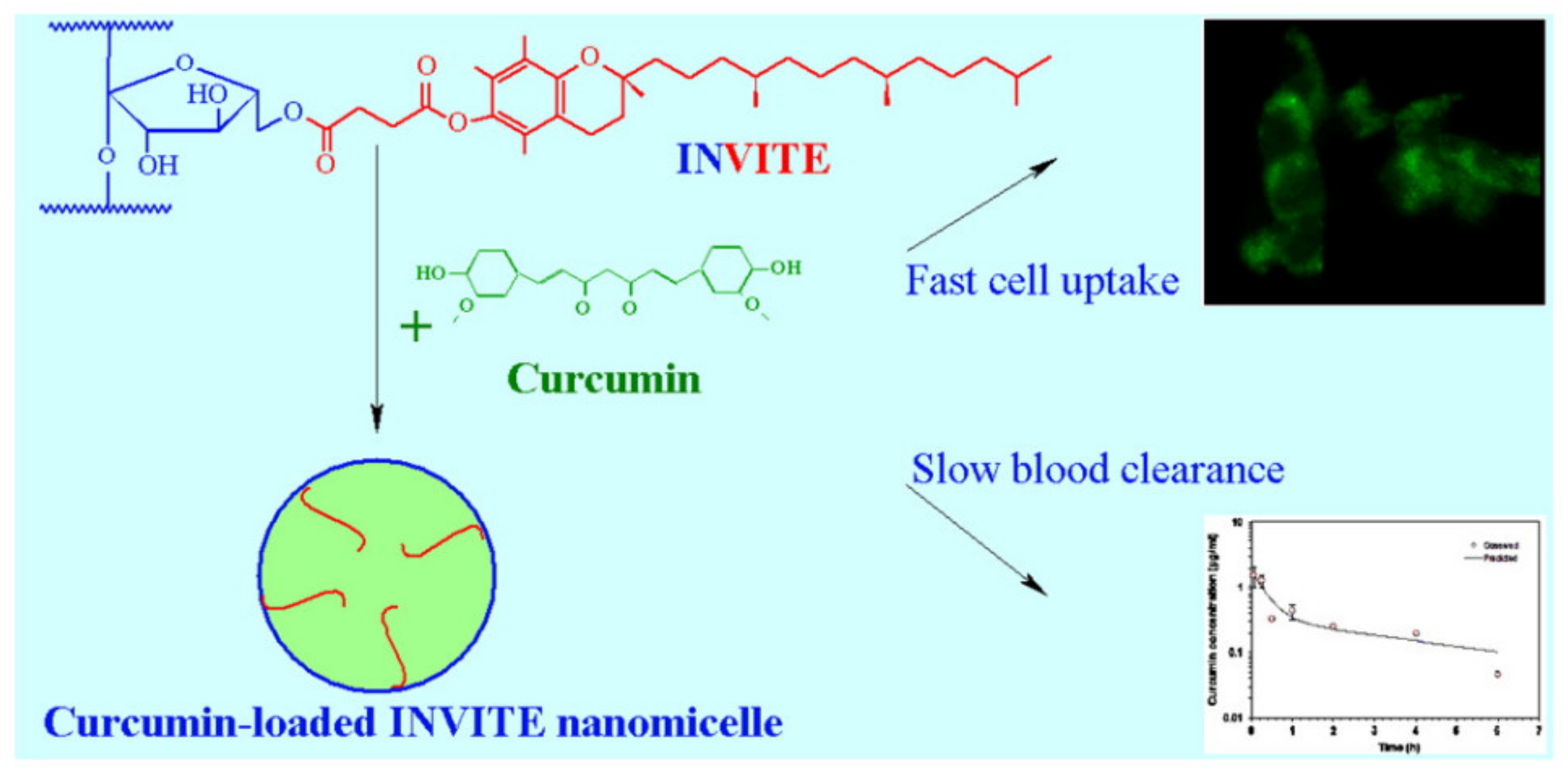
2.2. Inulin Micelles
2.3. Liposomes
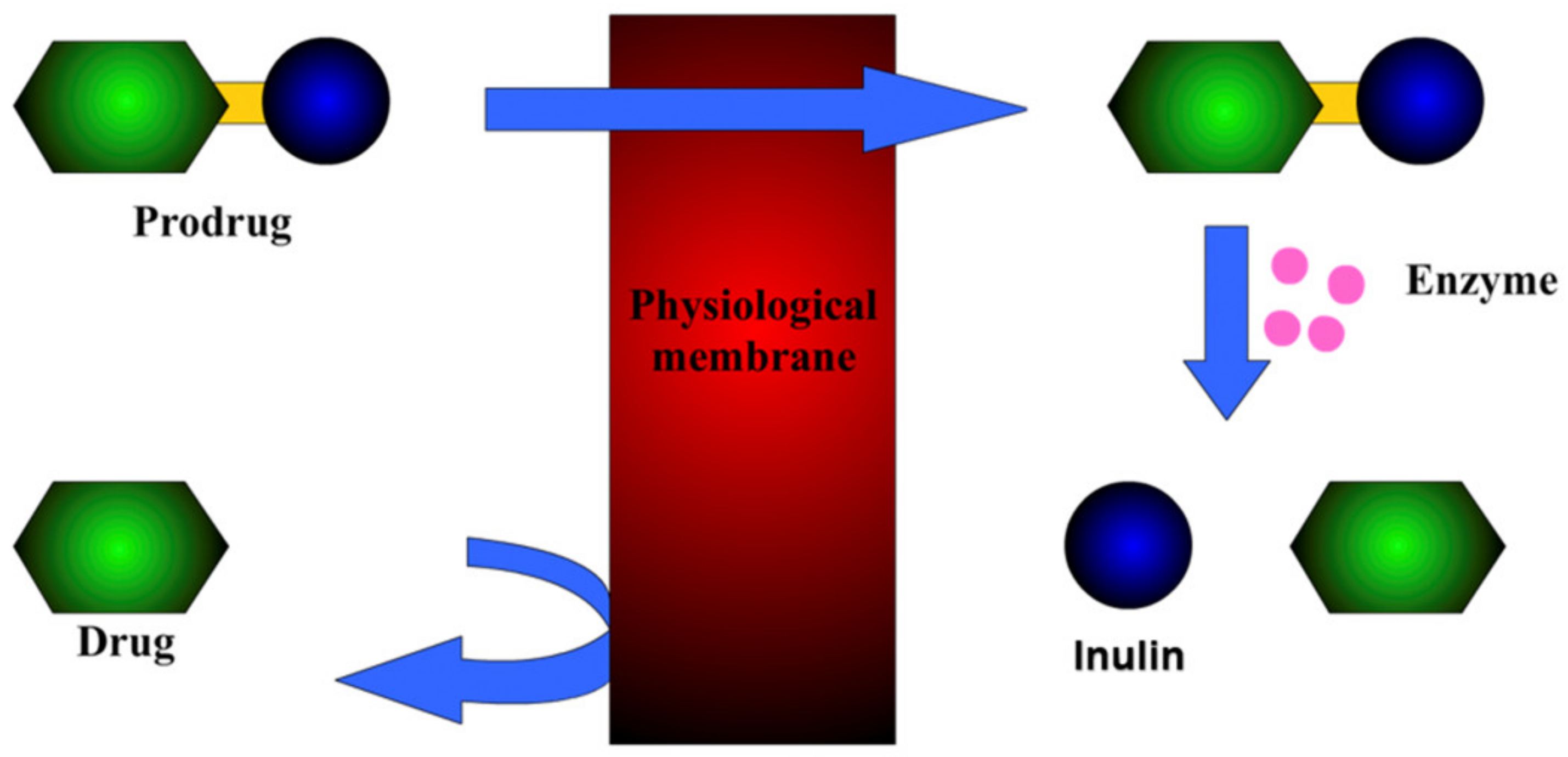
2.4. Prodrugs
- Prodrug with an ester linkage
- Bioactive Schiff bases Prodrug
2.4.1. Prodrug with an Ester Linkage
2.4.2. Bioactive Schiff Bases Prodrug
2.5. Inulin Chelating Agents/Complex/Polyplex
2.6. Microparticles
2.7. Inulin Nanoparticles
2.7.1. Active Targeting
Self-Assembling for Active Targeting
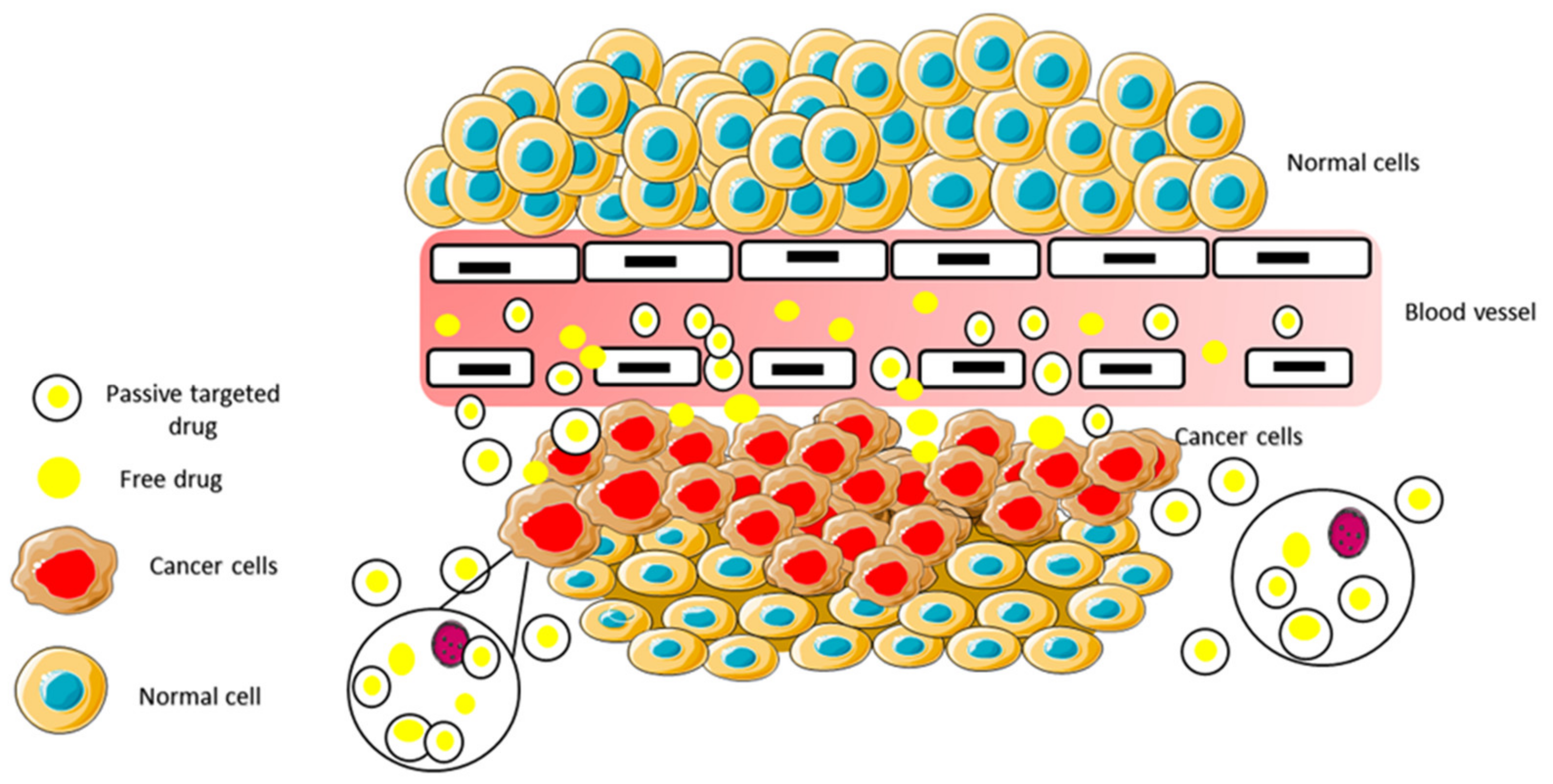
2.7.2. Passive Targeting
Gold Nanoparticles (GNPs)
Inulin Based Magnetic Nanoparticles
Functionalized Superparamagnetic Nanoparticles
Magnetoplexes
Self-Assembled Inulin Nanoparticles
Nanostructured Carbon-Nanosheets
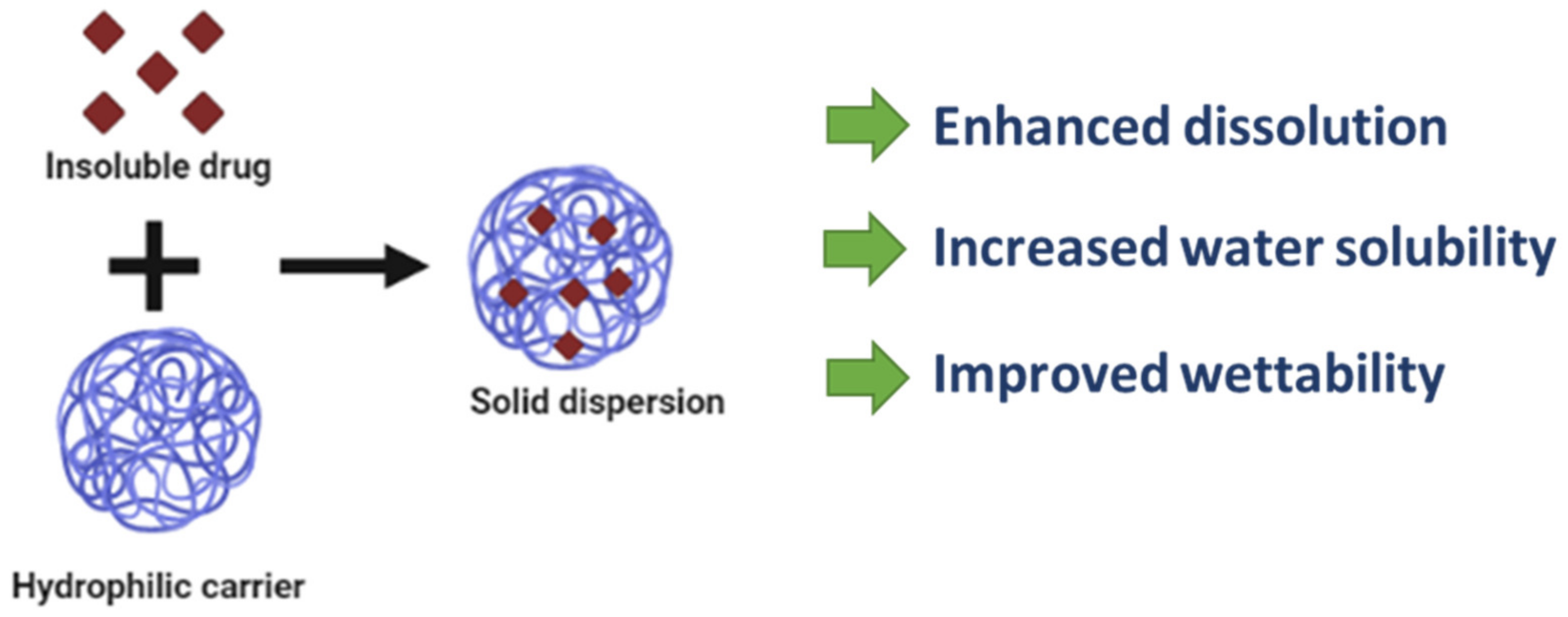
2.8. Solid Dispersion
3. Toxicological/Clinical Studies Performed Related to Inulin or Inulin-Containing Products
4. Conclusions and Expert Opinion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Lim, T.Y.; Poh, C.K.; Wang, W. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J. Mater. Sci. Mater. Electron. 2009, 20, 1669–1675. [Google Scholar] [CrossRef]
- Tardif, H.; Arnold, C.; Hayes, C.; Eagar, K. Establishment of the Australasian Electronic Persistent Pain Outcomes Collaboration. Pain Med. 2016, 18, 201–1018. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Im-munological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.D.V.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Van Arkel, J.; Sevenier, R.; Hakkert, J.C.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Tailor-made fructan synthesis in plants: A review. Carbohydr. Polym. 2013, 93, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T.; Mokoena, M.P.; Olaniran, A.O.; Wilhelmi, B.; Whiteley, C.G. Microbial enzymatic production and applications of short-chain fructooligosaccharides and inulooligosaccharides: Recent advances and current perspectives. J. Ind. Microbiol. Biotechnol. 2014, 41, 893–906. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A versatile polysaccharide with multiple pharmaceutical and food chemical uses. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of harvest time and storage temperature on characteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- Koch, K.; Andersson, R.; Rydberg, I.; Åman, P. Influence of harvest date on inulin chain length distribution and sugar profile for six chicory (Cichorium intybus L) cultivars. J. Sci. Food Agric. 1999, 79, 1503–1506. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.D.V.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Porras, C.; López-Pablos, A.; Alavrez, C.; Perez-Urizar, J.; Saavedra, Z. Physical Properties of Inulin and Technological Applications; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: New York, NY, USA, 2015; pp. 959–984. [Google Scholar]
- Keenan, D.F.; Resconi, V.C.; Kerry, J.P.; Hamill, R.M. Modelling the influence of inulin as a fat substitute in comminuted meat products on their physico-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014, 96, 1384–1394. [Google Scholar] [CrossRef]
- Rodriguez Furlan, L.T.; Padilla, A.P.; Campderros, M.E. Development of reduced fat minced meats using inulin and bovine plasma proteins as fat replacers. Meat Sci. 2014, 96, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Kocer, D.; Hicsasmaz, Z.; Bayindirli, A.; Katnas, S. Bubble and pore formation of the high-ratio cake formulation with polydextrose as a sugar- and fat-replacer. J. Food Eng. 2007, 78, 953–964. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Salvador, A.; Hernando, I. Replacing Fat and Sugar with Inulin in Cakes: Bubble Size Distribution, Physical and Sensory Properties. Food Bioprocess Technol. 2014, 7, 964–974. [Google Scholar] [CrossRef]
- Mittal, S.; Bajwa, U. Effect of fat and sugar substitution on the quality characteristics of low calorie milk drinks. J. Food Sci. Technol. 2011, 49, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Pintor, A.; Severiano-Pérez, P.; Totosaus, A. Optimization of fat-reduced ice cream formulation employing inulin as fat replacer via response surface methodology. Food Sci. Technol. Int. 2013, 20, 489–500. [Google Scholar] [CrossRef]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of Inulin and Oligofructose as natural in-gredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef]
- Coussement, P.A.A. Inulin and Oligofructose: Safe Intakes and Legal Status. J. Nutr. 1999, 129, 1412S–1417S. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Furlan, L.T.; Perez Padilla, A.; Campderros, M.E. Improvement of gluten-free bread properties by the incorporation of bovine plasma proteins and different saccharides into the matrix. Food Chem. 2015, 170, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Khomeiri, M.; Aalami, M.; Kashaninejad, M. Effect of inulin on the physicochemical properties, flow behavior and probiotic survival of frozen yogurt. J. Food Sci. Technol. 2014, 51, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Laguna, L.; Primo-Martín, C.; Salvador, A.; Sanz, T. Inulin and Erythritol as Sucrose Replacers in Short-dough Cookies: Sensory, Fracture, and Acoustic Properties. J. Food Sci. 2013, 78, S777–S784. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics—A review: Part 1. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 315–329. [Google Scholar]
- Matusek, A.; Merész, P.; Le, T.K.D.; Örsi, F. Effect of temperature and pH on the degradation of fructo-oligosaccharides. Eur. Food Res. Technol. 2009, 228, 355–365. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Chaito, C.; Judprasong, K.; Puwastien, P. Inulin content of fortified food products in Thailand. Food Chem. 2016, 193, 102–105. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef]
- Grasmeijer, N.; Stankovic, M.; de Waard, H.; Frijlink, H.W.; Hinrichs, W.L.J. Unraveling protein stabilization mechanisms: Vitrifica-tion and water replacement in a glass transition temperature controlled system. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ahmad, R.R.; Elkordy, A.A.; Chaw, C.S.; Moore, A. Compare and contrast the effects of surfactants (Pluronic®F-127 and Cremophor®EL) and sugars (β-cyclodextrin and inulin) on properties of spray dried and crystallised lysozyme. Eur. J. Pharm. Sci. 2013, 49, 519–534. [Google Scholar] [CrossRef]
- Saluja, V.; Amorij, J.-P.; Kapteyn, J.; de Boer, A.; Frijlink, H.; Hinrichs, W. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J. Control. Release 2010, 144, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.; Prinsen, M.; Frijlink, H.W. Inulin glasses for the stabilization of therapeutic proteins. Int. J. Pharm. 2001, 215, 163–174. [Google Scholar] [CrossRef]
- Wahjudi, M.; Murugappan, S.; van Merkerk, R.; Eissens, A.C.; Visser, M.R.; Hinrichs, W.L.; Quax, W.J. Development of a dry, stable and inhalable acyl–homoserine–lactone–acylase powder formulation for the treatment of pulmonary Pseudomonas aeruginosa infections. Eur. J. Pharm. Sci. 2013, 48, 637–643. [Google Scholar] [CrossRef]
- Srinarong, P.; Faber, J.; Visser, M.; Hinrichs, W.; Frijlink, H.W. Strongly enhanced dissolution rate of fenofibrate solid dispersion tablets by incorporation of superdisintegrants. Eur. J. Pharm. Biopharm. 2009, 73, 154–161. [Google Scholar] [CrossRef]
- Broesder, A.; Berends, J.M.E.; Scheepers, S.M.; Nguyen, D.N.; Frijlink, H.W.; Hinrichs, W.L.J. Ileo-Colon Targeting of the Poorly Water-Soluble Drug Celecoxib Using a pH-Dependent Coating in Combination with Self-Emulsifying Drug Delivery or Solid Dispersion Systems. Pharmaceutics 2021, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Swarup, P.; Agrawal, G.P. Solid Dispersion: A Mechanistic and Realistic Approach on Antihypertensive Drug as a Drug Carrier System. ASSAY Drug Dev. Technol. 2021, 19, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Traynor, J.; Mactier, R.; Geddes, C.C.; Fox, J.G. How to measure renal function in clinical practice. BMJ 2006, 333, 733–737. [Google Scholar] [CrossRef]
- Orlando, R.; Floreani, M.; Padrini, R.; Palatini, P. Determination of inulin clearance by bolus intravenous injection in healthy subjects and ascitic patients: Equivalence of systemic and renal clearances as glomerular filtration markers. Br. J. Clin. Pharmacol. 1998, 46, 605–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, M.H.; Maki, K.C. Effects of Dietary Inulin on Serum Lipids. J. Nutr. 1999, 129, 1474S–1477S. [Google Scholar] [CrossRef] [Green Version]
- Hond, E.D.; Geypens, B.; Ghoos, Y. Effect of high performance chicory inulin on constipation. Nutr. Res. 2000, 20, 731–736. [Google Scholar] [CrossRef]
- Marteau, P.; Jacobs, H.; Cazaubiel, M.; Signoret, C.; Prevel, J.-M.; Housez, B. Effects of chicory inulin in constipated elderly people: A double-blind controlled trial. Int. J. Food Sci. Nutr. 2010, 62, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Leenen, C.H.M.; Dieleman, L.A. Inulin and Oligofructose in Chronic Inflammatory Bowel Disease. J. Nutr. 2007, 137, 2572S–2575S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.G.; Cooper, P.D.; Petrovsky, N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol. Cell Biol. 2004, 82, 611–616. [Google Scholar] [CrossRef]
- Cooper, P.D.; Steele, E.J. The adjuvanticity of gamma inulin. Immunol. Cell Biol. 1988, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Cooper, P.D. Potentiation of photodynamic therapy of cancer by complement: The effect of γ-inulin. Br. J. Cancer 2007, 96, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.; Oksa, A.; Millar, J.; Alexander, M.; Kynoch, N.; Walton-Weitz, Z.; MacKenzie-Wood, P.; Tam, F.; Richards, H.; Naylor, R.; et al. The Safety of an Adjuvanted Autologous Cancer Vaccine Platform in Canine Cancer Patients. Vet. Sci. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, P.D.; Carter, M. The anti-melanoma activity of inulin in mice. Mol. Immunol. 1986, 23, 903–908. [Google Scholar] [CrossRef]
- Anda-Flores, D.; Carvajal-Millan, E.; Campa-Mada, A.; Lizardi-Mendoza, J.; Rascon-Chu, A.; Tanori-Cordova, J.; Martínez-López, A.L. Polysaccharide-Based Nanoparticles for Colon-Targeted Drug Delivery Systems. Polysaccharides 2021, 2, 626–647. [Google Scholar]
- Antunes, J.C.; Seabra, C.L.; Domingues, J.M.; Teixeira, M.O.; Nunes, C.; Costa-Lima, S.A.; Homem, N.C.; Reis, S.; Amorim, M.T.P.; Felgueiras, H.P. Drug Targeting of Inflammatory Bowel Diseases by Biomolecules. Nanomaterials 2021, 11, 2035. [Google Scholar] [CrossRef]
- Sun, Q.; Arif, M.; Chi, Z.; Li, G.; Liu, C.-G. Macrophages-targeting mannosylated nanoparticles based on inulin for the treatment of inflammatory bowel disease (IBD). Int. J. Biol. Macromol. 2021, 169, 206–215. [Google Scholar] [CrossRef]
- Pitarresi, G.; Tripodo, G.; Calabrese, R.; Craparo, E.F.; Licciardi, M.; Giammona, G. Hydrogels for Potential Colon Drug Release by Thiol-ene Conjugate Addition of a New Inulin Derivative. Macromol. Biosci. 2008, 8, 891–902. [Google Scholar] [CrossRef]
- Zijlstra, G.S.; Ponsioen, B.J.; Hummel, S.A.; Sanders, N.; Hinrichs, W.L.; de Boer, A.H.; Frijlink, H.W. Formulation and process development of (recombinant human) deoxyribonuclease I as a powder for inhalation. Pharm. Dev. Technol. 2009, 14, 358–368. [Google Scholar] [CrossRef]
- Giri, S.; Dutta, P.; Giri, T.K. Inulin-based carriers for colon drug targeting. J. Drug Deliv. Sci. Technol. 2021, 64, 102595. [Google Scholar] [CrossRef]
- Imran, S.; Gillis, R.B.; Kok, S.M.; Harding, S.E.; Adams, G.G. Application and use of Inulin as a tool for therapeutic drug delivery. Biotechnol. Genet. Eng. Rev. 2012, 28, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, W.; Joshi, R.; Garud, N. Inulin: A promising carrier for controlled and targeted drug delivery system. J. Drug Deliv. Ther. 2019, 9, 437–441. [Google Scholar] [CrossRef]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999, 185, 129–188. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Rudolph, A.; Wistrom, C.A.; Spargo, B.; Anchordoguy, T. Interactions of sugars with membranes. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Glass formation in plant anhydrobiotes: Survival in the dry state. Cryobiology 2004, 48, 215–528. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The Role of Vitrification in Anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Vereyken, I.J.; Chupin, V.; Islamov, A.; Kuklin, A.; Hincha, D.K.; de Kruijff, B. The Effect of Fructan on the Phospholipid Organization in the Dry State. Biophys. J. 2003, 85, 3058–3065. [Google Scholar] [CrossRef] [Green Version]
- Van den Mooter, G.; Vervoort, L.; Kinget, R. Characterization of methacrylated inulin hydrogels designed for colon targeting: In vitro release of BSA. Pharm. Res. 2003, 20, 303–307. [Google Scholar] [CrossRef]
- Hinrichs, W.L.; Sanders, N.N.; De Smedt, S.C.; Demeester, J.; Frijlink, H.W. Inulin is a promising cryo- and lyoprotectant for PEGylated lipoplexes. J. Control. Release 2005, 103, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, D.; Navarro-Martinez, M.D.; Rojas Melgarejo, F.; Hiner, A.N.; Chazarra, S.; Rodriguez-Lopez, J.N. Molecular prop-erties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and Oligofructose as Dietary Fiber: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef]
- Roberfroid, M.; Delzenne, N. Dietary Fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef]
- Vervoort, L.; Mooter, G.V.D.; Augustijns, P.; Kinget, R. Inulin hydrogels. I. Dynamic and equilibrium swelling properties. Int. J. Pharm. 1998, 172, 127–135. [Google Scholar] [CrossRef]
- Vervoort, L.; Vinckier, I.; Moldenaers, P.; Mooter, G.V.D.; Augustijns, P.; Kinget, R. Inulin hydrogels as carriers for colonic drug targeting. Rheological characterization of the hydrogel formation and the hydrogel network. J. Pharm. Sci. 1999, 88, 209–214. [Google Scholar] [CrossRef]
- Maris, B.; Verheyden, L.; Van Reeth, K.; Samyn, C.; Augustijns, P.; Kinget, R.; Mooter, G.V.D. Synthesis and characterisation of inulin-azo hydrogels designed for colon targeting. Int. J. Pharm. 2001, 213, 143–152. [Google Scholar] [CrossRef]
- Zabot, G.L.; Silva, E.K.; Azevedo, V.M.; Meireles, M.A.A. Replacing modified starch by inulin as prebiotic encapsulant matrix of lipophilic bioactive compounds. Food Res. Int. 2016, 85, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Denora, N.; Franco, M.; Pitarresi, G.; Giammona, G.; Trapani, G. New Biodegradable Hydrogels Based on Inulin and alpha, beta-Polyaspartylhydrazide Designed for Colonic Drug Delivery: In Vitro Release of Glutathione and Oxytocin. J. Biomater. Sci. Polym. Ed. 2011, 22, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Tripodo, G.; Pitarresi, G.; Palumbo, F.S.; Craparo, E.F.; Giammona, G. UV-photocrosslinking of inulin derivatives to produce hy-drogels for drug delivery application. Macromol. Biosci. 2005, 5, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Weber, L.M.; Lopez, C.G.; Anseth, K.S. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part. A 2008, 90, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervoort, L.; Mooter, G.V.D.; Augustijns, P.; Busson, R.; Toppet, S.; Kinget, R. Inulin hydrogels as carriers for colonic drug targeting: I. Synthesis and characterization of methacrylated inulin and hydrogel formation. Pharm. Res. 1997, 14, 1730–1737. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.; Song, Y.; Parikh, A.; Petrovsky, N.; Garg, S. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React. Funct. Polym. 2019, 134, 104–111. [Google Scholar] [CrossRef]
- Ferreira, L.; Carvalho, R.; Gil, M.H.; Dordick, J.S. Enzymatic synthesis of inulin-containing hydrogels. Biomacromolecules 2002, 3, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, F.; Sarpietro, M.G.; Micieli, D.; Ottimo, S.; Pitarresi, G.; Tripodo, G.; Carlisi, B.; Giammona, G. Differential scanning calorimetry study on drug release from an inulin-based hydrogel and its interaction with a biomembrane model: pH and loading effect. Eur. J. Pharm. Sci. 2008, 35, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-C.; Hsu, Y.-H.; Lin, P.-J. Synthesis of pH-sensitive inulin hydrogels and characterization of their swelling properties. J. Biomed. Mater. Res. 2002, 61, 146–152. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Fouladian, P.; Parikh, A.; Barclay, T.G.; Song, Y.; Garg, S. Preparation and Characterization of Oxidized Inulin Hy-drogel for Controlled Drug Delivery. Pharmaceutics 2019, 11, 356. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N.; Sagbas, S.; Yoshida, H.; Lyon, L.A. Synthesis and Properties of Inulin Based Microgels. Colloid Interface Sci. Commun. 2014, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Bae, Y.H.; Okano, T. Hydrogels: Swelling, Drug Loading, and Release. Pharm. Res. 1992, 09, 283–290. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Zhou, C.; Wang, C.; Zheng, Y.; Ye, K.; Li, C.; Zhou, G. Effects of gellan gum and inulin on mixed-gel properties and molecular structure of gelatin. Food Sci. Nutr. 2021, 9, 1336–1346. [Google Scholar] [CrossRef]
- Martinez, A.W.; Caves, J.M.; Ravi, S.; Li, W.S.; Chaikof, E.L. Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers. Acta Biomater. 2014, 10, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbe, B.; Maris, B.; Mooter, G.V.D.; De Smedt, S.C.; Demeester, J. The in vitro evaluation of ‘azo containing polysaccharide gels’ for colon delivery. J. Control. Release 2001, 75, 103–114. [Google Scholar] [CrossRef]
- Pitarresi, G.; Triolo, D.; Giorgi, M.; Fiorica, C.; Calascibetta, F.; Giammona, G. Inulin-Based Hydrogel for Oral Delivery of Flutamide: Preparation, Characterization, and in vivo Release Studies. Macromol. Biosci. 2012, 12, 770–778. [Google Scholar] [CrossRef] [PubMed]
- López-Molina, D.; Chazarra, S.; How, C.W.; Pruidze, N.; Navarro-Perán, E.; Garcia-Canovas, F.; Garcia-Ruiz, P.A.; Rojas-Melgarejo, F.; Rodríguez-López, J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int. J. Pharm. 2015, 479, 96–102. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fernandez, B.; Subirade, M.; Fliss, I. Study and Understanding Behavior of Alginate-Inulin Synbiotics Beads for Protection and Delivery of Antimicrobial-Producing Probiotics in Colonic Simulated Conditions. Probiotics Antimicrob. Proteins 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Bahadori, F.; Akinan, B.S.; Akyıl, S.; Eroğlu, M.S. Synthesis and engineering of sodium alginate/inulin core-shell nano-hydrogels for controlled-release oral delivery of 5-ASA. Org. Commun. 2019, 12, 132–142. [Google Scholar] [CrossRef]
- Kim, W.S.; Cho, C.S.; Hong, L.; Han, G.G.; Kil, B.J.; Kang, S.K.; Kim, D.D.; Choi, Y.J.; Huh, C.S. Oral Delivery of Probiotics Using pH-Sensitive Phthalyl Inulin Tablets. J. Microbiol. Biotechnol. 2019, 29, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Hufnagel, B.; Muellner, V.; Hlatky, K.; Tallian, C.; Vielnascher, R.; Guebitz, G.M.; Wirth, M.; Gabor, F. Chemically modified inulin for intestinal drug delivery—A new dual bioactivity concept for inflammatory bowel disease treatment. Carbohydr. Polym. 2021, 252, 117091. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2014, 16, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141–146. [Google Scholar] [CrossRef]
- Jain, R.K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 1997, 26, 71–90. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Mu, L.; Elbayoumi, T.; Torchilin, V. Mixed micelles made of poly(ethylene glycol)–phosphatidylethanolamine conjugate and d-α-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int. J. Pharm. 2005, 306, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, M.; Ahmed, S.; Kaur, K.; Lavasanifar, A. Decoration of polymeric micelles with cancer-specific peptide ligands for active targeting of paclitaxel. Biomaterials 2011, 32, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Eisenberg, A. Morphogenic Effect of Solvent on Crew-Cut Aggregates of Apmphiphilic Diblock Copolymers. Macromolecules 1998, 31, 1144–1154. [Google Scholar] [CrossRef]
- Choucair, A.; Eisenberg, A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur. Phys. J. E 2003, 10, 37–44. [Google Scholar] [CrossRef]
- Tripodo, G.; Pasut, G.; Trapani, A.; Mero, A.; Lasorsa, F.M.; Chlapanidas, T.; Trapani, G.; Mandracchia, D. Inulin-d-α-Tocopherol Succinate (INVITE) Na-nomicelles as a Platform for Effective Intravenous Administration of Curcumin. Biomacromolecules 2015, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Perteghella, S.; Grisoli, P.; Trapani, A.; Torre, M.L.; Mandracchia, D. Drug delivery of rifampicin by natural micelles based on inulin: Physicochemical properties, antibacterial activity and human macrophages uptake. Eur. J. Pharm. Biopharm. 2019, 136, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Rosato, A.; Trapani, A.; Chlapanidas, T.; Montagner, I.M.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Trapani, G.; Tripodo, G. Design, synthesis and evaluation of biotin decorated inulin-based polymeric micelles as long-circulating nanocarriers for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1245–1254. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Sardo, C.; Cavallaro, G.; Giammona, G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. J. Mater. Chem. B 2014, 2, 4262–4271. [Google Scholar] [CrossRef]
- Muley, P.; Kumar, S.; El Kourati, F.; Kesharwani, S.S.; Tummala, H. Hydrophobically modified inulin as an amphiphilic carbohy-drate polymer for micellar delivery of paclitaxel for intravenous route. Int. J. Pharm. 2016, 500, 32–41. [Google Scholar] [CrossRef]
- Di Prima, G.; Saladino, S.; Bongiovì, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel inulin-based mucoadhesive micelles loaded with corticosteroids as potential transcorneal permeation enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Campora, S.; Scialabba, C.; Adamo, G.; Licciardi, M.; Ghersi, G.; Giammona, G. Self-organized environment-sensitive inu-lin-doxorubicin conjugate with a selective cytotoxic effect towards cancer cells. Rsc. Adv. 2015, 5, 32421–32430. [Google Scholar] [CrossRef]
- Giammona, G.; Mauro, N.; Scialabba, C. Inulin for Cancer Therapy: Present and Perspectives. Int. J. Pharm. Res. Rev. 2016, 5, 63–69. [Google Scholar]
- Chehardoli, G.; Norouzian, P.; Firozian, F. Inulin-Grafted Stearate (In-g-St) as the Effective Self-Assembling Polymeric Micelle: Synthesis and Evaluation for the Delivery of Betamethasone. J. Nanomater. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Shivhare, K.; Garg, C.; Priyam, A.; Gupta, A.; Sharma, A.K.; Kumar, P. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. Int. J. Biol. Macromol. 2018, 106, 775–783. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [Green Version]
- Koning, G.A.; Storm, G. Targeted drug delivery systems for the intracellular delivery of macromolecular drugs. Drug Discov. Today 2003, 8, 482–483. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Storm, G. Liposomes in the treatment of inflammatory disorders. Expert Opin. Drug Deliv. 2005, 2, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K. A Review on Composite Liposomal Technologies for Specialized Drug Delivery. J. Drug Deliv. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Wu, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapra, P.; Allen, T. Ligand-targeted liposomal anticancer drugs. Prog. Lipid Res. 2003, 42, 439–462. [Google Scholar] [CrossRef]
- Kundu, S.K.; Sharma, A.R.; Lee, S.-S.; Sharma, G.; Doss, C.G.P.; Yagihara, S.; Kim, D.-Y.; Nam, J.-S.; Chakraborty, C. Recent Trends of Polymer Mediated Liposomal Gene Delivery System. BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allemann, E. Current methods for attaching targeting ligands to liposomes and nano-particles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering liposomal nanoparticles for targeted gene therapy. Gene. Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef]
- Essien, H.; Lai, J.Y.; Hwang, K.J. Synthesis of diethylenetriaminepentaacetic acid conjugated inulin and utility for cellular uptake of liposomes. J. Med. Chem. 1988, 31, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Nti-Addae, K.W. Prodrug strategies to overcome poor water solubility. Adv. Drug Deliv. Rev. 2007, 59, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem. Biodivers. 2009, 6, 2071–2083. [Google Scholar] [CrossRef]
- Peterson, L.W.; McKenna, C.E. Prodrug approaches to improving the oral absorption of antiviral nucleotide analogues. Expert Opin. Drug Deliv. 2009, 6, 405–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaman, R. Prodrugs for masking bitter taste of antibacterial drugs—a computational approach. J. Mol. Model. 2013, 19, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Choi, J.S. Enhanced bioavailability of atenolol by transdermal administration of the ethylene-vinyl acetate matrix in rabbits. Eur. J. Pharm. Biopharm. 2003, 56, 439–443. [Google Scholar] [CrossRef]
- D’Souza, M.; Venkataramanan, R.; D’Mello, A.; Niphadkar, P. An alternative prodrug approach for reducing presystemic metabolism of drugs. Int. J. Pharm. 1986, 31, 165–167. [Google Scholar] [CrossRef]
- Aungst, B.J.; Matz, N. Prodrugs to reduce presystemic metabolism. In Solvent Systems and Their Selection in Pharmaceutics and Biopharmaceutics; Augustijns, J., Brewste, M.E., Eds.; Springer: Amsterdam, The Netherlands, 2007; pp. 339–355. [Google Scholar]
- Min, Y.; Mao, C.; Chen, S.; Ma, G.; Wang, J.; Liu, Y. Combating the Drug Resistance of Cisplatin Using a Platinum Prodrug Based Delivery System. Angew. Chem. Int. Ed. 2012, 51, 6742–6747. [Google Scholar] [CrossRef]
- Placzek, A.T.; Ferrara, S.J.; Hartley, M.D.; Sanford-Crane, H.S.; Meinig, J.M.; Scanlan, T.S. Sobetirome prodrug esters with en-hanced blood-brain barrier permeability. Bioorg. Med. Chem. 2016, 24, 5842–5854. [Google Scholar] [CrossRef]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug Approaches for CNS Delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giang, I.; Boland, E.L.; Poon, G.M.K. Prodrug Applications for Targeted Cancer Therapy. AAPS J. 2014, 16, 899–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remon, J.; Duncan, R.; Schacht, E. Polymer-drug combinations: Pinocytic uptake of modified polysaccharides containing procainamide moieties by rat visceral yolk sacs cultured -itin vitro. J. Control. Release 1984, 1, 47–56. [Google Scholar] [CrossRef]
- Schacht, E.; Buys, L.; Vermeersch, J.; Remon, J.P. Polymer-drug combinations: Synthesis and characterization of modified polysaccharides containing procainamide moieties. J. Control. Release 1984, 1, 33–46. [Google Scholar] [CrossRef]
- Hartzell, A.L.; Maldonado-Gómez, M.X.; Yang, J.; Hutkins, R.W.; Rose, D.J. In vitro digestion and fermentation of 5-formyl-aminosailcylate-inulin: A potential prodrug of 5-aminosalicylic acid. Bioact. Carbohydr. Diet. Fibre 2013, 2, 8–14. [Google Scholar] [CrossRef]
- Stevens, C.V.; Meriggi, A.; Booten, K. Chemical Modification of Inulin, a Valuable Renewable Resource, and Its Industrial Applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef]
- Mura, C.; Valenti, D.; Floris, C.; Sanna, R.; De Luca, M.A.; Fadda, A.M.; Loy, G. Metronidazole prodrugs: Synthesis, physico-chemical properties, stability, and ex vivo release studies. Eur. J. Med. Chem. 2011, 46, 4142–4150. [Google Scholar] [CrossRef]
- Vermeersch, J.; Vandoorne, F.; Permentier, D.; Schacht, E. Macromolecular Prodrugs of Metronidazole. Esterification of Hydroxyl Containing Polymers With Metronidazole Monosuccinate. Bull. Des. Sociétés Chim. Belg. 2010, 94, 591–596. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, J.; Ballard, E.C.; Wang, B. Prodrug approaches to the improved delivery of peptide drugs. Curr. Pharm. Des. 1999, 5, 265–287. [Google Scholar]
- Simplício, A.L.; Clancy, J.M.; Gilmer, J.F. Prodrugs for Amines. Molecules 2008, 13, 519–547. [Google Scholar] [CrossRef] [Green Version]
- Barsanti, C.; Lenzarini, F.; Kusmic, C. Diagnostic and prognostic utility of non-invasive imaging in diabetes management. World J. Diabetes 2015, 6, 792–806. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Mohs, A.M.; Lu, Z.R. Gadolinium(III)-based blood-pool contrast agents for magnetic resonance imaging: Status and clinical po-tential. Expert Opin Drug Deliv. 2007, 4, 149–164. [Google Scholar] [CrossRef]
- Weinmann, H.J.; Brasch, R.C.; Press, W.R.; Wesbey, G.E. Characteristics of gadolinium-DTPA complex: A potential NMR contrast agent. Am. J. Roentgenol. 1984, 142, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, B.A.; Enyeart, J.J. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am. J. Physiol. Physiol. 1990, 259, C515–C520. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Fasano, M.; Terreno, E. Lanthanide(III) chelates for NMR biomedical applications. Chem. Soc. Rev. 1998, 27, 19–29. [Google Scholar] [CrossRef]
- Peters, J.; Huskens, J.; Raber, D. Lanthanide induced shifts and relaxation rate enhancements. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 283–350. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tsourkas, A. Gd-based macromolecules and nanoparticles as magnetic resonance contrast agents for mo-lecular imaging. Curr. Top. Med. Chem. 2013, 13, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Baxter, A.B.; Lazarus, S.C.; Brasch, R.C. In Vitro Histamine Release Induced by Magnetic Resonance Imaging and Iodinated Contrast Media. Investig. Radiol. 1993, 28, 308–312. [Google Scholar] [CrossRef]
- Corsi, D.M.; Elst, L.V.; Muller, R.N.; van Bekkum, H.; Peters, J.A. Inulin as a carrier for contrast agents in magnetic resonance imaging. Chemistry 2001, 7, 64–71. [Google Scholar] [CrossRef]
- Rebizak, R.; Schaefer, M.; Dellacherie, É. Macromolecular contrast agents for magnetic resonance imaging: Influence of polymer content in ligand on the paramagnetic properties. Eur. J. Pharm. Sci. 1999, 7, 243–248. [Google Scholar] [CrossRef]
- Lebduskova, P.; Kotek, J.; Hermann, P.; Vander, E.L.; Muller, R.N.; Lukes, I.; Peters, J.A. A gadolinium(III) complex of a carbox-ylic-phosphorus acid derivative of diethylenetriamine covalently bound to inulin, a potential macromolecular MRI contrast agent. Bioconjug. Chem. 2004, 15, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Granato, L.; Laurent, S.; Elst, L.V.; Djanashvili, K.; Peters, J.A.; Muller, R.N. The Gd3+ complex of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(p-isothiocyanatoanilide) conjugated to inulin: A potential stable macromolecular contrast agent for MRI. Contrast Media Mol. Imaging 2011, 6, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M. Promises and Challenges in Developing RNAi as a Research Tool and Therapy. Pept. Microarrays 2011, 703, 173–187. [Google Scholar] [CrossRef]
- Cavallaro, G.; Sardo, C.; Scialabba, C.; Licciardi, M.; Giammona, G.; Lamberti, G. Smart inulin-based polycationic nanodevices for siRNA delivery. Curr. Drug Deliv. 2016, 13, 1. [Google Scholar] [CrossRef]
- Sardo, C.; Farra, R.; Licciardi, M.; Dapas, B.; Scialabba, C.; Giammona, G.; Grassi, M.; Grassi, G.; Cavallaro, G. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. Eur. J. Pharm. Sci. 2015, 75, 60–71. [Google Scholar] [CrossRef]
- Sardo, C.; Craparo, E.F.; Porsio, B.; Giammona, G.; Cavallaro, G. Improvements in Rational Design Strategies of Inulin De-rivative Polycation for siRNA Delivery. Biomacromolecules 2016, 17, 2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fu, X.; Bao, Y. Cationization of Inulin via Atom Transfer Radical Polymerization for Gene Delivery. Chem. J. Chin. Univ. 2014, 35, 2124–2130. [Google Scholar]
- Pitarresi, G.; Tripodo, G.; Cavallaro, G.; Palumbo, F.S.; Giammona, G. Inulin–iron complexes: A potential treatment of iron deficiency anaemia. Eur. J. Pharm. Biopharm. 2008, 68, 267–276. [Google Scholar] [CrossRef]
- Cooper, P.D.; Barclay, T.; Ginic-Markovic, M.; Petrovsky, N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology 2013, 23, 1164–1174. [Google Scholar] [CrossRef]
- Dolter, K.E.; Evans, C.F.; Ellefsen, B.; Song, J.; Boente-Carrera, M.; Vittorino, R.; Rosenberg, T.J.; Hannaman, D.; Vasan, S. Immunogenicity, safety, biodistribution and persistence of ADVAX, a prophylactic DNA vaccine for HIV-1, delivered by in vivo electroporation. Vaccine 2011, 29, 795–803. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax, a polysaccharide adjuvant derived from delta inulin, provides im-proved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [Green Version]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef]
- Petrovsky, N.; Cooper, P.D. Carbohydrate-based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef]
- Nishiyama, A.; Tsuji, S.; Yamashita, M.; Henriksen, R.A.; Myrvik, Q.N.; Shibata, Y. Phagocytosis of N-acetyl-d-glucosamine particles, a Th1 adjuvant, by RAW 264.7 cells results in MAPK activation and TNF-α, but not IL-10, production. Cell. Immunol. 2006, 239, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Wang, J.P.; Specht, C.A.; Levitz, S.M. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect. Immun. 2009, 77, 1774–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielefeldt-Ohmann, H.; Prow, A.N.; Wang, W.; Tan, C.S.; Coyle, M.; Douma, A.; Hobson-Peters, J.; Kidd, L.; Hall, A.R.; Petrovsky, N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Veter. Res. 2014, 45, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, D.L.; Sajkov, D.; Woodman, R.J.; Honda-Okubo, Y.; Cox, M.M.; Heinzel, S.; Petrovsky, N. Randomized clinical trial of immu-nogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Tummala, H. Development of Soluble Inulin Microparticles as a Potent and Safe Vaccine Adjuvant and Delivery System. Mol. Pharm. 2013, 10, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Gallovic, M.D.; Montjoy, D.G.; Collier, M.A.; Do, C.; Wyslouzil, B.E.; Bachelder, E.M.; Ainslie, K.M. Chemically modified inulin micro-particles serving dual function as a protein antigen delivery vehicle and immunostimulatory adjuvant. Biomater. Sci. 2016, 4, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kesharwani, S.S.; Kuppast, B.; Rajput, M.; Bakkari, M.A.; Tummala, H. Discovery of inulin acetate as a novel immune-active polymer and vaccine adjuvant: Synthesis, material characterization, and biological evaluation as a toll-like receptor-4 agonist. J. Mater. Chem. B 2016, 4, 7950–7960. [Google Scholar] [CrossRef]
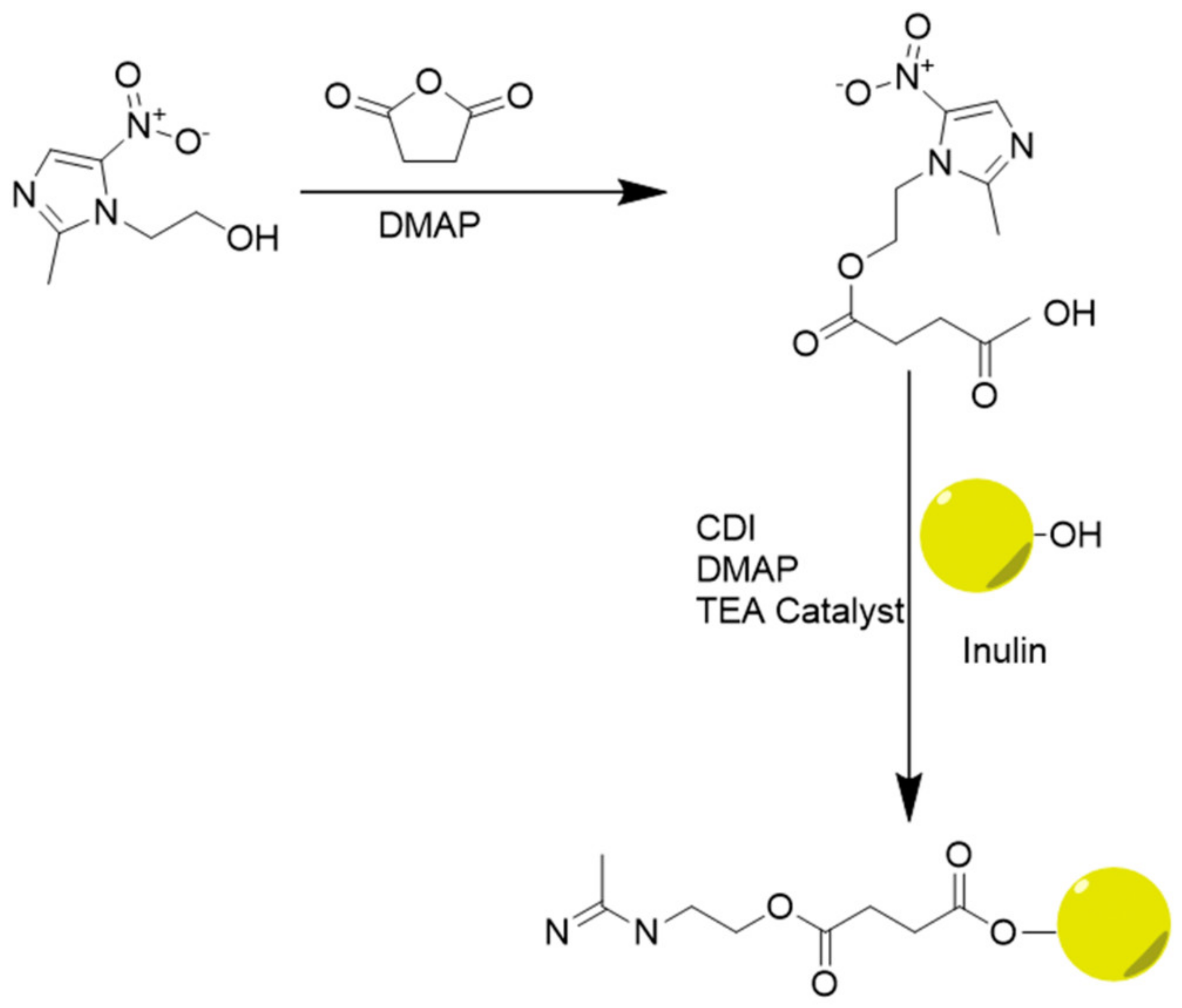
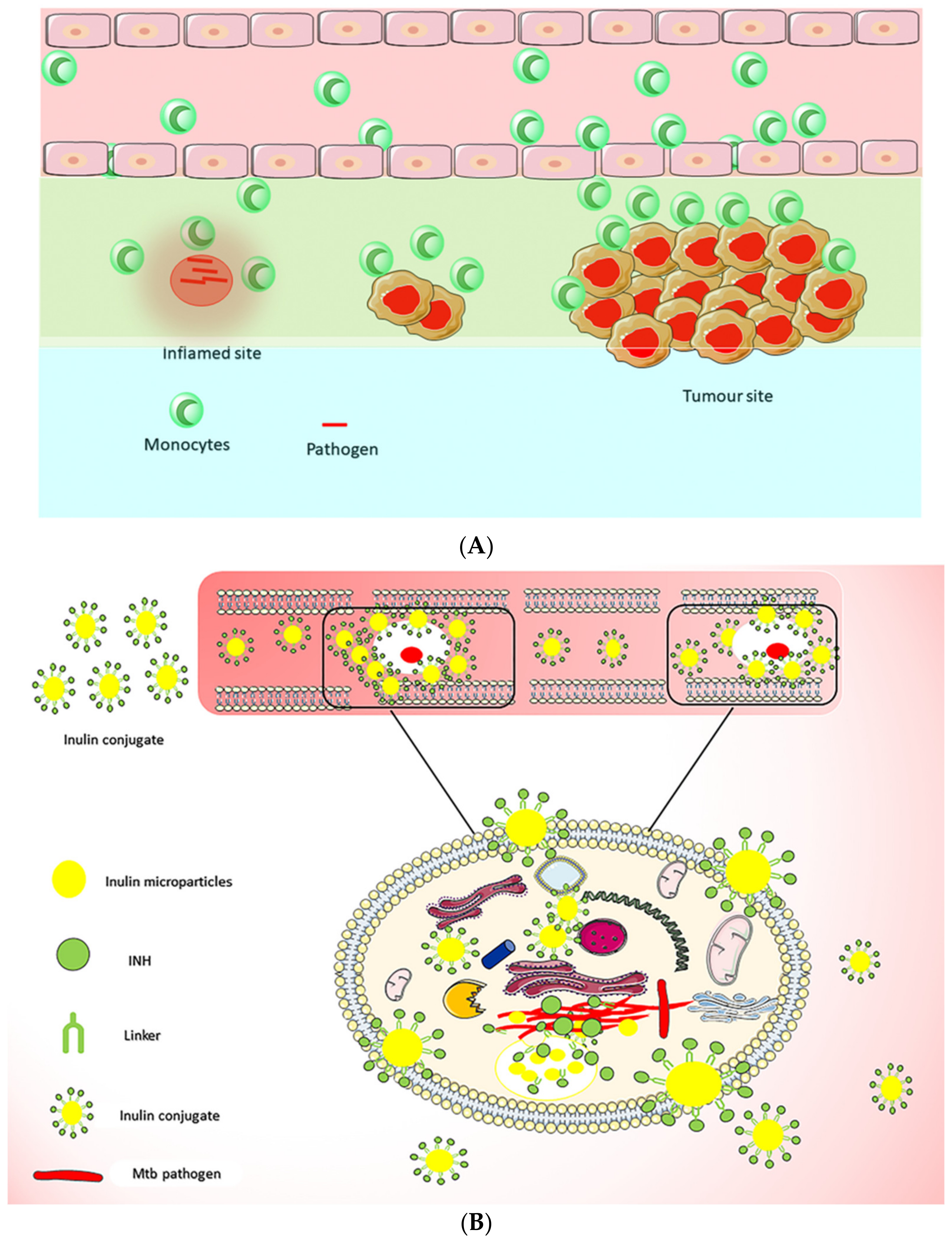
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Chung, R.; Song, Y.M.; Nagalingam, G.; Triccas, J.; Wang, L.; Liu, L.; Hayball, J.D.; et al. Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery. Pharmaceutics 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Song, Y.; Chung, R.; Wang, L.; Liu, L.; Hayball, J.D.; Petrovsky, N.; Garg, S. Design and Characterization of Inulin Con-jugate for Improved Intracellular and Targeted Delivery of Pyrazinoic Acid to Monocytes. Pharmaceutics 2019, 11, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Song, Y.; Parikh, A.; Joyce, P.; Chung, R.; Liu, L.; Afinjuomo, F.; Hayball, J.D.; Petrovsky, N.; Barclay, T.G.; et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics 2019, 11, 581. [Google Scholar] [CrossRef] [Green Version]
- Robert, P.; García, P.; Reyes, N.; Chávez, J.; Santos, J. Acetylated starch and inulin as encapsulating agents of gallic acid and their release behaviour in a hydrophilic system. Food Chem. 2012, 134, 1–8. [Google Scholar] [CrossRef]
- Wu, X.Y.; Lee, P.I. Preparation and characterization of inulin ester microspheres as drug carriers. J. Appl. Polym. Sci. 2000, 77, 833–840. [Google Scholar] [CrossRef]
- Poulain, N.; Dez, I.; Perrio, C.; Lasne, M.-C.; Prud’Homme, M.-P.; Nakache, E. Microspheres based on inulin for the controlled release of serine protease inhibitors: Preparation, characterization and in vitro release. J. Control. Release 2003, 92, 27–38. [Google Scholar] [CrossRef]
- Jain, A.K.; Sood, V.; Bora, M.; Vasita, R.; Katti, D.S. Electrosprayed inulin microparticles for microbiota triggered targeting of colon. Carbohydr. Polym. 2014, 112, 225–234. [Google Scholar] [CrossRef]
- Samuli, H.; Catherine, P.; Jean-Pierre, B. Passive and Active Tumour Targeting with Nanocarriers. Curr. Drug Dis. Cover. Technol. 2011, 8, 188–196. [Google Scholar]
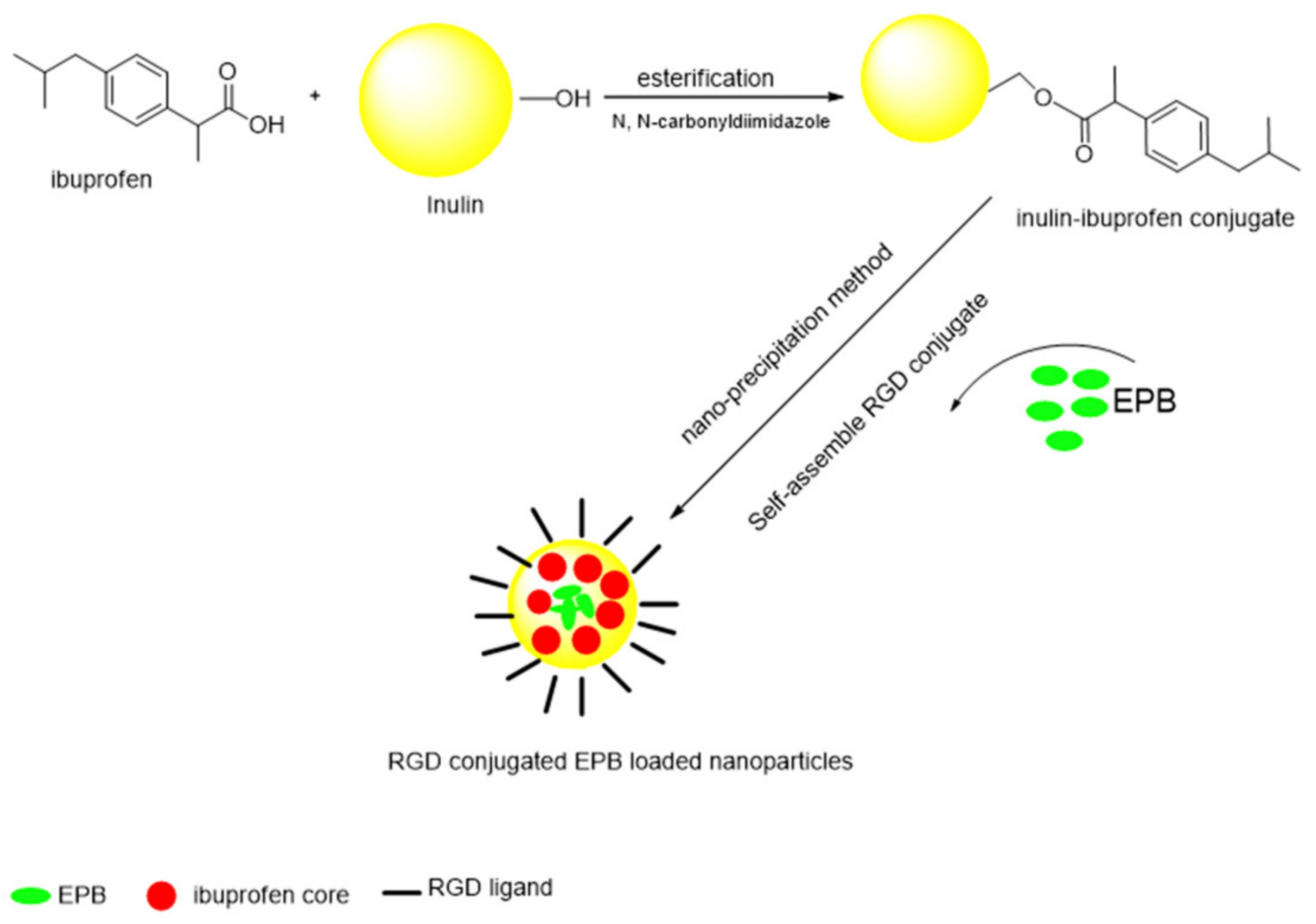
- Zhang, L.; Li, G.; Gao, M.; Liu, X.; Ji, B.; Hua, R.; Zhou, Y.; Yang, Y. RGD-peptide conjugated inulin-ibuprofen nanoparticles for targeted delivery of Epirubicin. Colloids Surf. B Biointerfaces 2016, 144, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Volsi, A.L.; Mauro, N.; Scialabba, C.; Cavallaro, G.; Giammona, G. Preparation and Characterization of Inulin Coated Gold Nanoparticles for Selective Delivery of Doxorubicin to Breast Cancer Cells. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mandal, S.; Selvakannan, P.R.; Pasricha, R.; Mandale, A.B.; Sastry, M. Investigation into the Interaction between Surface-Bound Alkylamines and Gold Nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thünemann, A.F.; Kraehnert, R. Mechanism of Gold Nanoparticle Formation in the Classical Citrate Synthesis Method Derived from Coupled In Situ XANES and SAXS Evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Volsi, A.L.; De Aberasturi, D.J.; Henriksen-Lacey, M.; Giammona, G.; Licciardi, M.; Liz-Marzán, L.M. Inulin coated plasmonic gold nanoparticles as a tumor-selective tool for cancer therapy. J. Mater. Chem. B 2016, 4, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Hitchens, T.K.; Ye, Q.; Wu, Y.; Barbe, B.; Prior, D.E.; Li, W.F.; Yeh, F.-C.; Foley, L.M.; Bain, D.J.; et al. Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano- and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3447–3453. [Google Scholar] [CrossRef] [Green Version]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef]
- Durán, J.; Arias, J.L.; Gallardo, V.; Delgado, A. Magnetic Colloids as Drug Vehicles. J. Pharm. Sci. 2008, 97, 2948–2983. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.-M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Kawasaki, E.S.; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 101–109. [Google Scholar] [CrossRef]
- Liang, X.-J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. CRISPR Cas Methods 2010, 596, 467–488. [Google Scholar]
- Laurent, S.; Saei, A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
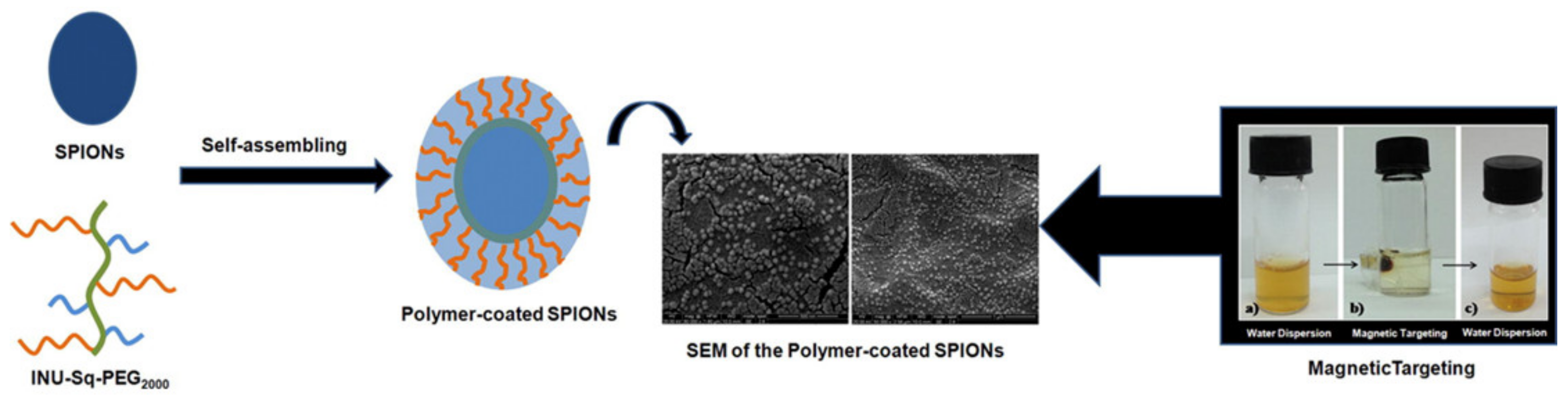
- Scialabba, C.; Licciardi, M.; Mauro, N.; Rocco, F.; Ceruti, M.; Giammona, G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur. J. Pharm. Biopharm. 2014, 88, 695–705. [Google Scholar] [CrossRef]
- Santiago-Rodríguez, L.; Lafontaine, M.M.; Castro, C.; Méndez-Vega, J.; Latorre-Esteves, M.; Juan, E.J.; Mora, E.; Torres-Lugo, M.; Rinaldi, C. Synthesis, stability, cellular uptake, and blood circulation time of carboxymethyl-inulin coated magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 2807–2817. [Google Scholar] [CrossRef] [Green Version]
- Scialabba, C.; Puleio, R.; Peddis, D.; Varvaro, G.; Calandra, P.; Cassata, G.; Cicero, L.; Licciardi, M.; Giammona, G. Folate targeted coated SPIONs as efficient tool for MRI. Nano Res. 2017, 10, 3212–3227. [Google Scholar] [CrossRef]
- Riley, M.K.; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery—A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy- An Overview. J. Clin. Diagn. Res. JCDR 2015, 9, GE01–GE6. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [Green Version]
- Licciardi, M.; Volsi, A.L.; Sardo, C.; Mauro, N.; Cavallaro, G.; Giammona, G. Inulin-Ethylenediamine Coated SPIONs Magnetoplexes: A Promising Tool for Improving siRNA Delivery. Pharm. Res. 2015, 32, 3674–3687. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, C.; Li, G.; Zhao, Y.; Yang, Y. Synthesis of methylprednisolone loaded ibuprofen modified inulin based nanoparticles and their application for drug delivery. Mater. Sci. Eng. C 2014, 42, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Scialabba, C.; Cavallaro, G.; Licciardi, M.; Giammona, G. Biotin-Containing Reduced Graphene Oxide-Based Nanosystem as a Multieffect Anticancer Agent: Combining Hyperthermia with Targeted Chemotherapy. Biomacromolecules 2015, 16, 2766–2775. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Srinarong, P.; Kouwen, S.; Visser, M.R.; Hinrichs, W.; Frijlink, H.W. Effect of drug-carrier interaction on the dissolution behavior of solid dispersion tablets. Pharm. Dev. Technol. 2009, 15, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Srinarong, P.; Hamalainen, S.; Visser, M.R.; Hinrichs, W.L.; Ketolainen, J.; Frijlink, H.W. Surface-active derivative of inulin (Inutec(R) SP1) is a superior carrier for solid dispersions with a high drug load. J. Pharm. Sci. 2011, 100, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Van Drooge, D.J.; Hinrichs, W.L.; Frijlink, H.W. Anomalous dissolution behaviour of tablets prepared from sugar glass-based solid dispersions. J. Control. Release 2004, 97, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.S.; Rijkeboer, M.; Jan van Drooge, D.; Sutter, M.; Jiskoot, W.; van de Weert, M.; Hinrichs, W.L.; Frijlink, H.W. Characterization of a cyclo-sporine solid dispersion for inhalation. AAPS J. 2007, 9, E190–E199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fares, M.M.; Salem, M.t.S.; Khanfar, M. Inulin and poly(acrylic acid) grafted inulin for dissolution enhancement and pre-liminary controlled release of poorly water-soluble Irbesartan drug. Int. J. Pharm. 2011, 410, 206–211. [Google Scholar] [CrossRef]
- Van Drooge, D.-J.; Hinrichs, W.L.J.; Dickhoff, B.H.J.; Elli, M.N.A.; Visser, M.R.; Zijlstra, G.S.; Frijlink, H.W. Spray freeze drying to produce a stable Δ9-tetrahydrocannabinol containing inulin-based solid dispersion powder suitable for inhalation. Eur. J. Pharm. Sci. 2005, 26, 231–240. [Google Scholar] [CrossRef]
- Fares, M.M.; Salem, M.T.S. Dissolution enhancement of curcumin via curcumin–prebiotic inulin nanoparticles. Drug Dev. Ind. Pharm. 2015, 41, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Weuts, I.; De Ridder, T.; Blaton, N. Evaluation of Inutec SP1 as a new carrier in the formulation of solid dispersions for poorly soluble drugs. Int. J. Pharm. 2006, 316, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.R.; Baert, L.; Klooster, G.V.T.; Schueller, L.; Geldof, M.; Vanwelkenhuysen, I.; De Kock, H.; De Meyer, S.; Frijlink, H.W.; Rosier, J.; et al. Inulin solid dispersion technology to improve the absorption of the BCS Class IV drug TMC. Eur. J. Pharm. Biopharm. 2010, 74, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Van Drooge, D.J.; Hinrichs, W.L.J.; Wegman, K.A.M.; Visser, M.R.; Eissens, A.C.; Frijlink, H.W. Solid dispersions based on inulin for the stabilisation and formulation of Δ9-tetrahydrocannabinol. Eur. J. Pharm. Sci. 2004, 21, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Kiumarsi, M.; Majchrzak, D.; Jäger, H.; Song, J.; Lieleg, O.; Shahbazi, M. Comparative study of instrumental properties and sensory profiling of low-calorie chocolate containing hydrophobically modified inulin. Part II: Proton mobility, topological, tribological and dynamic sensory properties. Food Hydrocol. 2021, 110, 106144. [Google Scholar] [CrossRef]
- Srinarong, P.; Pham, B.T.; Holen, M.; Van Der Plas, A.; Schellekens, R.C.; Hinrichs, W.L.; Frijlink, H.W. Preparation and physicochemical evaluation of a new tacrolimus tablet formulation for sublingual administration. Drug Dev. Ind. Pharm. 2011, 38, 490–500. [Google Scholar] [CrossRef]


| Delivery System | Drug Delivered | Method | Key Outcomes |
|---|---|---|---|
| Hydrogels and Beads, Vesicles, Pellets, tablets, and nano-hydrogel | NSAIDs, hormones, peptides, corticosteroids, anticancer, immunoglobulin G (IgG), bovine serum albumin, and lysozyme, MTX, 5-aminosalicylic acid, probiotics strains | radical polymerization radical copolymerization Michael addition crosslinking UV radiation, chemical crosslinkers solvent precipitation | Allow targeted and localized delivery of drugs to the colon |
| Micelles | curcumin and celecoxib rifampicin doxorubicin | Self-assembly micelles using modified inulin Amphiphilic micelles | Delivers high payload to cancer cells Use of biotin can also improve targeting |
| liposomes | Quercetin | Shealth Coating | Stabilizes liposome Coat liposome Liposome uptake marker |
| prodrugs | Metronidazole Procainamide 5-ASA | Conjugation chemistry ester and Schiff base prodrug | Improve stability Increase plasma half-life |
| Complexes/chelating agent | Gd–inulin complex iron supplements | Chelation and complex formation | Better contrasting agents |
| Microparticles | Vaccine adjuvants Isoniazid, pyrazinoic acid Doxorubicin Gallic acid Chlorhexidine Indomethacin | Conjugation chemistry coacervation technique Spray drying | Better cellular uptake Targeted delivery to infected cells |
| Nanoparticles Gold nanoparticles Supermagnetic nanoparticles (SPION) Self-assembly Nanostructured carbon sheet | Ibuprofen Doxorubicin Methylprednisolone | Modification of inulin via chemistry | Attaching ligand such as RGD, biotin improves cellular uptake Better targeting to cancer cells |
| Solid dispersion | Nifedipine Diazepam, fenofibrates Ritonavir, itraconazole, curcumin, Irbesartan | Inulin as hydrophilic polymer | Result in high loading without recrystallization Improve dissolution and physical stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. https://doi.org/10.3390/ph14090855
Afinjuomo F, Abdella S, Youssef SH, Song Y, Garg S. Inulin and Its Application in Drug Delivery. Pharmaceuticals. 2021; 14(9):855. https://doi.org/10.3390/ph14090855
Chicago/Turabian StyleAfinjuomo, Franklin, Sadikalmahdi Abdella, Souha H. Youssef, Yunmei Song, and Sanjay Garg. 2021. "Inulin and Its Application in Drug Delivery" Pharmaceuticals 14, no. 9: 855. https://doi.org/10.3390/ph14090855
APA StyleAfinjuomo, F., Abdella, S., Youssef, S. H., Song, Y., & Garg, S. (2021). Inulin and Its Application in Drug Delivery. Pharmaceuticals, 14(9), 855. https://doi.org/10.3390/ph14090855







