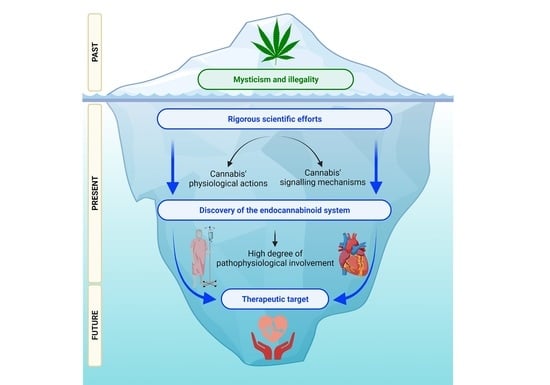
The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases
Abstract
:1. Introduction
2. The Endogenous Cannabinoid System
2.1. Endocannabinoid Metabolism
2.2. Cannabinoid Receptors
2.3. Beyond CBRs
2.4. CBR Signalling
2.5. Functions of the ECS
3. The ECS and CVDs
3.1. Myocardial Ischaemia/Reperfusion Syndrome
3.2. Atherosclerosis
3.3. Hypertension
3.4. Cardiomyopathy and Heart Failure
3.4.1. Diabetic Cardiomyopathy
3.4.2. Chemotherapy-Linked Cardiomyopathy
3.4.3. Hepatic Cirrhosis Cardiomyopathy
3.4.4. Septic Shock Cardiomyopathy and Myocarditis
3.4.5. Stress Cardiomyopathy
3.5. Arrhythmias
4. Clinical Trials
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, T.R. A Dictionary of Assyrian Botany; The British Academy: London, UK, 1949. [Google Scholar]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Gerard, C.M.; Mollereau, C.; Vassart, G.; Parmentier, M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 1991, 279 Pt 1, 129–134. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Steffens, S.; Hasko, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Montecucco, F.; Di Marzo, V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol. Sci. 2012, 33, 331–340. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Biro, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.M.; Tesmer, J.J. Structural insights into phospholipase C-beta function. Mol. Pharmacol. 2013, 84, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.J. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013, 280, 1895–1904. [Google Scholar] [CrossRef]
- Fowler, C.J. Anandamide uptake explained? Trends Pharmacol. Sci. 2012, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Dainese, E.; Oddi, S. Intracellular trafficking of anandamide: New concepts for signaling. Trends Biochem. Sci. 2010, 35, 601–608. [Google Scholar] [CrossRef]
- Rimmerman, N.; Hughes, H.V.; Bradshaw, H.B.; Pazos, M.X.; Mackie, K.; Prieto, A.L.; Walker, J.M. Compartmentalization of endocannabinoids into lipid rafts in a dorsal root ganglion cell line. Br. J. Pharmacol. 2008, 153, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Barnett-Norris, J.; Lynch, D.; Reggio, P.H. Lipids, lipid rafts and caveolae: Their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005, 77, 1625–1639. [Google Scholar] [CrossRef]
- McFarland, M.J.; Porter, A.C.; Rakhshan, F.R.; Rawat, D.S.; Gibbs, R.A.; Barker, E.L. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J. Biol. Chem. 2004, 279, 41991–41997. [Google Scholar] [CrossRef] [Green Version]
- Yates, M.L.; Barker, E.L. Organized trafficking of anandamide and related lipids. Vitam. Horm. 2009, 81, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.R.; Satomi, S.; Kato, Y.; Patel, H.H. The caveolar-mitochondrial interface: Regulation of cellular metabolism in physiology and pathophysiology. Biochem. Soc. Trans. 2020, 48, 165–177. [Google Scholar] [CrossRef]
- Gratton, J.P.; Bernatchez, P.; Sessa, W.C. Caveolae and caveolins in the cardiovascular system. Circ. Res. 2004, 94, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Judina, A.; Gorelik, J.; Wright, P.T. Studying signal compartmentation in adult cardiomyocytes. Biochem. Soc. Trans. 2020, 48, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanon, V.P.; Sawaki, D.; Mjaatvedt, C.H.; Jourdan-Le Saux, C. Myocardial tissue caveolae. Compr. Physiol. 2015, 5, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Busquets Garcia, A.; Soria-Gomez, E.; Bellocchio, L.; Marsicano, G. Cannabinoid receptor type-1: Breaking the dogmas. F1000Research 2016, 5, F1000 Faculty Rev-990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Chin, C.L.; Tovcimak, A.E.; Hradil, V.P.; Seifert, T.R.; Hollingsworth, P.R.; Chandran, P.; Zhu, C.Z.; Gauvin, D.; Pai, M.; Wetter, J.; et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. Br. J. Pharmacol. 2008, 153, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Kasacka, I.; Piotrowska, Z.; Filipek, A.; Lebkowski, W. Comparative evaluation of cannabinoid receptors, apelin and S100A6 protein in the heart of women of different age groups. BMC Cardiovasc. Disord. 2018, 18, 190. [Google Scholar] [CrossRef] [Green Version]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Piotrowska, Z.; Niezgoda, M.; Lebkowski, W.; Filipek, A.; Domian, N.; Kasacka, I. Sex differences in distribution of cannabinoid receptors (CB1 and CB2), S100A6 and CacyBP/SIP in human ageing hearts. Biol. Sex Differ. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benyo, Z.; Ruisanchez, E.; Leszl-Ishiguro, M.; Sandor, P.; Pacher, P. Endocannabinoids in cerebrovascular regulation. Am. J. Physiol. Heart C 2016, 310, H785–H801. [Google Scholar] [CrossRef] [Green Version]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef] [Green Version]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv. Pharmacol. 2017, 80, 223–247. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.K.; Devi, L.A. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol. Rev. 2011, 63, 461–470. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Denovan-Wright, E.M. Cannabinoid receptor ligand bias: Implications in the central nervous system. Curr. Opin. Pharmacol. 2017, 32, 32–43. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Starowicz, K.; Moriello, A.S.; Vivese, M.; Orlando, P.; Di Marzo, V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): Effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007, 313, 1911–1920. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Ruiz-Llorente, L.; Sanchez, A.M.; Diaz-Laviada, I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell. Signal. 2003, 15, 851–859. [Google Scholar] [CrossRef]
- Patel, M.; Finlay, D.B.; Glass, M. Biased agonism at the cannabinoid receptors—Evidence from synthetic cannabinoid receptor agonists. Cell. Signal. 2021, 78, 109865. [Google Scholar] [CrossRef]
- Kearn, C.S.; Greenberg, M.J.; DiCamelli, R.; Kurzawa, K.; Hillard, C.J. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J. Neurochem. 1999, 72, 2379–2387. [Google Scholar] [CrossRef] [Green Version]
- Vasquez, C.; Lewis, D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 9271–9280. [Google Scholar] [CrossRef] [Green Version]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Zottola, A.C.P.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Irving, A.J.; Hundal, H.S. Mitochondria: A possible nexus for the regulation of energy homeostasis by the endocannabinoid system? Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1–E13. [Google Scholar] [CrossRef] [Green Version]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Delta9-THC, CBN, CBGA, CBDA and Delta9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimian Azari, E.; Kerrigan, A.; O’Connor, A. Naturally Occurring Cannabinoids and their Role in Modulation of Cardiovascular Health. J. Diet. Suppl. 2020, 17, 625–650. [Google Scholar] [CrossRef]
- Liu, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Wagner, J.A.; Cravatt, B.F.; Gao, B.; Kunos, G. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J. Biol. Chem. 2003, 278, 45034–45039. [Google Scholar] [CrossRef] [Green Version]
- Lepicier, P.; Bouchard, J.F.; Lagneux, C.; Lamontagne, D. Endocannabinoids protect the rat isolated heart against ischaemia. Br. J. Pharmacol. 2003, 139, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, J.F.; Lepicier, P.; Lamontagne, D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003, 72, 1859–1870. [Google Scholar] [CrossRef] [Green Version]
- Kaschina, E. Cannabinoid CB1/CB2 Receptors in the Heart: Expression, Regulation, and Function. In Cannabinoids in Health and Disease; InTechOpen: London, UK, 2016. [Google Scholar]
- Fulmer, M.L.; Thewke, D.P. The Endocannabinoid System and Heart Disease: The Role of Cannabinoid Receptor Type 2. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 34–51. [Google Scholar] [CrossRef]
- Di Filippo, C.; Rossi, F.; Rossi, S.; D’Amico, M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 2004, 75, 453–459. [Google Scholar] [CrossRef]
- Lagneux, C.; Lamontagne, D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br. J. Pharmacol. 2001, 132, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Joyeux, M.; Arnaud, C.; Godin-Ribuot, D.; Demenge, P.; Lamontagne, D.; Ribuot, C. Endocannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc. Res. 2002, 55, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Duerr, G.D.; Heinemann, J.C.; Suchan, G.; Kolobara, E.; Wenzel, D.; Geisen, C.; Matthey, M.; Passe-Tietjen, K.; Mahmud, W.; Ghanem, A.; et al. The endocannabinoid-CB2 receptor axis protects the ischemic heart at the early stage of cardiomyopathy. Basic Res. Cardiol. 2014, 109, 425. [Google Scholar] [CrossRef]
- Heinemann, J.C.; Duerr, G.D.; Keppel, K.; Breitbach, M.; Fleischmann, B.K.; Zimmer, A.; Wehner, S.; Welz, A.; Dewald, O. CB2 receptor-mediated effects of pro-inflammatory macrophages influence survival of cardiomyocytes. Life Sci. 2015, 138, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Neumann, D.; Glatz, J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids 2019, 140, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Karsak, M.; Zimmer, A.; Frossard, J.L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef]
- Takeda, S.; Jiang, R.; Aramaki, H.; Imoto, M.; Toda, A.; Eyanagi, R.; Amamoto, T.; Yamamoto, I.; Watanabe, K. Delta9-tetrahydrocannabinol and its major metabolite Delta9-tetrahydrocannabinol-11-oic acid as 15-lipoxygenase inhibitors. J. Pharm. Sci. 2011, 100, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.K.; Srivastava, I.; Szlenk, C.T.; Muench, T.R.; Natesan, S.; Ahmed, S. Cannabinoid Receptor 2 Agonist JWH-015 Inhibits Interleukin-1beta-Induced Inflammation in Rheumatoid Arthritis Synovial Fibroblasts and in Adjuvant Induced Arthritis Rat via Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Alfulaij, N.; Meiners, F.; Michalek, J.; Small-Howard, A.L.; Turner, H.C.; Stokes, A.J. Cannabinoids, the Heart of the Matter. J. Am. Heart Assoc. 2018, 7, e009099. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Hasko, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dol-Gleizes, F.; Paumelle, R.; Visentin, V.; Mares, A.M.; Desitter, P.; Hennuyer, N.; Gilde, A.; Staels, B.; Schaeffer, P.; Bono, F. Rimonabant, a selective cannabinoid CB1 receptor antagonist, inhibits atherosclerosis in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinmark, S.J.; Cornicelli, J.A. Is there a role for 15-lipoxygenase in atherogenesis? Biochem. Pharmacol. 1997, 54, 953–959. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Martin Gimenez, V.M.; Noriega, S.E.; Kassuha, D.E.; Fuentes, L.B.; Manucha, W. Anandamide and endocannabinoid system: An attractive therapeutic approach for cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Huestis, M.A. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am. Heart J. 2006, 151, 754.e1–754.e5. [Google Scholar] [CrossRef]
- Lake, K.D.; Compton, D.R.; Varga, K.; Martin, B.R.; Kunos, G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997, 281, 1030–1037. [Google Scholar]
- Batkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertaler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, K.D.; Martin, B.R.; Kunos, G.; Varga, K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 1997, 29, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Bohme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef]
- Pacher, P.; Batkai, S.; Osei-Hyiaman, D.; Offertaler, L.; Liu, J.; Harvey-White, J.; Brassai, A.; Jarai, Z.; Cravatt, B.F.; Kunos, G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart C 2005, 289, H533–H541. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Kwolek, G.; Gothert, M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2001, 364, 562–569. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef]
- van Esbroeck, A.C.M.; Janssen, A.P.A.; Cognetta, A.B., 3rd; Ogasawara, D.; Shpak, G.; van der Kroeg, M.; Kantae, V.; Baggelaar, M.P.; de Vrij, F.M.S.; Deng, H.; et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 2017, 356, 1084–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Sidhu, P.; Singh, S. What failed BIA 10-2474 Phase I clinical trial? Global speculations and recommendations for future Phase I trials. J. Pharmacol. Pharmacother. 2016, 7, 120–126. [Google Scholar] [CrossRef]
- Bauer, M.; Chicca, A.; Tamborrini, M.; Eisen, D.; Lerner, R.; Lutz, B.; Poetz, O.; Pluschke, G.; Gertsch, J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012, 287, 36944–36967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedonio, G.; Stefanucci, A.; Maccallini, C.; Mirzaie, S.; Novellino, E.; Mollica, A. Hemopressin Peptides as Modulators of the Endocannabinoid System and their Potential Applications as Therapeutic Tools. Protein Pept. Lett. 2016, 23, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhao, L.; Jing, Y. Signaling molecules targeting cannabinoid receptors: Hemopressin and related peptides. Neuropeptides 2020, 79, 101998. [Google Scholar] [CrossRef]
- Zubrzycki, M.; Liebold, A.; Janecka, A.; Zubrzycka, M. A new face of endocannabinoids in pharmacotherapy. Part II: Role of endocannabinoids in inflammation-derived cardiovaascular diseases. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 183–191. [Google Scholar]
- Rajesh, M.; Mukhopadhyay, P.; Batkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horvath, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.V.; Giricz, Z.; Liaudet, L.; Hasko, G.; Ferdinandy, P.; Pacher, P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta 2015, 1852, 232–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, I.; Gonthier, M.P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Pagano, C.; Rossato, M.; Vettor, R. Endocannabinoids, adipose tissue and lipid metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 124–129. [Google Scholar] [CrossRef]
- Rajesh, M.; Batkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horvath, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Batkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; Mackie, K.; Hasko, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdelyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Hasko, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.; Giannone, F.A.; Napoli, L.; Tovoli, A.; Ricci, C.S.; Tufoni, M.; Caraceni, P. The endocannabinoid system in advanced liver cirrhosis: Pathophysiological implication and future perspectives. Liver Int. 2013, 33, 1298–1308. [Google Scholar] [CrossRef]
- Batkai, S.; Jarai, Z.; Wagner, J.A.; Goparaju, S.K.; Varga, K.; Liu, J.; Wang, L.; Mirshahi, F.; Khanolkar, A.D.; Makriyannis, A.; et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 2001, 7, 827–832. [Google Scholar] [CrossRef]
- Matyas, C.; Erdelyi, K.; Trojnar, E.; Zhao, S.; Varga, Z.V.; Paloczi, J.; Mukhopadhyay, P.; Nemeth, B.T.; Hasko, G.; Cinar, R.; et al. Interplay of Liver-Heart Inflammatory Axis and Cannabinoid 2 Receptor Signaling in an Experimental Model of Hepatic Cardiomyopathy. Hepatology 2020, 71, 1391–1407. [Google Scholar] [CrossRef]
- Gaskari, S.A.; Liu, H.Q.; Moezi, L.; Li, Y.; Baik, S.K.; Lee, S.S. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br. J. Pharmacol. 2005, 146, 315–323. [Google Scholar] [CrossRef]
- Gaskari, S.A.; Liu, H.Q.; D’Mello, C.; Kunos, G.; Lee, S.S. Blunted cardiac response to hemorrhage in cirrhotic rats is mediated by local macrophage-released endocannabinoids. J. Hepatol. 2015, 62, 1272–1277. [Google Scholar] [CrossRef] [Green Version]
- Godlewski, G.; Malinowska, B.; Schlicker, E. Presynaptic cannabinoid CB(1) receptors are involved in the inhibition of the neurogenic vasopressor response during septic shock in pithed rats. Br. J. Pharmacol. 2004, 142, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Katsimpoulas, M.; Kadoglou, N.E.; Moustardas, P.; Kapelouzou, A.; Dede, E.; Kostomitsopoulos, N.; Karayannacos, P.E.; Kostakis, A.; Liapis, C.D. The role of exercise training and the endocannabinoid system in atherosclerotic plaque burden and composition in Apo-E-deficient mice. Hell. J. Cardiol. 2016, 57, 417–425. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ito, Y.; Hashiguchi, T.; Kitajima, I.; Yamakuchi, M.; Shimizu, H.; Matsuo, S.; Imaizumi, H.; Maruyama, I. Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin B-selective adsorption and subsequent high-performance liquid chromatography analysis: Increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal. Biochem. 2001, 294, 73–82. [Google Scholar] [CrossRef]
- Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Kuwano, H.; Saito, S.; Goto, F. Effects of AM281, a cannabinoid antagonist, on systemic haemodynamics, internal carotid artery blood flow and mortality in septic shock in rats. Br. J. Anaesth. 2005, 94, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, K.; Wagner, J.A.; Bridgen, D.T.; Kunos, G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1998, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, K.; Sarker, K.P.; Kawahara, K.; Iino, S.; Yamakuchi, M.; Abeyama, K.; Hashiguchi, T.; Maruyama, I. Anandamide induces apoptosis in human endothelial cells: Its regulation system and clinical implications. Thromb. Haemost. 2003, 89, 875–884. [Google Scholar] [CrossRef]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Hasku, G.; Cihakova, D.; Mechoulam, R.; Pacher, P. Cannabidiol Limits T Cell-Mediated Chronic Autoimmune Myocarditis: Implications to Autoimmune Disorders and Organ Transplantation. Mol. Med. 2016, 22, 136–146. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Jenabian, M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020, 53, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Sharma, V. Cannabis for COVID-19: Can cannabinoids quell the cytokine storm? Future Sci. OA 2020, 6, FSO625. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Argenziano, M.; Di Paola, A.; Punzo, F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int. J. Mol. Sci. 2020, 21, 3809. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Dawson, A.J.; Krastev, Y.; Parsonage, W.A.; Peek, M.; Lust, K.; Sullivan, E.A. Experiences of women with cardiac disease in pregnancy: A systematic review and metasynthesis. BMJ Open 2018, 8, e022755. [Google Scholar] [CrossRef] [Green Version]
- Nogi, M.; Fergusson, D.; Chiaco, J.M. Mid-ventricular variant takotsubo cardiomyopathy associated with Cannabinoid Hyperemesis Syndrome: A case report. Hawaii J. Med. Public Health 2014, 73, 115. [Google Scholar]
- Aryana, A.; Williams, M.A. Marijuana as a trigger of cardiovascular events: Speculation or scientific certainty? Int. J. Cardiol. 2007, 118, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular Complications of Marijuana and Related Substances: A Review. Cardiol. Ther. 2018, 7, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amitoj Singh, S.A.; Fegley, M.; Manda, Y.; Nanda, S.; Shirani, J. Marijuana (Cannabis) Use is an Independent Predictor of Stress Cardiomyopathy in Younger Men. Ciculation 2018, 134, 14100. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Bajaj, N.S.; Singh, A.; Malloy, R.; Givertz, M.M.; Blankstein, R.; Bhatt, D.L.; Vaduganathan, M. Marijuana Use in Patients With Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Shamim, S.; Patel, K.; Sadolikar, A.; Kaur, V.P.; Bhivandkar, S.; Patel, S.; Savani, S.; Mansuri, Z.; Mahuwala, Z. Primary Causes of Hospitalizations and Procedures, Predictors of In-hospital Mortality, and Trends in Cardiovascular and Cerebrovascular Events Among Recreational Marijuana Users: A Five-year Nationwide Inpatient Assessment in the United States. Cureus 2018, 10, e3195. [Google Scholar] [CrossRef] [Green Version]
- Desai, R.; Desai, A.; Fong, H.K.; Mahmood, A.; Shah, K.; Varakantam, V.R.; Haque, F.A.; Savani, S.; Gangani, K.; Kumar, G.; et al. Prevalence, trends and in-hospital outcomes of takotsubo syndrome among United States cannabis users. Int. J. Cardiol. 2020, 316, 43–46. [Google Scholar] [CrossRef]
- Desai, R.; Patel, U.; Deshmukh, A.; Sachdeva, R.; Kumar, G. Burden of arrhythmia in recreational marijuana users. Int. J. Cardiol. 2018, 264, 91–92. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Lip, G.Y.; Lane, D.A. Alcohol and illicit drug use as precipitants of atrial fibrillation in young adults: A case series and literature review. Am. J. Med. 2009, 122, 851–856.e3. [Google Scholar] [CrossRef] [PubMed]
- Charbonney, E.; Sztajzel, J.M.; Poletti, P.A.; Rutschmann, O. Paroxysmal atrial fibrillation after recreational marijuana smoking: Another “holiday heart”? Swiss Med. Wkly. 2005, 135, 412–414. [Google Scholar]
- Lee, J.D.; Schatz, D.; Hochman, J. Cannabis and Heart Disease: Forward Into the Great Unknown? J. Am. Coll. Cardiol. 2018, 71, 2552–2554. [Google Scholar] [CrossRef]
- Baranchuk, A.; Johri, A.M.; Simpson, C.S.; Methot, M.; Redfearn, D.P. Ventricular fibrillation triggered by marijuana use in a patient with ischemic cardiomyopathy: A case report. Cases J. 2008, 1, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez Artiles, A.E.; Awan, A.; Karl, M.; Santini, A. Cardiovascular effects of cannabis (marijuana): A timely update. Phytother. Res. PTR 2019, 33, 1592–1594. [Google Scholar] [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- Krylatov, A.V.; Maslov, L.N.; Lasukova, O.V.; Pertwee, R.G. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull. Exp. Biol. Med. 2005, 139, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Khanji, M.Y.; Jensen, M.T.; Kenawy, A.A.; Raisi-Estabragh, Z.; Paiva, J.M.; Aung, N.; Fung, K.; Lukaschuk, E.; Zemrak, F.; Lee, A.M.; et al. Association between Recreational Cannabis Use and Cardiac Structure and Function. JACC Cardiovasc. Imaging 2020, 13, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Draz, E.I.; Oreby, M.M.; Elsheikh, E.A.; Khedr, L.A.; Atlam, S.A. Marijuana use in acute coronary syndromes. Am. J. Drug Alcohol Abus. 2017, 43, 576–582. [Google Scholar] [CrossRef]
- Hodcroft, C.J.; Rossiter, M.C.; Buch, A.N. Cannabis-associated myocardial infarction in a young man with normal coronary arteries. J. Emerg. Med. 2014, 47, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Latif, Z.; Garg, N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. J. Clin. Med. 2020, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.R.; Bing, M.L.; Moulin, A.K.; Elder, J.W.; Rominski, R.T.; Summers, P.J.; Laurin, E.G. Cannabis use and acute coronary syndrome. Clin. Toxicol. 2019, 57, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.G.; Yu, B. Investigating Cumulative Marijuana Use and Risk of Cardiovascular Disease in Middle Age with Longitudinal Data. Am. J. Public Health 2018, 108, e11–e12. [Google Scholar] [CrossRef]
- Reis, J.P.; Auer, R.; Bancks, M.P.; Goff, D.C., Jr.; Lewis, C.E.; Pletcher, M.J.; Rana, J.S.; Shikany, J.M.; Sidney, S. Cumulative Lifetime Marijuana Use and Incident Cardiovascular Disease in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Public Health 2017, 107, 601–606. [Google Scholar] [CrossRef]
- Reis, J.P.; Auer, R.; Bancks, M.P.; Goff, D.C., Jr.; Lewis, C.E.; Pletcher, M.J.; Rana, J.S.; Shikany, J.M.; Sidney, S. Reis et al. Respond. Am. J. Public Health 2018, 108, e12. [Google Scholar] [CrossRef] [PubMed]
- Hunault, C.C.; Mensinga, T.T.; Bocker, K.B.; Schipper, C.M.; Kruidenier, M.; Leenders, M.E.; de Vries, I.; Meulenbelt, J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology 2009, 204, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Malcolm, R.J.; Babalonis, S.; Nuzzo, P.A.; Cooper, Z.D.; Bedi, G.; Gray, K.M.; McRae-Clark, A.; Lofwall, M.R.; Sparenborg, S.; et al. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlovic, D.; Dujic, Z.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef]
- Samsamikor, M.; Mackay, D.; Mollard, R.C.; Aluko, R.E. A double-blind, randomized, crossover trial protocol of whole hemp seed protein and hemp seed protein hydrolysate consumption for hypertension. Trials 2020, 21, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejot, Y.; Delpont, B.; Giroud, M. Rising Stroke Incidence in Young Adults: More Epidemiological Evidence, More Questions to Be Answered. J. Am. Heart Assoc. 2016, 5, e003661. [Google Scholar] [CrossRef] [Green Version]
- Boot, E.; Ekker, M.S.; Putaala, J.; Kittner, S.; De Leeuw, F.E.; Tuladhar, A.M. Ischaemic stroke in young adults: A global perspective. J. Neurol. Neurosurg. Psychiatry 2020, 91, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Sultan, S.; Elkind, M.S. The growing problem of stroke among young adults. Curr. Cardiol. Rep. 2013, 15, 421. [Google Scholar] [CrossRef] [PubMed]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Quercioli, A.; Pataky, Z.; Montecucco, F.; Carballo, S.; Thomas, A.; Staub, C.; Di Marzo, V.; Vincenti, G.; Ambrosio, G.; Ratib, O.; et al. Coronary vasomotor control in obesity and morbid obesity: Contrasting flow responses with endocannabinoids, leptin, and inflammation. JACC Cardiovasc. Imaging 2012, 5, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Quercioli, A.; Pataky, Z.; Vincenti, G.; Makoundou, V.; Di Marzo, V.; Montecucco, F.; Carballo, S.; Thomas, A.; Staub, C.; Steffens, S.; et al. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur. Heart J. 2011, 32, 1369–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baye, T.M.; Zhang, Y.; Smith, E.; Hillard, C.J.; Gunnell, J.; Myklebust, J.; James, R.; Kissebah, A.H.; Olivier, M.; Wilke, R.A. Genetic variation in cannabinoid receptor 1 (CNR1) is associated with derangements in lipid homeostasis, independent of body mass index. Pharmacogenomics 2008, 9, 1647–1656. [Google Scholar] [CrossRef] [Green Version]
- Benzinou, M.; Chevre, J.C.; Ward, K.J.; Lecoeur, C.; Dina, C.; Lobbens, S.; Durand, E.; Delplanque, J.; Horber, F.F.; Heude, B.; et al. Endocannabinoid receptor 1 gene variations increase risk for obesity and modulate body mass index in European populations. Hum. Mol. Genet. 2008, 17, 1916–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luis, D.A.; Sagrado, M.G.; Aller, R.; Izaola, O.; Conde, R.; Romero, E. C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance in patients with diabetes mellitus type 2. Diabetes Res. Clin. Pract. 2010, 88, 76–80. [Google Scholar] [CrossRef]
- Feng, Q.; Vickers, K.C.; Anderson, M.P.; Levin, M.G.; Chen, W.; Harrison, D.G.; Wilke, R.A. A common functional promoter variant links CNR1 gene expression to HDL cholesterol level. Nat. Commun. 2013, 4, 1973. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.; Strazzullo, P.; Cappuccio, F.P.; Tregouet, D.A.; Lauria, F.; Loguercio, M.; Barba, G.; Versiero, M.; Siani, A. Genetic variations at the endocannabinoid type 1 receptor gene (CNR1) are associated with obesity phenotypes in men. J. Clin. Endocrinol. Metab. 2007, 92, 2382–2386. [Google Scholar] [CrossRef] [Green Version]
- Silver, H.J.; Niswender, K.D.; Keil, C.D.; Jiang, L.; Feng, Q.; Chiu, S.; Krauss, R.M.; Wilke, R.A. CNR1 genotype influences HDL-cholesterol response to change in dietary fat intake. PLoS ONE 2012, 7, e36166. [Google Scholar] [CrossRef] [Green Version]
- Sipe, J.C.; Waalen, J.; Gerber, A.; Beutler, E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int. J. Obes. 2005, 29, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, W.; Stark, K.; Neureuther, K.; Sedlacek, K.; Fischer, M.; Baessler, A.; Weber, S.; Kaess, B.; Wiedmann, S.; Erdmann, J.; et al. Common polymorphisms in the cannabinoid CB2 receptor gene (CNR2) are not associated with myocardial infarction and cardiovascular risk factors. Int. J. Mol. Med. 2008, 22, 165–174. [Google Scholar]
- Van Gaal, L.; Pi-Sunyer, X.; Despres, J.P.; McCarthy, C.; Scheen, A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: Pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 2008, 31 (Suppl. S2), S229–S240. [Google Scholar] [CrossRef] [Green Version]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Rodes-Cabau, J.; Cannon, C.P.; Deanfield, J.E.; Despres, J.P.; Kastelein, J.J.; Steinhubl, S.R.; Kapadia, S.; et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: The STRADIVARIUS randomized controlled trial. JAMA 2008, 299, 1547–1560. [Google Scholar] [CrossRef] [Green Version]
- Despres, J.P.; Ross, R.; Boka, G.; Almeras, N.; Lemieux, I. Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despres, J.P.; Moorjani, S.; Lupien, P.J.; Tremblay, A.; Nadeau, A.; Bouchard, C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990, 10, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, D.H.; Reuwer, A.Q.; Nissen, S.E.; Despres, J.P.; Deanfield, J.E.; Brown, M.W.; Zhou, R.; Zabbatino, S.M.; Job, B.; Kastelein, J.J.; et al. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: The AUDITOR Trial. Heart 2011, 97, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Topol, E.J.; Bousser, M.G.; Fox, K.A.; Creager, M.A.; Despres, J.P.; Easton, J.D.; Hamm, C.W.; Montalescot, G.; Steg, P.G.; Pearson, T.A.; et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet 2010, 376, 517–523. [Google Scholar] [CrossRef]
- Mechoulam, R. Plant cannabinoids: A neglected pharmacological treasure trove. Br. J. Pharmacol. 2005, 146, 913–915. [Google Scholar] [CrossRef]
- Delling, F.N.; Vittinghoff, E.; Dewland, T.A.; Pletcher, M.J.; Olgin, J.E.; Nah, G.; Aschbacher, K.; Fang, C.D.; Lee, E.S.; Fan, S.M.; et al. Does cannabis legalisation change healthcare utilisation? A population-based study using the healthcare cost and utilisation project in Colorado, USA. BMJ Open 2019, 9, e027432. [Google Scholar] [CrossRef] [Green Version]
- Hall, W. A Summary of Reviews of Evidence on the Efficacy and Safety of Medical Use of Cannabis and Cannabinoids; EMCDDA: Lisbon, Portugal, 2018. [Google Scholar]


| Trial | ECS Modulator | Readouts | Findings | Phase |
|---|---|---|---|---|
| “A Double-Blind, Randomised, Placebo-Controlled, Cross-Over Study on the Pharmacokinetics and Effects of Cannabis”-NCT00225407, 2005. | Smoking cannabis cigarettes (different Δ9-THC strengths). | Primary: Serum Δ9-THC concentration over time; physical parameters (HR, BP); psychomotor tests (e.g., continuous attention); event related potentials. Secondary: Self-reporting questionnaires. | HR and intoxication were positively linearly associated with increasing Δ9-THC doses (29.3, 49.1, 69.4 mg). | Phase 1. |
| “Laboratory Study of the Influence of Oral Cannabidiol on the Subjective, Reinforcing and Cardiovascular Effects of Smoked Marijuana”-NCT01844687, 2013. | Active cannabis with 0, 200, 400 or 800 mg CBD; inactive cannabis (containing 0.56% Δ9-THC) with 0, 200, 400 or 800 mg CBD. | Primary: “Feeling high”mood scale-subscale. Secondary: rating form assessing, experience, strength, additional puffs taken, HR, plasma concentration of CBD. | Oral CBD pre-treatment does not alter the subjective, reinforcing, or cardiovascular effects of smoked cannabis. Active cannabis produced significant increases in ratings of “High” and “Good Effect” as well as assessments of the cannabis cigarette (e.g., “Strength”, “Liking”, “Desire to take again”) and HR. | Phase 2. |
| “A single dose of cannabidiol reduces BP in healthy volunteers in a randomized crossover study”. University of Nottingham (UK) study reference code: E18102012, c.2017. | CBD (600 mg). | Cardiovascular parameters: Systolic, diastolic and mean arterial BP, HR, stroke volume, CO, ejection time, total peripheral resistance, and forearm blood flow. | Acute administration of CBD reduces resting BP. | Phase 1. |
| “The Effects of Cannabinoids on Vascular and Cognitive Function in Young and Old Healthy Adults”-NCT03295903, 2018. | CBD and TurboCBD™. | Primary: Circulating CBD and nitric oxide markers and vascular function. Secondary: Height, weight, body mass index, systolic and diastolic BP, HR, respiration, questionnaires (medical history, gastrointestinal distress, anxiety), cognitive and exercise performance evaluations. | TurboCBD™ had higher bioavailability than CBD and at 90 mg was associated with increased cerebral perfusion and slight reduction in BP. | Phase 1. |
| “Hemp Seed Protein Consumption for Hypertension“-NCT03508895, 2018. | Hemp seed protein. | Primary: Change in 24 h ambulatory BP. Secondary: Change in BP, pulse wave velocity, augmentation index, body weight, waist circumference, hip circumference, body composition, total serum cholesterol, HDL/LDL cholesterol, serum triglycerides, serum glucose, serum creatinine, plasma insulin concentrations, insulin homeostasis modelling assessment, renal panel. | No data to date. | Phase 2. |
| CANNASTROKE “Prevalence of Strokes Secondary to a Reversible Cerebral Vasoconstriction Attributable to Cannabis Consumption in Young Subjects (≤45 Years) Hospitalized for an Ischaemic Stroke”-NCT03379857, 2017. | Cannabis. | Primary: Evaluation of cannabis use, reversible vasoconstriction on medical imaging of intracranial arteries. | No data to date. Estimated primary completion date: January 2025. | Not applicable (behavioural study). |
| “Atherosclerotic Plaque Texture-Experimental and Clinical Study on the Diagnostic and Therapeutic Strategies of Atherosclerotic Plaque Vulnerability”-NCT00636766, 2005. | Rimonabant combined with exercise. | Primary: Ultrasound and immuno-histochemical parameters of plaque stability and novel cardiovascular risk factors. Secondary: Long-term cardiovascular outcomes. | Rimonabant and exercise induced plaque regression and promoted plaque stability. A combination of the two interventions failed to show additive or synergistic benefits. | Phase 3. |
| RIO-Europe “A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Fixed-Dose, Multicenter Study of Weight-Reducing Effect and Safety of SR141716 in Obese Patients With or Without Comorbidities”-NCT00386061, 2001. | Rimonabant (5/20 mg daily), reduced caloric intake and exercise promotion. | Primary: Change in body weight, waist circumference, and BP from baseline to 1 year. Secondary: Lipid profile, HDL cholesterol and triglycerides; patients (%) with improvement of glucose tolerance, patients (%) with NCEP-ATPIII metabolic syndrome. | Rimonabant produced weight loss and significant improvements in multiple cardiometabolic risk factors. | Phase 3. |
| STRADIVARIUS “Strategy To Reduce Atherosclerosis Development InVolving Administration of Rimonabant-the Intravascular Ultrasound Study”-NCT00124332, 2005. | Rimonabant (20 mg, daily for 18–20 months). | Primary: Change from baseline in percent atheroma volume. Secondary: Change from baseline in normalized TAV. | After 18 months of treatment, no effect of rimonabant on the primary efficacy parameter. A statistically significant favourable effect on the secondary end point was observed. | Phase 3. |
| ADAGIO-Lipids Trial “Effect of Rimonabant on the High-Triglyceride/ Low–HDL-Cholesterol Dyslipidemia, Intra-abdominal Adiposity, and Liver Fat”-NCT00239967, 2005. | Rimonabant (20 mg daily for 12 months). | Measurements of LDL particle size, HDL quantity, quality and subfractions, and apo B/apo A1 ratio; assessments of visceral and liver fat (by a computed tomography sub-study). | Rimonabant significantly improved multiple cardiometabolic risk markers and induced significant reductions in both intra-abdominal and liver fat. | Phase 3. |
| AUDITOR “Atherosclerosis Underlying Development Assessed by Intima-Media Thickness in Patients on Rimonabant”-NCT00228176, 2005. | Rimonabant (20 mg daily for 30 months). | Primary: Absolute change from baseline in averaged per patient CIMT. Secondary: First occurrence of any component of stroke/MI/cardiovascular death. First occurrence of any component of stroke/MI/cardiovascular death/hospitalization for revascularization procedure, unstable angina, transient ischaemic attack. | No difference in atherosclerosis progression between patients receiving rimonabant for 30 months and those receiving placebo for the primary efficacy measure (absolute change in CIMT). | Phase 3. |
| CRESCENDO “Comprehensive Rimonabant Evaluation Study of Cardiovascular ENDpoints and Outcomes”-NCT00263042, 2005. | Rimonabant (20 mg daily up to 13.4 months). | Primary: First occurrence of any of myocardial infarction, stroke or cardiovascular death. Secondary: First occurrence of any of myocardial infarction, stroke, cardiovascular death, cardiovascular hospitalization and all-cause mortality. | No evidence for the efficacy of prevention of adverse cardiovascular outcomes by rimonabant. Rimonabant was associated with serious side-effects (e.g., neuropsychiatric, gastrointestinal) and the trial was discontinued. | Phase 3. |
| PRIMARIA “Early Detection of Atherosclerosis in the Primary Care Setting: a Randomized Trial to Assess the Efficacy of a Novel Strategy in the Primary Prevention of Cardiovascular Diseases”-NCT00734123, 2008. | Rimonabant. | Primary: CIMT progression/regression. Secondary: Cardiac and cerebrovascular events. | No data to date. | Phase 4. |
| CAPITAL-AC “Cannabidiol in Patients With Heart Failure in AHA/ACC Stages A-C”-NCT03634189, 2021. | CBD. | Primary: Number of participants with treatment-related serious adverse events and events of interest as assessed by MedDRA v5.1. | No data to date. Estimated study completion date: December 2021. | Phase 1. |
| “Study to Evaluate the Efficacy and Safety of CardiolRx™ in Patients With COVID-19 and Cardiovascular Disease or Risk Factors A Double-blind, Placebo-controlled Trial”-NCT04615949, 2021. | CBD, pharmaceutically produced with <5 ppm THC. | Primary: All-cause mortality, ICU admission, ventilator support, cardiovascular complications. | No data to date. Estimated study completion date: September 2021. | Phase 2, 3. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936. https://doi.org/10.3390/ph14090936
Rabino M, Mallia S, Castiglioni E, Rovina D, Pompilio G, Gowran A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals. 2021; 14(9):936. https://doi.org/10.3390/ph14090936
Chicago/Turabian StyleRabino, Martina, Sara Mallia, Elisa Castiglioni, Davide Rovina, Giulio Pompilio, and Aoife Gowran. 2021. "The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases" Pharmaceuticals 14, no. 9: 936. https://doi.org/10.3390/ph14090936
APA StyleRabino, M., Mallia, S., Castiglioni, E., Rovina, D., Pompilio, G., & Gowran, A. (2021). The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals, 14(9), 936. https://doi.org/10.3390/ph14090936