New Molecular Targets for Antidepressant Drugs
Abstract
1. Introduction
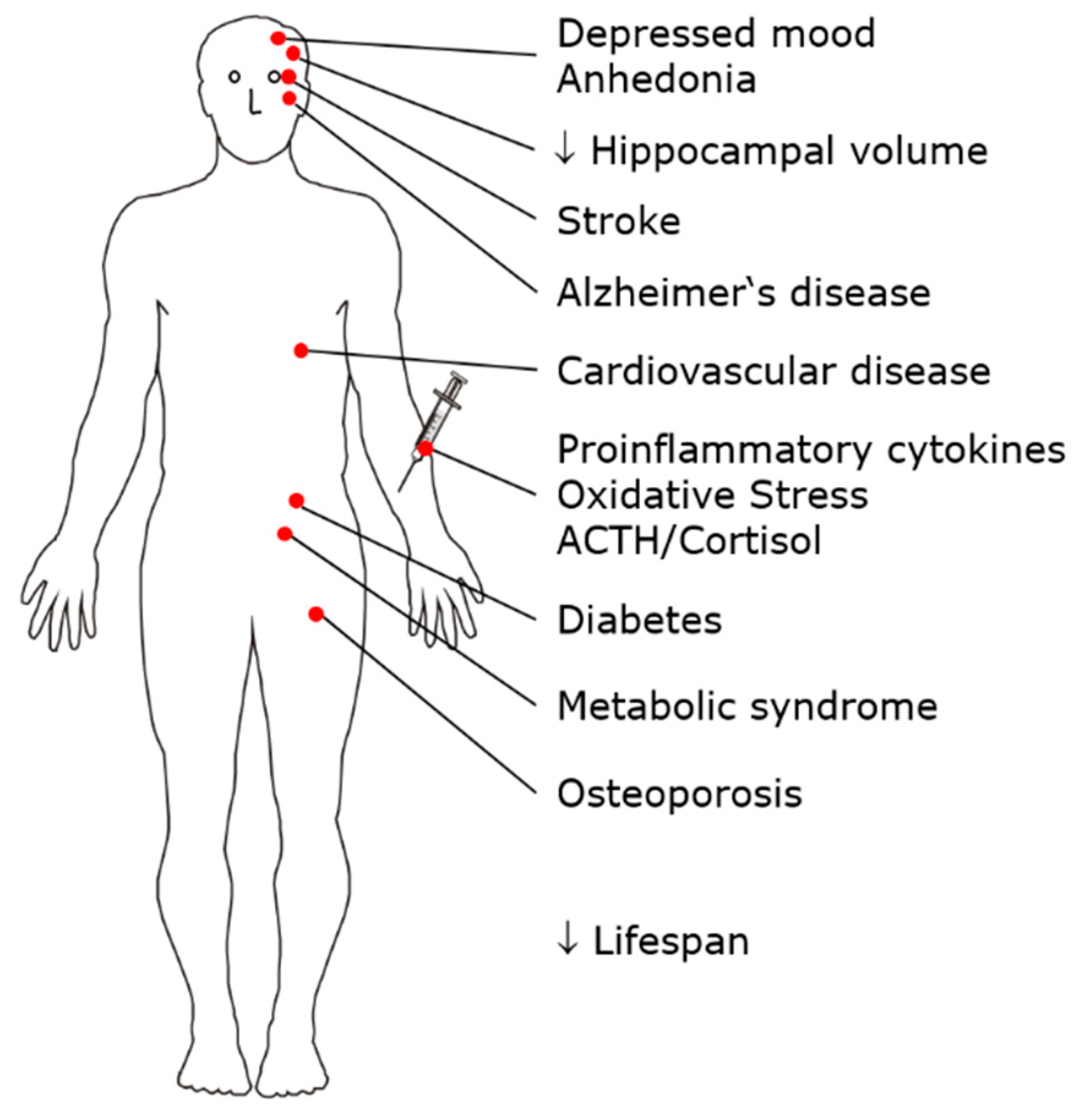
2. MDD as a Whole-Body Multisystem Disease
3. Current Pharmacological Treatments for MDD
4. Neurobiological Hypotheses of Depression
5. The Sphingolipid System
5.1. Altered Biophysical Properties of the Plasma Membrane
5.2. Direct Effects of Sphingolipids on Membrane Proteins
5.3. Mechanisms That Activate the ASM/Ceramide System
5.4. Functional Inhibitors of ASM (FIASMAs)
6. Sphingolipids in MDD
6.1. A Priori Arguments for Involvement of the ASM/Ceramide System in MDD
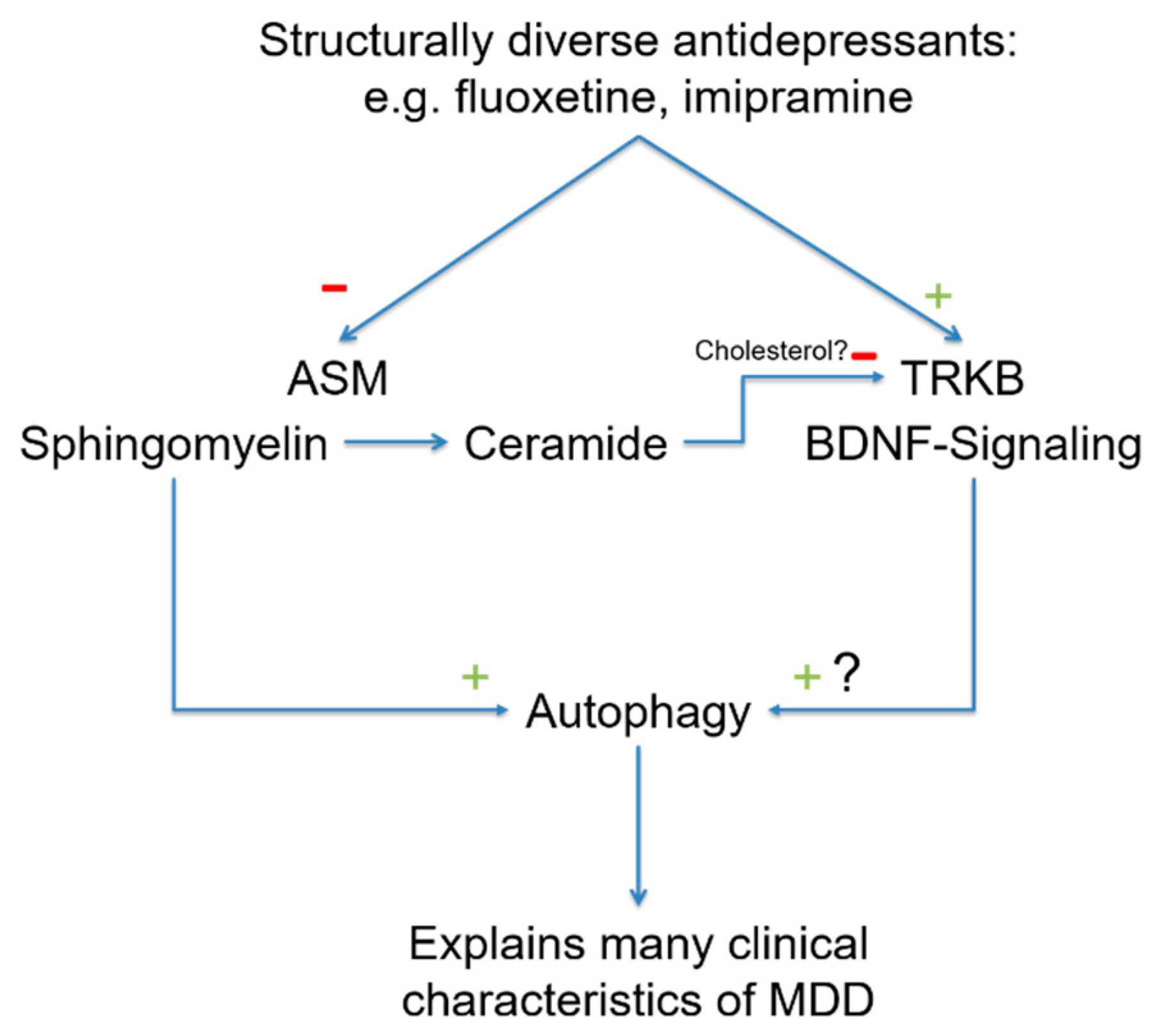
6.2. Behavioral Effects of Antidepressant Drugs Are Mediated by the ASM/Ceramide System
6.3. Autophagy
6.3.1. The Sphingomyelin-Ceramide System
6.3.2. Autophagy and the Clinical Characteristics of MDD
7. Sphingolipids as Therapeutic Targets
8. Other Mechanisms of Action
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornhuber, J.; Bormann, J.; Retz, W.; Hübers, M.; Riederer, P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur. J. Pharmacol. 1989, 166, 589–590. [Google Scholar] [CrossRef]
- Kornhuber, J.; Bormann, J.; Hübers, M.; Rusche, K.; Riederer, P. Effects of the 1-amino-adamantanes at the MK-801-binding site of the NMDA-receptor-gated ion channel: A human postmortem brain study. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 206, 297–300. [Google Scholar] [CrossRef]
- Kornhuber, J.; Herr, B.; Thome, J.; Riederer, P. The antiparkinsonian drug budipine binds to NMDA and sigma receptors in postmortem human brain tissue. J. Neural Transm. Suppl. 1995, 46, 131–137. [Google Scholar]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; Macdonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nat. Cell Biol. 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Ban, T.A. Pharmacotherapy of depression: A historical analysis. J. Neural Transm. 2001, 108, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.-F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nat. Cell Biol. 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kanes, S.; Colquhoun, H.; Gunduz-Bruce, H.; Raines, S.; Arnold, R.; Schacterle, A.; Doherty, J.; Epperson, C.N.; Deligiannidis, K.; Riesenberg, R.; et al. Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet 2017, 390, 480–489. [Google Scholar] [CrossRef]
- Zheng, W.; Cai, D.-B.; Sim, K.; Ungvari, G.S.; Peng, X.-J.; Ning, Y.-P.; Wang, G.; Xiang, Y.-T. Brexanolone for postpartum depression: A meta-analysis of randomized controlled studies. Psychiatry Res. 2019, 279, 83–89. [Google Scholar] [CrossRef]
- Gunduz-Bruce, H.; Silber, C.; Kaul, I.; Rothschild, A.J.; Riesenberg, R.; Sankoh, A.J.; Li, H.; Lasser, R.; Zorumski, C.F.; Rubinow, D.R.; et al. Trial of SAGE-217 in patients with major depressive disorder. N. Engl. J. Med. 2019, 381, 903–911. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Meltzer-Brody, S.; Gunduz-Bruce, H.; Doherty, J.; Jonas, J.; Li, S.; Sankoh, A.J.; Silber, C.; Campbell, A.D.; Werneburg, B.; et al. Effect of zuranolone vs placebo in postpartum depression: A randomized clinical trial. JAMA Psychiatry 2021, 78, 951–959. [Google Scholar] [CrossRef]
- Sanches, M.; Quevedo, J.; Soares, J.C. New agents and perspectives in the pharmacological treatment of major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110157. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Kornhuber, J.; Reichel, M.; Tripal, P.; Groemer, T.W.; Henkel, A.W.; Mühle, C.; Gulbins, E. The role of ceramide in major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013, 11, 129. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Cipriani, A.; Furukawa, T.; Salanti, G.; Chaimani, A.; Atkinson, L.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Fournier, J.C.; DeRubeis, R.J.; Hollon, S.D.; Dimidjian, S.; Amsterdam, J.D.; Shelton, R.C.; Fawcett, J. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA 2010, 303, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.; Werbeloff, N.; Mandel, F.S.; Menard, F.; Marangell, L.; Kapur, S. Initial depression severity and response to antidepressants v. placebo: Patient-level data analysis from 34 randomised controlled trials. Br. J. Psychiatry 2016, 209, 427–428. [Google Scholar] [CrossRef]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: Systematic review and network meta-analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef]
- Gelenberg, A.J.; Chesen, C.L. How fast are antidepressants? J. Clin. Psychiatry 2000, 61, 712–721. [Google Scholar] [CrossRef]
- Frazer, A.; Benmansour, S. Delayed pharmacological effects of antidepressants. Mol. Psychiatry 2002, 7, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J. Two-week delay in onset of action of antidepressants: New evidence. Br. J. Psychiatry 2006, 188, 105–106. [Google Scholar] [CrossRef]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 4–6. [Google Scholar]
- Brink, C.B.; Harvey, B.H.; Brand, L. Tianeptine: A novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Pat. CNS Drug Discov. 2006, 1, 29–41. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2019, 25, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Duman, R.S. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus 2006, 16, 239–249. [Google Scholar] [CrossRef]
- Marazziti, D.; Baroni, S.; Catena-Dell’Osso, M.; Schiavi, E.; Ceresoli, D.; Conversano, C.; Dell’Osso, L.; Picano, E. Cognitive, psychological and psychiatric effects of ionizing radiation exposure. Curr. Med. Chem. 2012, 19, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.V.; Meyer, J.P.; Farber, K.G.; Chen, X.R.; Rosenthal, B.D.; Kellner, C.H. Rapid response to electroconvulsive therapy: A case report and literature review. J. ECT 2017, 33, e20–e21. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; A Oren, D.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Gulbins, E.; Palmada, M.; Reichel, M.; Lüth, A.; Böhmer, C.; Amato, D.; Müller, C.P.; Tischbirek, C.H.; Groemer, T.W.; Tabatabai, G.; et al. Acid sphingomyelinase/ceramide system mediates effects of antidepressant drugs. Nat. Med. 2013, 19, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Tripal, P.; Reichel, M.; Mühle, C.; Rhein, C.; Muehlbacher, M.; Groemer, T.; Gulbins, E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A novel pharmacological group of drugs with broad clinical applications. Cell. Physiol. Biochem. 2010, 26, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 Signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; Von Kürthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The role of ceramide and sphingosine-1-phosphate in Alzheimer’s disease and other neurodegenerative disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Gulbins, E.; Kolesnick, R. Raft ceramide in molecular medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef]
- Kolesnick, R.N.; Goni, F.M.; Alonso, A. Compartmentalization of ceramide signaling: Physical foundations and biological effects. J. Cell. Physiol. 2000, 184, 285–300. [Google Scholar] [CrossRef]
- Nurminen, T.A.; Holopainen, J.M.; Zhao, H.; Kinnunen, P.K.J. Observation of topical catalysis by sphingomyelinase coupled to microspheres. J. Am. Chem. Soc. 2002, 124, 12129–12134. [Google Scholar] [CrossRef]
- Zeitler, S.; Ye, L.; Andreyeva, A.; Schumacher, F.; Monti, J.; Nürnberg, B.; Nowak, G.; Kleuser, B.; Reichel, M.; Fejtová, A.; et al. Acid sphingomyelinase—A regulator of canonical transient receptor potential channel 6 (TRPC6) activity. J. Neurochem. 2019, 150, 678–690. [Google Scholar] [CrossRef]
- Heiser, J.H.; Schuwald, A.M.; Sillani, G.; Ye, L.; Müller, W.E.; Leuner, K. TRPC6 channel-mediated neurite outgrowth in PC12 cells and hippocampal neurons involves activation of RAS/MEK/ERK, PI3K, and CAMKIV signaling. J. Neurochem. 2013, 127, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived neurotrophic factor and glucocorticoid stress in neurogenesis. Int. J. Mol. Sci. 2017, 18, 2312. [Google Scholar] [CrossRef]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, S.; Schumacher, F.; Monti, J.; Anni, D.; Guhathakurta, D.; Kleuser, B.; Friedland, K.; Fejtová, A.; Kornhuber, J.; Rhein, C. Acid sphingomyelinase impacts canonical Transient Receptor Potential Channels 6 (TRPC6) activity in primary neuronal systems. Cells 2020, 9, 2502. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Ernst, A.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nat. Cell Biol. 2012, 481, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jafurulla, M.; Tiwari, S.; Chattopadhyay, A. Identification of sphingolipid-binding motif in G protein-coupled receptors. Adv. Exp. Med. Biol. 2018, 1112, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Won, J.-S.; Singh, I. Sphingolipid signaling and redox regulation. Free Radic. Biol. Med. 2006, 40, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Li, P.L.; Zhang, Y. Cross talk between ceramide and redox signalling: Implications for endothelial dysfunction and renal disease. In Sphingolipids in Disease; Gulbins, E., Petrache, I., Eds.; Springer: Wien, Austria, 2013; pp. 171–197. [Google Scholar]
- Li, H.; Junk, P.; Huwiler, A.; Burkhardt, C.; Wallerath, T.; Pfeilschifter, J.; Forstermann, U. Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Carpinteiro, A.; Gulbins, E. Acid sphingomyelinase amplifies redox signaling in pseudomonas aeruginosa-induced macrophage apoptosis. J. Immunol. 2008, 181, 4247–4254. [Google Scholar] [CrossRef]
- Gulbins, E.; Li, P.L. Physiological and pathophysiological aspects of ceramide. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R11–R26. [Google Scholar] [CrossRef]
- Vaena, S.; Chakraborty, P.; Lee, H.G.; Janneh, A.H.; Kassir, M.F.; Beeson, G.; Hedley, Z.; Yalcinkaya, A.; Sofi, M.H.; Li, H.; et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep. 2021, 35, 109076. [Google Scholar] [CrossRef]
- Sakuragawa, N.; Kuwabara, T.; Pentchev, P.; Barranger, J.; Brady, R. Niemann-Pick disease experimental model: Sphingomyelinase reduction induced by AY-9944. Science 1977, 196, 317–319. [Google Scholar] [CrossRef]
- Albouz, S.; Hauw, J.J.; Berwald-Netter, Y.; Boutry, J.M.; Bourdon, R.; Baumann, N. Tricyclic antidepressants induce sphingomyelinase deficiency in fibroblast and neuroblastoma cell cultures. Biomedicine/[Publiee Pour l’A.A.I.C.I.G.] 1981, 35, 218–220. [Google Scholar]
- Yoshida, Y.; Arimoto, K.; Sato, M.; Sakuragawa, N.; Arima, M.; Satoyoshi, E. Reduction of acid sphingomyelinase activity in human fibroblasts induced by AY-9944 and other cationic amphiphilic drugs. J. Biochem. (Tokyo) 1985, 98, 1669–1679. [Google Scholar] [CrossRef]
- Kornhuber, J.; Tripal, P.; Reichel, M.; Terfloth, L.; Bleich, S.; Wiltfang, J.; Gulbins, E. Identification of new functional inhibitors of acid sphingomyelinase using a structure−property−activity relation model. J. Med. Chem. 2008, 51, 219–237. [Google Scholar] [CrossRef]
- Kornhuber, J.; Muehlbacher, M.; Trapp, S.; Pechmann, S.; Friedl, A.; Reichel, M.; Mühle, C.; Terfloth, L.; Groemer, T.; Spitzer, G.M.; et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 2011, 6, e23852. [Google Scholar] [CrossRef]
- Trapp, S.; Rosania, G.R.; Horobin, R.W.; Kornhuber, J. Quantitative modeling of selective lysosomal targeting for drug design. Eur. Biophys. J. 2008, 37, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Kölzer, M.; Werth, N.; Sandhoff, K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004, 559, 96–98. [Google Scholar] [CrossRef]
- Hurwitz, R.; Ferlinz, K.; Sandhoff, K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol. Chem. Hoppe-Seyler 1994, 375, 447–450. [Google Scholar] [CrossRef]
- Cassano, G.B.; Hansson, E. Distribution and fate of C14-amitriptyline in mice and rats. Psychopharmacol. 1965, 8, 1–11. [Google Scholar] [CrossRef]
- Bynum, N.D.; Poklis, J.L.; Gaffney-Kraft, M.; Garside, D.; Ropero-Miller, J.D. Postmortem distribution of tramadol, amitriptyline, and their metabolites in a suicidal overdose. J. Anal. Toxicol. 2005, 29, 401–406. [Google Scholar] [CrossRef]
- Hilberg, T.; Mørland, J.; Bjørneboe, A. Postmortem release of amitriptyline from the lungs; a mechanism of postmortem drug redistribution. Forensic Sci. Int. 1994, 64, 47–55. [Google Scholar] [CrossRef]
- Rhein, C.; Mühle, C.; Tüfek, Ö.; Muehlbacher, M.; Loeber, S.; Pettermann, L.; Thomas, H.; Gmeiner, P.; Tripal, P.; Kornhuber, J. Derivatizations of antidepressant drugs improve inhibition of acid sphingomyelinase and risk of phospholipidosis. J. Neural Transm. 2018, 125, 1837–1845. [Google Scholar] [CrossRef]
- Riethmüller, J.; Anthonysamy, J.; Serra, E.; Schwab, M.; Döring, G.; Gulbins, E. Therapeutic efficacy and safety of amitriptyline in patients with cystic fibrosis. Cell. Physiol. Biochem. 2009, 24, 65–72. [Google Scholar] [CrossRef]
- Nährlich, L.; Mainz, J.G.; Adams, C.; Engel, C.; Herrmann, G.; Icheva, V.; Lauer, J.; Deppisch, C.; Wirth, A.; Unger, K.; et al. Therapy of CF-patients with amitriptyline and placebo—A randomised, double-blind, placebo-controlled phase IIb multicenter, cohort-study. Cell. Physiol. Biochem. 2013, 31, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Riethmüller, J.; Gulbins, E. Biological aspects of ceramide-enriched membrane domains. Prog. Lipid Res. 2007, 46, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Lanctôt, K.L. Ceramides and depression: A systematic review. J. Affect. Disord. 2017, 213, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Saleem, M.; Herrmann, N.; Mielke, M.M.; Oh, P.I.; Venkata, S.L.V.; Haughey, N.J.; Lanctôt, K.L. Plasma sphingolipids and depressive symptoms in coronary artery disease. Brain Behav. 2017, 7, e00836. [Google Scholar] [CrossRef]
- Reichel, M.; Rhein, C.; Hofmann, L.M.; Monti, J.; Japtok, L.; Langgartner, D.; Füchsl, A.M.; Kleuser, B.; Gulbins, E.; Hellerbrand, C.; et al. Chronic psychosocial stress in mice is associated with increased acid sphingomyelinase activity in liver and serum and with hepatic C16:0-ceramide accumulation. Front. Psychiatry 2018, 9, 496. [Google Scholar] [CrossRef]
- Brunkhorst-Kanaan, N.; Klatt-Schreiner, K.; Hackel, J.; Schröter, K.; Trautmann, S.; Hahnefeld, L.; Wicker, S.; Reif, A.; Thomas, D.; Geisslinger, G.; et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism 2019, 95, 65–76. [Google Scholar] [CrossRef]
- Mühle, C.; Wagner, C.J.; Färber, K.; Richter-Schmidinger, T.; Gulbins, E.; Lenz, B.; Kornhuber, J. Secretory acid sphingomyelinase in the serum of medicated patients predicts the prospective course of depression. J. Clin. Med. 2019, 8, 846. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Schumacher, F.; Kleuser, B.; Reichel, M.; Gulbins, E.; Fejtová, A.; Kornhuber, J.; Rhein, C. The forebrain-specific overexpression of acid sphingomyelinase induces depressive-like symptoms in mice. Cells 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Jaddoa, E.; Masania, J.; Masiero, E.; Sgamma, T.; Arroo, R.; Sillence, D.; Zetterström, T. Effect of antidepressant drugs on the brain sphingolipid system. J. Psychopharmacol. 2020, 34, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, J.; Nong, K.; Wang, Y.; Niu, A.; Chen, W.; Dong, J.; Wang, J. Discovery of potent, selective, and direct acid sphingomyelinase inhibitors with antidepressant activity. J. Med. Chem. 2020, 63, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Hongmei, W.; Leijie, X.; Dandan, Z.; Peng, L.; Zhifei, H.; Ruimin, M.; Yijun, S.; Guanghui, Z.; Guojun, Z. Serum ceramide concentrations are associated with depression in patients after ischemic stroke-A two-center case-controlled study. Clin. Chim. Acta 2021, 518, 110–115. [Google Scholar] [CrossRef]
- Berkowitz, L.; Henríquez, M.P.; Salazar, C.; Rojas, E.; Echeverría, G.; Love, G.D.; Rigotti, A.; Coe, C.L.; Ryff, C.D. Association between serum sphingolipids and eudaimonic well-being in white U.S. adults. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef]
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Illes, K.; Heinz, L.; Superti-Furga, L.X.H.G.; Nagar, A.G.K.I.B. Crystal structure of mammalian acid sphingomyelinase. Nat. Commun. 2016, 7, 12196. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-J.; Huang, J.; Poda, G.; Pomès, R.; Privé, G.G. Structure of human acid sphingomyelinase reveals the role of the saposin domain in activating substrate hydrolysis. J. Mol. Biol. 2016, 428, 3026–3042. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Metcalf, M.C.; Garman, S.C.; Edmunds, T.; Qiu, H.; Wei, R.R. Human acid sphingomyelinase structures provide insight to molecular basis of Niemann–Pick disease. Nat. Commun. 2016, 7, 13082. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, A.; Schumacher, F.; Becker, K.A.; Wilker, B.; Soddemann, M.; Boldrin, F.; Müller, C.P.; Edwards, M.J.; Goodman, M.; Caldwell, C.; et al. Antidepressants act by inducing autophagy controlled by sphingomyelin–ceramide. Mol. Psychiatry 2018, 23, 2324–2346. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Zschocke, J.; Stepan, J.; Hafner, K.; Zellner, A.; Kirmeier, T.; Kollmannsberger, L.; Wagner, K.V.; Dedic, N.; et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: Evidence in cells, mice, and humans. PLoS Med. 2014, 11, e1001755. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, L.; Guo, X.-D.; Cao, L.-L.; Xue, T.-F.; Zhao, X.-J.; Yang, D.-D.; Yang, J.; Xiao-Jie, Z.; Huang, J.-Y.; et al. Rosiglitazone exerts an anti-depressive effect in unpredictable chronic mild-stress-induced depressive mice by maintaining essential neuron autophagy and inhibiting excessive astrocytic apoptosis. Front. Mol. Neurosci. 2017, 10, 293. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Z.; Du, X.; Davies, H.; Huo, X.; Fang, M. miR-16 and fluoxetine both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Front. Neurosci. 2017, 11, 428. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Jiang, R.; Sun, G.; Shen, J.; Ma, M.; Ma, C.; Zhang, S.; Huang, Z.; Wu, Q.; et al. The antidepressant effects of α-tocopherol are related to activation of autophagy via the AMPK/mTOR pathway. Eur. J. Pharmacol. 2018, 833, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.B.; Dhamija, S. Beclin 1 phosphorylation–at the center of autophagy regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef]
- Pasquier, B. Autophagy inhibitors. Cell. Mol. Life Sci. 2016, 73, 985–1001. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F.; Gusain, A. Tricyclodecan-9-yl-xanthogenate (D609) mechanism of actions: A mini-review of literature. Neurochem. Res. 2012, 37, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Luberto, C.; Hannun, Y.A. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. J. Biol. Chem. 1998, 273, 14550–14559. [Google Scholar] [CrossRef] [PubMed]
- Milhas, D.; Andrieu-Abadie, N.; Levade, T.; Benoist, H.; Ségui, B. The tricyclodecan-9-yl-xanthogenate D609 triggers ceramide increase and enhances FasL-induced caspase-dependent and -independent cell death in T lymphocytes. Int. J. Mol. Sci. 2012, 13, 8834–8852. [Google Scholar] [CrossRef]
- Adachi, R.; Ogawa, K.; Matsumoto, S.-I.; Satou, T.; Tanaka, Y.; Sakamoto, J.; Nakahata, T.; Okamoto, R.; Kamaura, M.; Kawamoto, T. Discovery and characterization of selective human sphingomyelin synthase 2 inhibitors. Eur. J. Med. Chem. 2017, 136, 283–293. [Google Scholar] [CrossRef]
- Mo, M.; Yang, J.; Jiang, X.-C.; Cao, Y.; Fei, J.; Chen, Y.; Qi, X.; Chu, Y.; Zhou, L.; Ye, D. Discovery of 4-benzyloxybenzo[d]isoxazole-3-amine derivatives as highly selective and orally efficacious human sphingomyelin synthase 2 inhibitors that reduce chronic inflammation in db/db Mice. J. Med. Chem. 2018, 61, 8241–8254. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Lou, B.; Ye, D.; Qi, X.; Li, X.; Hu, S.; Ding, T.; Chen, Y.; Cao, Y.; et al. Discovery, synthesis and anti-atherosclerotic activities of a novel selective sphingomyelin synthase 2 inhibitor. Eur. J. Med. Chem. 2019, 163, 864–882. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, T.; Zhen, X.; Li, Y.; Mo, M.; Ye, D.; Cheng, N. A selective sphingomyelin synthase 2 inhibitor ameliorates diet induced insulin resistance via the IRS-1/Akt/GSK-3b signaling pathway. Pharmazie 2019, 74, 553–558. [Google Scholar]
- Sentelle, R.D.; Senkal, C.E.; Jiang, W.; Ponnusamy, S.; Gencer, S.; Selvam, S.P.; Ramshesh, V.K.; Peterson, Y.K.; LeMasters, J.J.; Szulc, Z.M.; et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012, 8, 831–838. [Google Scholar] [CrossRef]
- Casares-Crespo, L.; Calatayud-Baselga, I.; García-Corzo, L.; Mira, H. On the role of basal autophagy in adult neural stem cells and neurogenesis. Front. Cell. Neurosci. 2018, 12, 339. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cornelissen-Guillaume, G.G.; He, J.; Kastin, A.J.; Harrison, L.M.; Pan, W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiol. Int. 2016, 33, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef]
- Nakamura, S.; Yoshimori, T. Autophagy and longevity. Mol. Cells 2018, 41, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, S.; Yuan, Z.; Shen, L. Weighted gene coexpression network analysis identifies specific modules and hub genes related to major depression. Neuropsychiatr. Dis. Treat. 2020, 16, 703–713. [Google Scholar] [CrossRef]
- Zhao, K.-X.; Huang, C.-Q.; Xiao, Q.; Gao, Y.; Liu, Q.-X.; Wang, Z.-R.; Li, Y.-H.; Xie, Y.-Z. Age and risk for depression among the elderly: A meta-analysis of the published literature. CNS Spectr. 2012, 17, 142–154. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef]
- Loeffler, D.A. Influence of normal aging on brain autophagy: A complex scenario. Front. Aging Neurosci. 2019, 11, 49. [Google Scholar] [CrossRef]
- Shang, D.; Wang, L.; Klionsky, D.J.; Cheng, H.; Zhou, R. Sex differences in autophagy-mediated diseases: Toward precision medicine. Autophagy 2021, 17, 1065–1076. [Google Scholar] [CrossRef]
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human umbilical endothelial cells (HUVECs) have a sex: Characterisation of the phenotype of male and female cells. Biol. Sex Differ. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Zschocke, J.; Zimmermann, N.; Berning, B.; Ganal, V.; Holsboer, F.; Rein, T. Antidepressant drugs diversely affect autophagy pathways in astrocytes and neurons—Dissociation from cholesterol homeostasis. Neuropsychopharmacology 2011, 36, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Rein, T. Is there a role of autophagy in depression and antidepressant action? Front. Psychiatry 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Heiseke, A.; Aguib, Y.; Riemer, C.; Baier, M.; Schatzl, H. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J. Neurochem. 2009, 109, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wei, L.; Yu, S.; Jiang, S.; Ma, Y.; Zhang, C.; Wang, J.; Gao, Z.; Wan, F.; Zhuang, G.; et al. Ketamine induces reactive oxygen species and enhances autophagy in SV-HUC-1 human uroepithelial cells. J. Cell. Physiol. 2019, 234, 2778–2787. [Google Scholar] [CrossRef]
- Li, P.; Hao, X.-C.; Luo, J.; Lv, F.; Wei, K.; Min, S. Propofol mitigates learning and memory impairment after electroconvulsive shock in depressed rats by inhibiting autophagy in the hippocampus. Med. Sci. Monit. 2016, 22, 1702–1708. [Google Scholar] [CrossRef][Green Version]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Hallgren, M.; Meyer, J.D.; Lyons, M.; Herring, M.P. Association of efficacy of resistance exercise training with depressive symptoms: Meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry 2018, 75, 566–576. [Google Scholar] [CrossRef]
- He, C.; Sumpter, J.R.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551. [Google Scholar] [CrossRef]
- Josefsson, T.; Lindwall, M.; Archer, T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scand. J. Med. Sci. Sports 2013, 24, 259–272. [Google Scholar] [CrossRef]
- Andreotti, D.Z.; Silva, J.D.N.; Matumoto, A.M.; Orellana, A.M.; De Mello, P.S.; Kawamoto, E.M. Effects of physical exercise on autophagy and apoptosis in aged brain: Human and animal studies. Front. Nutr. 2020, 7, 94. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef]
- Shafiei, F.; Moghaddam, A.S.; Larijani, B.; Esmaillzadeh, A. Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta-analysis of observational studies. Nutr. Rev. 2019, 77, 230–239. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.; Solmi, M.; Stubbs, B.; Schuch, F.; Carvalho, A.F.; Jacka, F.; et al. The Effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Fang, X.; Ge, K.; Song, C.; Ge, Y.; Zhang, J. Effects of n-3PUFAs on autophagy and inflammation of hypothalamus and body weight in mice. Biochem. Biophys. Res. Commun. 2018, 501, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Corella, L.; Coltell, O.; Macian, F.; Ordovás, J.M. Advances in understanding the molecular basis of the Mediterranean diet effect. Annu. Rev. Food Sci. Technol. 2018, 9, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Roth, L.; Schrijvers, D.M.; De Meyer, G.R.Y.; Martinet, W. Defective autophagy in atherosclerosis: To die or to senesce? Oxidative Med. Cell Longev. 2018, 2018, 7687083. [Google Scholar] [CrossRef]
- Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Ein, N.; Armstrong, B.; Vickers, K. The effect of a very low calorie diet on subjective depressive symptoms and anxiety: Meta-analysis and systematic review. Int. J. Obes. 2018, 43, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Fan, J.; Ge, T.; Tang, L.; Li, B. The mechanism of acute fasting-induced antidepressant-like effects in mice. J. Cell. Mol. Med. 2017, 22, 223–229. [Google Scholar] [CrossRef]
- Lozano, J.; Morales, A.; Cremesti, A.; Fuks, Z.; Tilly, J.L.; Schuchman, E.; Gulbins, E.; Kolesnick, R. Niemann–Pick Disease versus acid sphingomyelinase deficiency. Cell Death Differ. 2001, 8, 100–102. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef] [PubMed]
- London, M.; London, E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): Implications for lipid raft structure and function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar]
- Liu, H.Y.; Wei, H.J.; Wu, L.; Liu, S.M.; Tang, Y.Y.; Zou, W.; Wang, C.Y.; Zhang, P.; Tang, X.Q. BDNF-TrkB pathway mediates antidepressant-like roles of H2S in diabetic rats via promoting hippocampal autophagy. Clin. Exp. Pharmacol. Physiol. 2020, 47, 302–312. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornhuber, J.; Gulbins, E. New Molecular Targets for Antidepressant Drugs. Pharmaceuticals 2021, 14, 894. https://doi.org/10.3390/ph14090894
Kornhuber J, Gulbins E. New Molecular Targets for Antidepressant Drugs. Pharmaceuticals. 2021; 14(9):894. https://doi.org/10.3390/ph14090894
Chicago/Turabian StyleKornhuber, Johannes, and Erich Gulbins. 2021. "New Molecular Targets for Antidepressant Drugs" Pharmaceuticals 14, no. 9: 894. https://doi.org/10.3390/ph14090894
APA StyleKornhuber, J., & Gulbins, E. (2021). New Molecular Targets for Antidepressant Drugs. Pharmaceuticals, 14(9), 894. https://doi.org/10.3390/ph14090894





