Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
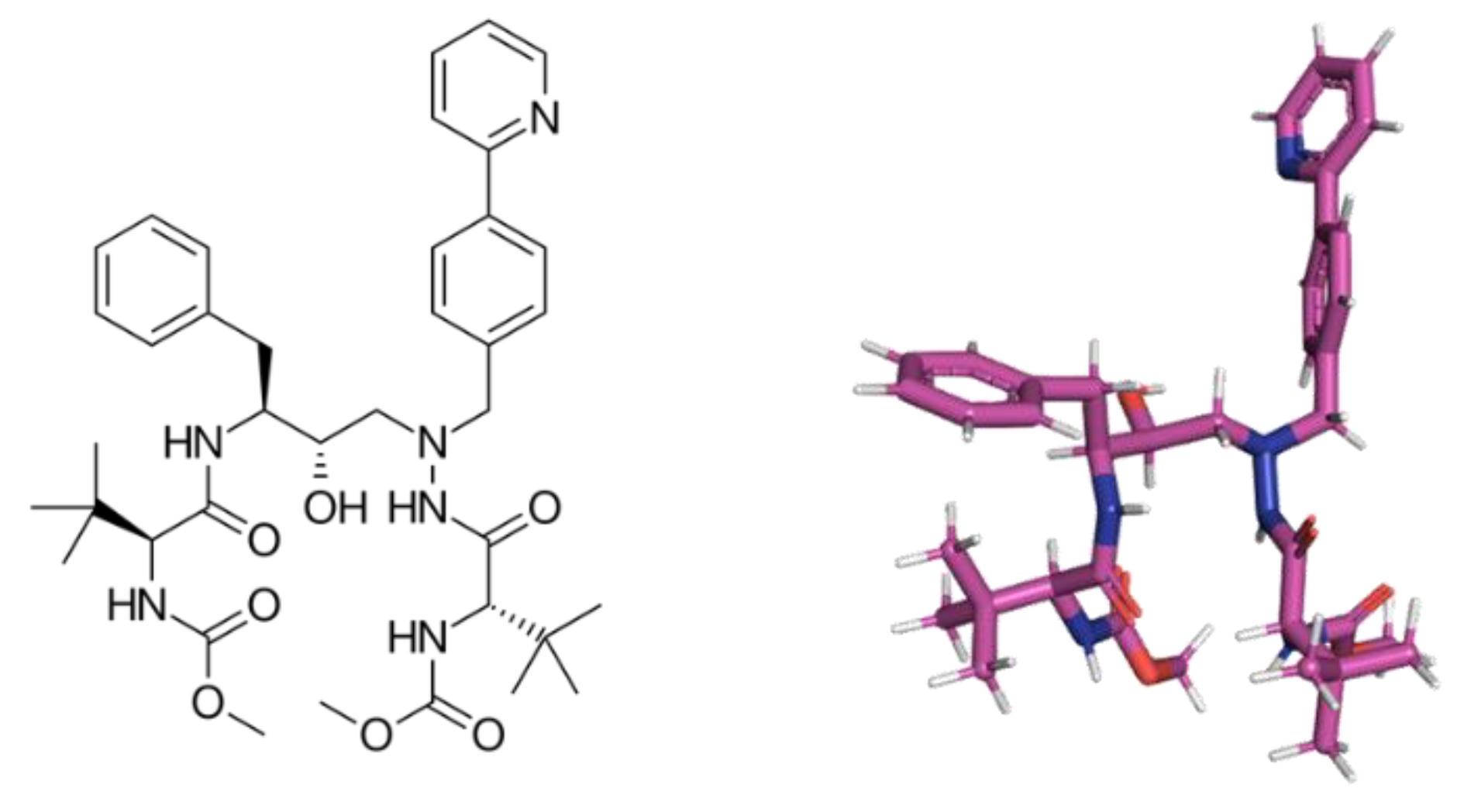
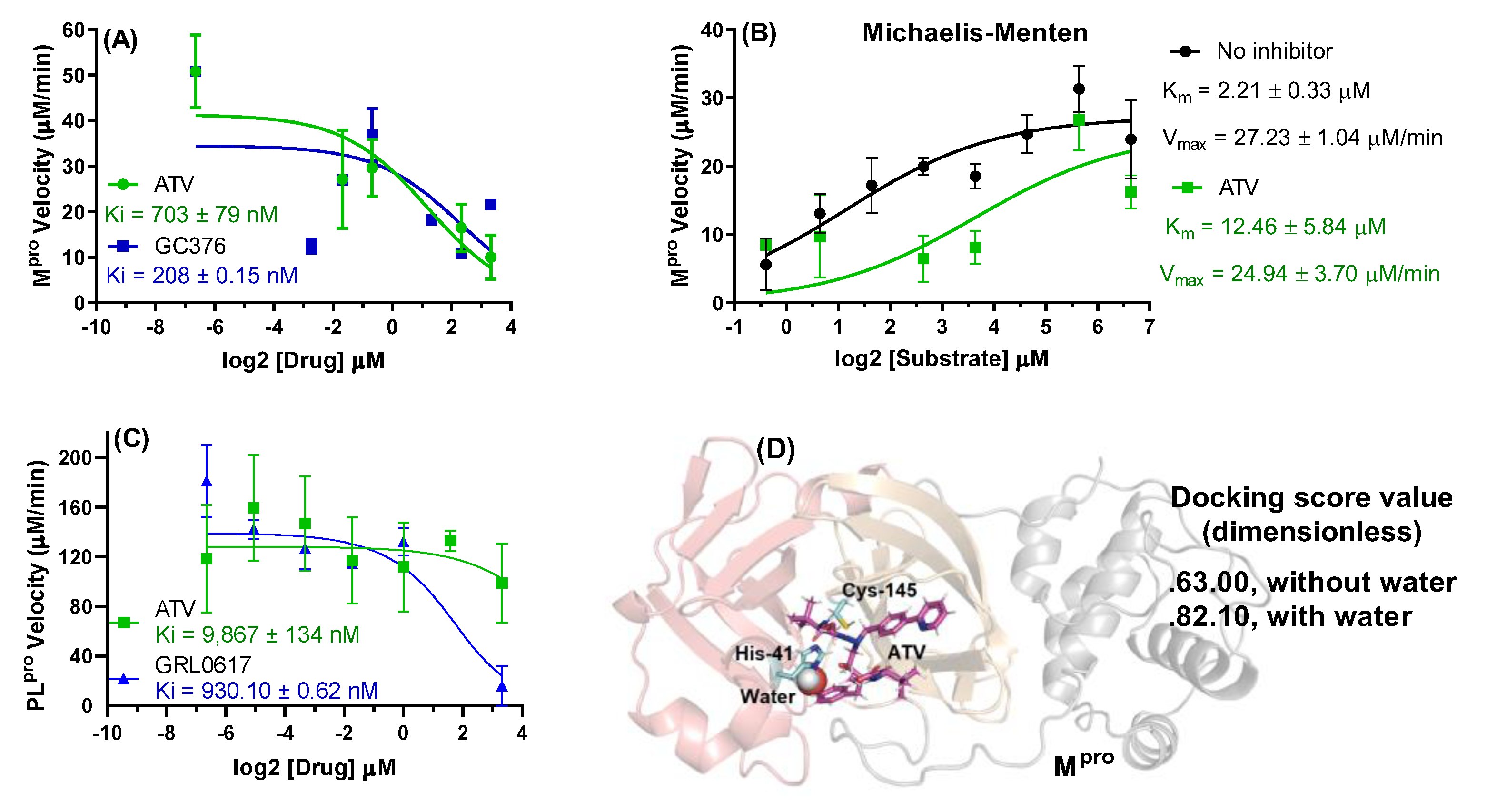
2.1. Enzymatic and Cell-Based Assays for ATV in SARS-CoV-2 D614G and Gamma Strains
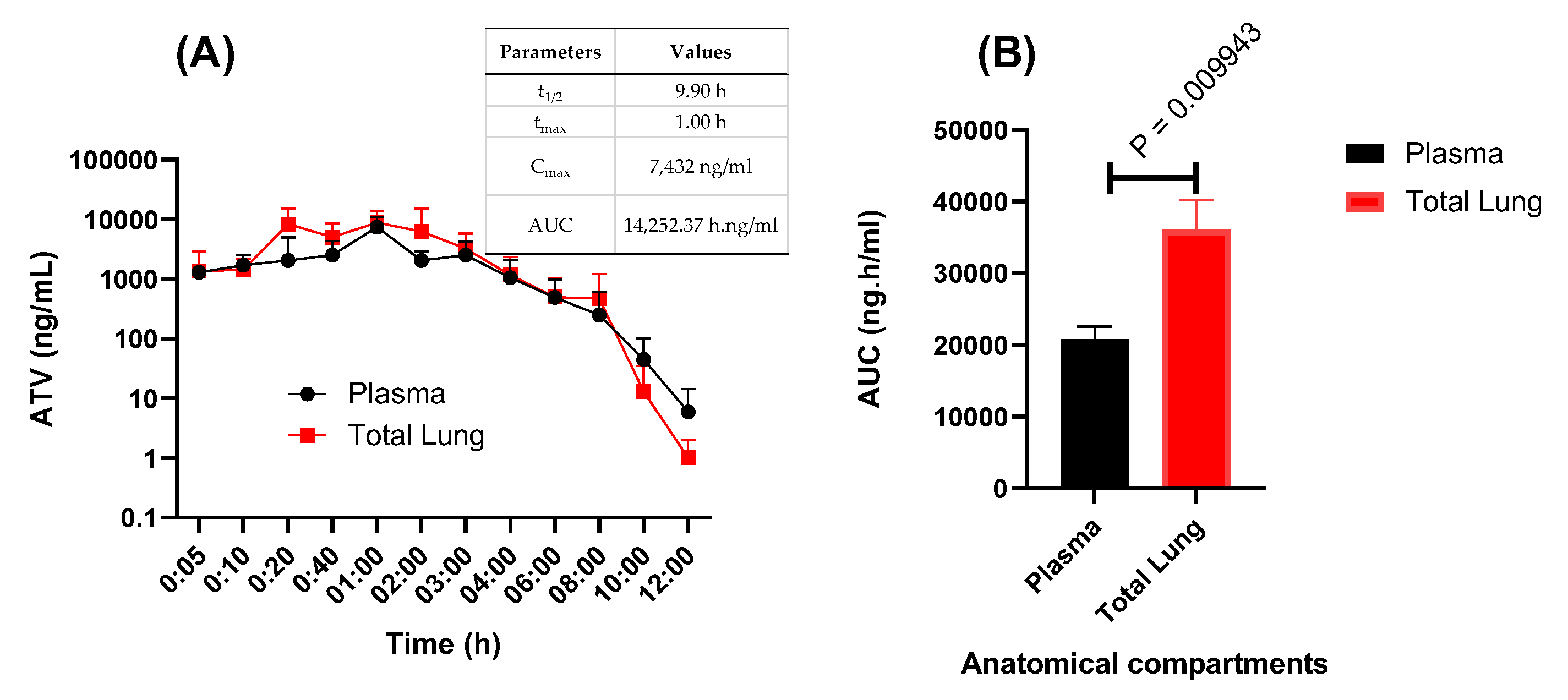
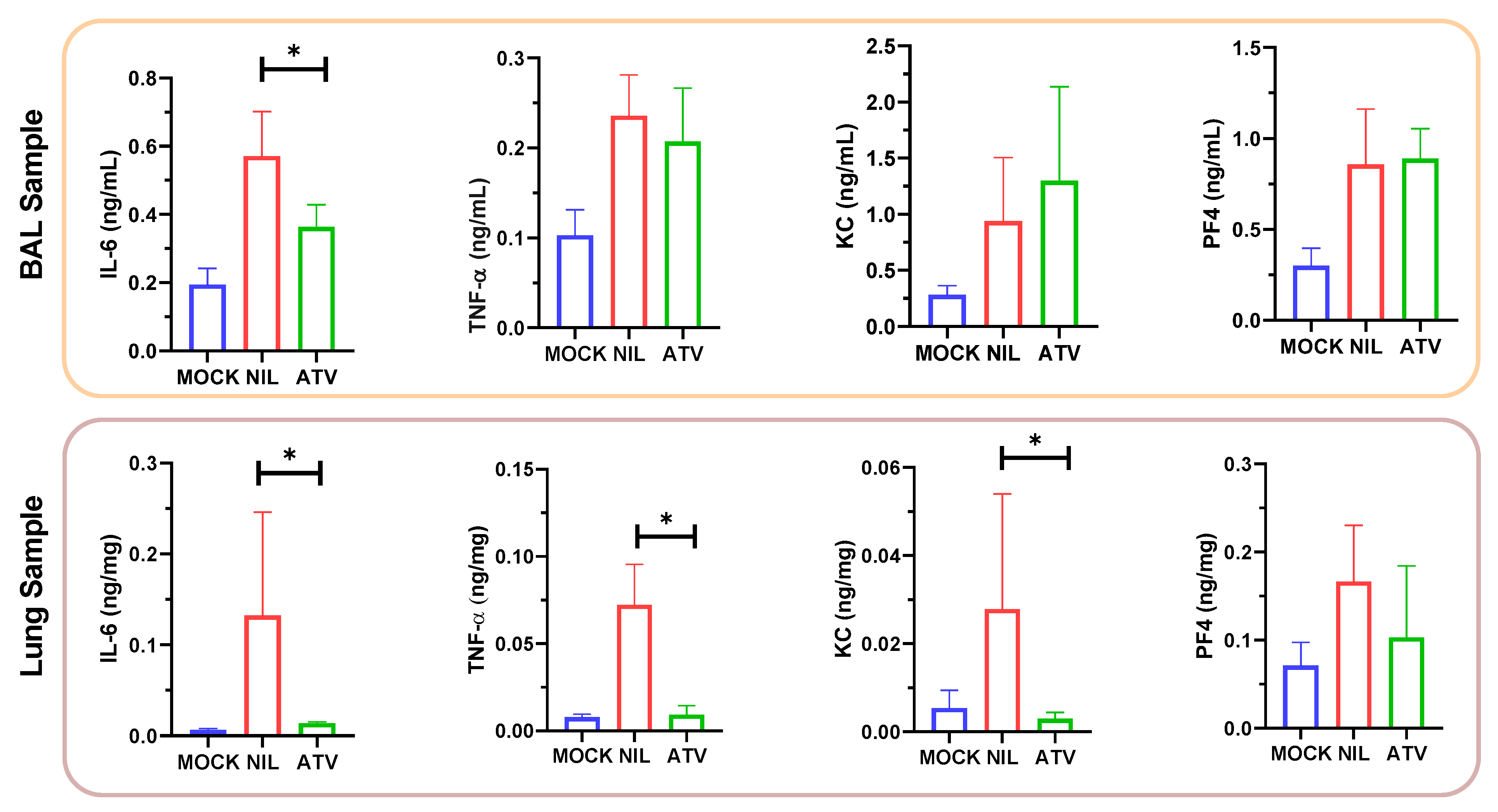
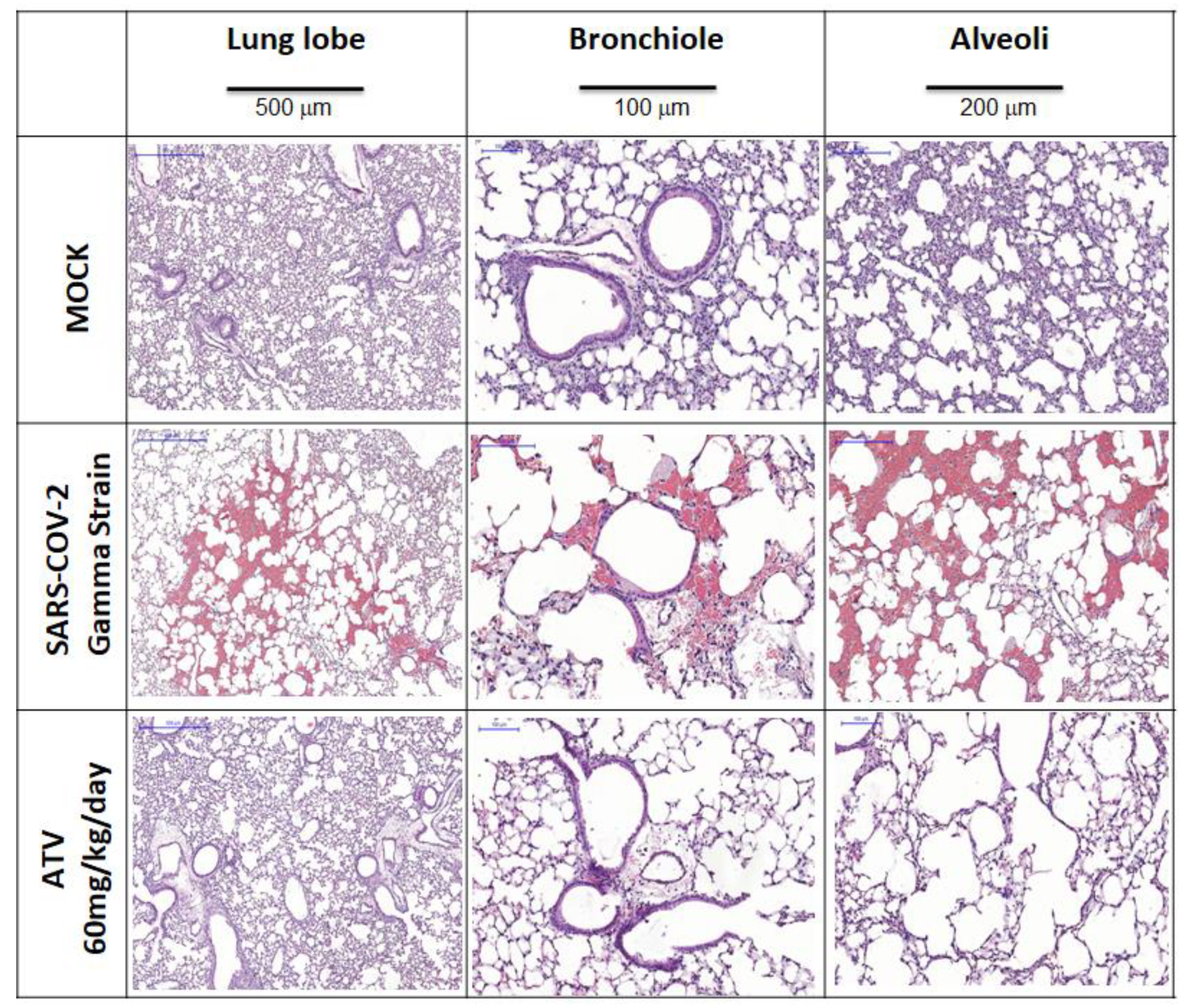
2.2. In Vivo Antiviral Activity of ATV against SARS-CoV-2 Gamma Variant
3. Discussion
4. Materials and Methods
4.1. General Materials
4.2. Cells and Virus
4.3. Enzymatic Assays
4.4. Molecular Docking Procedure
4.5. Yield-Reduction Assays and Virus Titration
4.6. Cytotoxic Assays
4.7. Pharmacokinetic Assays
4.8. In Vivo Assays—Mice Treatment and Infections
4.9. Quantification of Viral RNA
4.10. Measurements of Inflammatory Mediators and Cell Death
4.11. Histological Procedure
4.12. Clotting Time
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaplin, S. COVID-19: A brief history and treatments in development. Prescriber 2020, 31, 23–28. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 6 November 2021).
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Venkatesan, P. Repurposing drugs for treatment of COVID-19. Lancet 2021, 9, E63. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Wood, R. Atazanavir: Its role in HIV treatment. Expert Rev. Anti-Infect. Ther. 2008, 6, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fintelman-Rodrigues, N.; Sacramento, C.Q.; Lima, C.R.; da Silva, F.S.; Ferreira, A.C.; Mattos, M.; de Freitas, C.S.; Soares, V.C.; Dias, S.S.G.; Temerozo, J.R.; et al. Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob. Agents Chemother. 2020, 64, e00825-20. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.; Wang, Y.; Yuen, T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020, 71, 1400–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Chan, J.F.; Yuen, T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, 14–23. [Google Scholar] [CrossRef]
- The Nitazoxanide Plus Atazanavir for COVID-19 Study (NACOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04459286 (accessed on 6 November 2021).
- NA-831, Atazanavir and Dexamethasone Combination Therapy for the Treatment of COVID-19 Infection (NATADEX). Available online: https://clinicaltrials.gov/ct2/show/NCT04452565?term=atazanavir&cond=COVID-19&draw=2&rank=2 (accessed on 6 November 2021).
- Li, Z.; Li, X.; Huang, Y.Y.; Wu, Y.; Liu, R.; Zhou, L.; Lin, Y.; Wu, D.; Zhang, L.; Liu, H.; et al. Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual careening of existing drugs. Proc. Natl. Acad. Sci. USA 2020, 117, 27381–27387. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 Epic-HR Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-COVID-19-oral-antiviral-treatment-candidate (accessed on 23 November 2021).
- Ma, C.; Wang, J. Dipyridamole, chloroquine, montelukast sodium, candesartan, oxytetracycline, and atazanavir are not SARS-CoV-2 main protease inhibitors. Proc. Natl. Acad. Sci. USA 2021, 118, e2024420118. [Google Scholar] [CrossRef]
- Oerlemans, R.; Ruiz-Moreno, A.J.; Cong, Y.; Kumar, N.D.; Velasco-Velazquez, M.A.; Neochoritis, C.G.; Smith, J.; Reggiori, F.; Groves, M.R.; Domling, A. Repurposing the HCV NS3–4A protease drug boceprevir as COVID-19 therapeutics. RSC Med. Chem. 2021, 12, 370–379. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Jedrzejczak, R.; Stols, L.; Langan, P.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Structural plasticity of SARS-CoV-2 3CL Mpro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020, 11, 3202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Worrall, L.J.; Vuckovic, M.; Rosell, F.I.; Gentile, F.; Ton, A.-T.; Caveney, N.A.; Ban, F.; Cherkasov, A.; Paetzel, M.; et al. Crystallographic structure of wild-type SARS-CoV2 main protease acyl-enzyme intermediate with physiological C-terminal autoprocessing site. Nat. Commun. 2020, 11, 5877. [Google Scholar] [CrossRef]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Hobartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Product Monograph Atazanavir Capsules. Available online: https://www.bms.com/assets/bms/ca/documents/productmonograph/REYATAZ_EN_PM.pdf (accessed on 6 November 2021).
- Ferreira, A.C.; Soares, V.C.; de Azevedo-Quintanilha, I.G.; Dias, S.S.G.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; de Freitas, C.S.; Temerozo, J.R.; Teixeira, L.; et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef]
- Trezza, A.; Lovinelli, D.; Santucci, A.; Prischi, F.; Spiga, O. An integrated drug repurposing strategy for the rapid identification of potential SARS-CoV-2 viral inhibitors. Sci. Rep. 2020, 10, 13866. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.S.; Rangel, F.S.; Santos, N.O.; Freitas, A.S.; Soares, W.R.A.; Siqueira, S.; Barh, D.; Góes-Neto, A.; Birbrair, A.; Azevedo, V.A.C. Repurposing approved drugs for guiding COVID-19 prophylaxis: A systematic review. Front. Pharmacol. 2020, 11, 590598. [Google Scholar] [CrossRef] [PubMed]
- Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. Available online: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/ (accessed on 23 November 2021).
- Ma, C.; Sacco, M.D.; Hurst, B.; Townsend, J.A.; Hu, Y.; Szeto, T.; Zhang, X.; Tarbet, B.; Marty, M.T.; Chen, Y.; et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020, 30, 678–692. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, C.; Zhang, Q.; Zhang, R.; Zhao, X.; Duan, Y.; Wang, H.; Zhu, Y.; Feng, L.; Zhao, J.; et al. Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein Cell 2021, 10, 1–5. [Google Scholar] [CrossRef]
- Pavan, M.; Bolcato, G.; Bassani, D.; Sturlese, M.; Moro, S. Supervised molecular dynamics (SuMD) insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332. J. Enzyme Inhib. Med. Chem. 2021, 36, 1646–1650. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Graf, T.; Barcia, C.A.L.; Costa, V.F.; de Oliveira, J.L.; Passos, R.H.; Bastos, I.N.; de Santana, M.C.B.; Santos, I.M.; de Souza, K.A.F.; et al. SARS-CoV-2 variant of concern P.1 (Gamma) infection in young and middle-aged patients admitted to the intensive care units of a single hospital in Salvador, Northeast Brazil, February 2021. Int. J. Infect. Dis. 2021, 111, 47–54. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.E.; Sales, F.C.S.; Hawryluk, I.; Mccrone, J.T.; et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.; Fard, S.R.; Maleki, D.; Taher, M.T.; Yassin, Z.; Alimohamadi, Y.; Minaeian, S. Comparing the effectiveness of atazanavir/ritonavir/dolutegravir/hydroxychloroquine and lopinavir/ritonavir/hydroxychloroquine treatment regimens in COVID-19 patients. J. Med. Virol. 2021, 93, 6557–6565. [Google Scholar] [CrossRef]
- Antiviral Agents Against COVID-19 Infection (REVOLUTIOn). Available online: https://clinicaltrials.gov/ct2/show/NCT04468087?term=Atazanavir&cond=SARS-CoV+Infection&draw=2&rank=3 (accessed on 6 November 2021).
- Bain, W.; Lee, J.S.; Watson, A.M.; Stitt-Fischer, M.S. Practical guidelines for collection, manipulation and inactivation of SARS-CoV-2 and COVID-19 clinical specimens. Curr. Protoc. Cytom. 2020, 93, e77. [Google Scholar] [CrossRef] [PubMed]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identifcation of ebselen and its analogues as potent covalente inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2020, 11, 3640. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the past: Possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem 2020, 21, 730–738. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafna, K.; Krug, R.M.; Montelione, G.T. Structural similarity of SARS-CoV2 Mpro and HCV NS3/4A proteases suggests new approaches for identifying existing drugs useful as COVID-19 therapeutics. ChemRxiv 2020, 1–38. [Google Scholar]
- Wavefunction, Inc. Available online: https://www.wavefun.com/ (accessed on 6 November 2021).
- GOLD: Protein-Ligand Docking Software. Available online: http://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/ (accessed on 6 November 2021).
- PyMOL by Schrödinger. Available online: https://pymol.org/2/ (accessed on 6 November 2021).
- Zentrum für Bioinformatik: Universität Hamburg—ProteinsPlus. Available online: https://proteins.plus/ (accessed on 16 November 2021).
- Puhl, A.C.; Fritch, E.J.; Lane, T.R.; Tse, L.V.; Yount, B.L.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Tavella, T.A.; Costa, F.T.M.; Weston, S.; et al. Repurposing the ebola and marburg virus inhibitors tilorone, quinacrine, and pyronaridine: In vitro activity against SARS-CoV-2 and potential mechanisms. ACS Omega 2021, 6, 7454–7468. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, C.Q.; Fintelman-Rodrigues, N.; Temerozo, J.R.; da Silva, A.P.D.; Días, S.S.G.; da Silva, C.S.; Ferreira, A.C.; Mattos, M.; Pão, C.R.R.; de Freitas, C.S.; et al. In vitro antiviral activity of the anti-HCV drugs daclatasvir and sofosbuvir against SARS-CoV-2, the etiological agent of COVID-19. J. Antimicrob. Chemother. 2021, 76, 1874–1885. [Google Scholar] [CrossRef] [PubMed]

| ATV | RDV | ||||||
|---|---|---|---|---|---|---|---|
| Strain | MOI | EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI |
| B.1 | 0.1 | 0.490 ± 0.020 | 312 ± 8 | 637 | 0.461 ± 0.038 | 512 ± 30 | 1111 |
| B.1 | 0.001 | 0.321 ± 0.016 | 972 | 0.225 ± 0.019 | 2276 | ||
| P1 (formally B.1.1.28) | 0.1 | 0.399 ± 0.020 | 782 | 0.113 ± 0.010 | 4530 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, O.A.; Sacramento, C.Q.; Ferreira, A.C.; Mattos, M.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Vazquez, L.; Pinto, D.P.; da Silveira, G.P.E.; da Fonseca, L.B.; et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals 2022, 15, 21. https://doi.org/10.3390/ph15010021
Chaves OA, Sacramento CQ, Ferreira AC, Mattos M, Fintelman-Rodrigues N, Temerozo JR, Vazquez L, Pinto DP, da Silveira GPE, da Fonseca LB, et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals. 2022; 15(1):21. https://doi.org/10.3390/ph15010021
Chicago/Turabian StyleChaves, Otávio Augusto, Carolina Q. Sacramento, André C. Ferreira, Mayara Mattos, Natalia Fintelman-Rodrigues, Jairo R. Temerozo, Leonardo Vazquez, Douglas Pereira Pinto, Gabriel P. E. da Silveira, Laís Bastos da Fonseca, and et al. 2022. "Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo" Pharmaceuticals 15, no. 1: 21. https://doi.org/10.3390/ph15010021
APA StyleChaves, O. A., Sacramento, C. Q., Ferreira, A. C., Mattos, M., Fintelman-Rodrigues, N., Temerozo, J. R., Vazquez, L., Pinto, D. P., da Silveira, G. P. E., da Fonseca, L. B., Pereira, H. M., Carlos, A. S., d’Avila, J. C., Viola, J. P. B., Monteiro, R. Q., Bozza, P. T., Castro-Faria-Neto, H. C., & Souza, T. M. L. (2022). Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals, 15(1), 21. https://doi.org/10.3390/ph15010021









