Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing
Abstract
:1. Introduction
2. Results
2.1. Datasets
2.2. Molecular Descriptors
2.3. Murcko Frameworks Profile
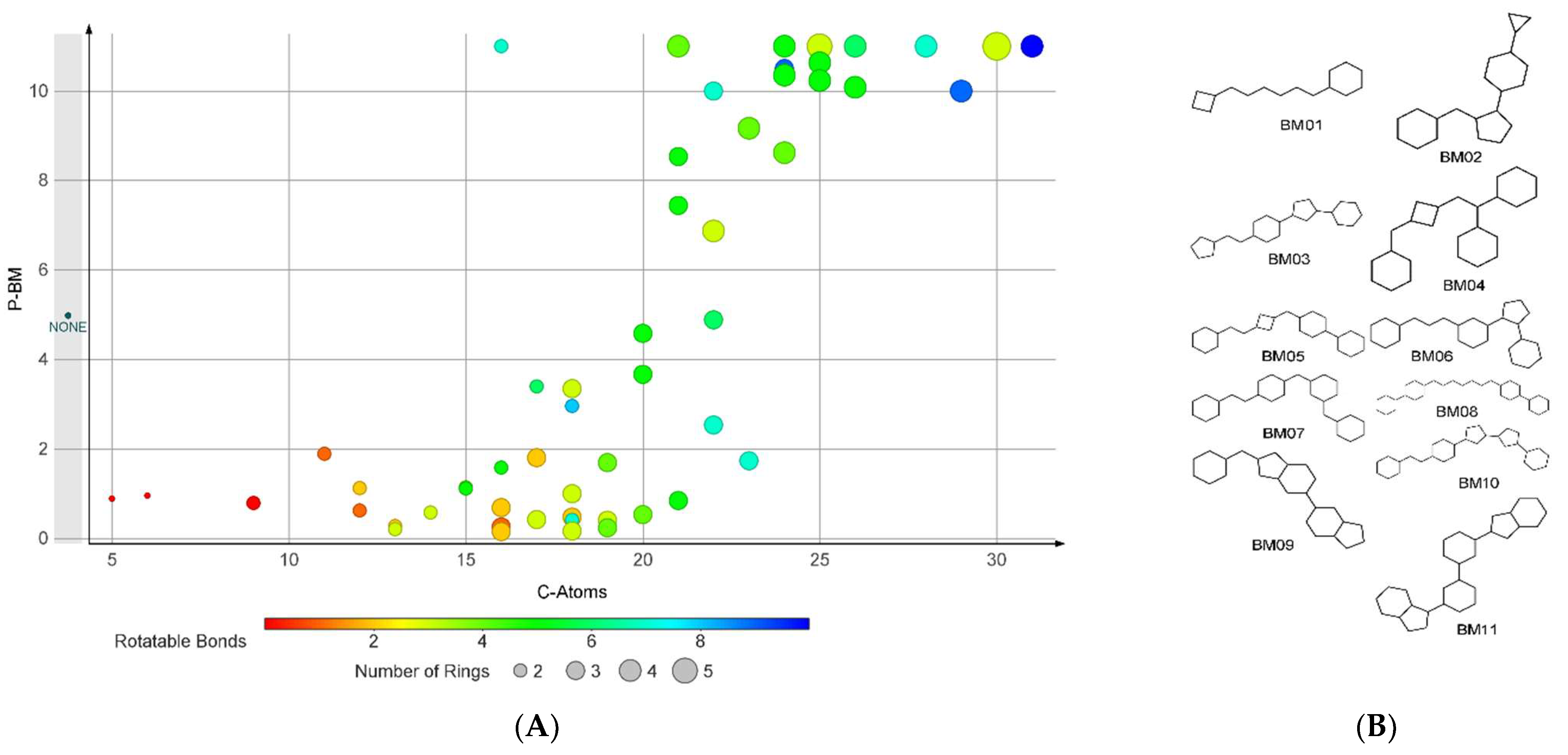
2.4. Bemis-Murcko Skeletons Profile
2.5. Plain Ring Analysis
2.6. Classification Model
2.7. Molecular Docking
2.8. Repurposing Study
2.9. Simulation of FAAH-Montelukast Complex
3. Discussion
4. Materials and Methods
4.1. Datasets Preparation
4.2. Molecular Descriptors
4.3. Bemis-Murcko Skeletons and Murcko Frameworks Analysis
4.4. Plain Ring Analysis
4.5. Molecular Docking
4.6. Molecular Dynamics Simulation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burston, J.J.; Woodhams, S.G. Endocannabinoid system and pain: An introduction. Proc. Nutr. Soc. 2014, 73, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Console-Bram, L.; Mundy, C.; Popoff, S.N.; Kapur, A.; Abood, M.E. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J. Neuroimmune Pharmacol. 2012, 7, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Iannotti, F.A.; Vitale, R.M. The endocannabinoid system and PPARs: Focus on their signalling crosstalk, action and transcriptional regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Nie, Y.; Tian, Y.; Xiao, X.; Yang, F. Endocannabinoid activation of the TRPV1 ion channel is distinct from activation by capsaicin. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef] [PubMed]
- Anthony, A.T.; Rahmat, S.; Sangle, P.; Sandhu, O.; Khan, S. Cannabinoid receptors and their relationship with chronic pain: A narrative review. Cureus 2020, 12, e10436. [Google Scholar] [CrossRef]
- Mielnik, C.A.; Lam, V.M.; Ross, R.A. CB1 allosteric modulators and their therapeutic potential in CNS disorders. Prog. Neuro. Psychopharmacol. Biol. Psychiatry 2021, 106, 110163. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Le Foll, B. Targeting the endocannabinoid CB1 receptor to treat body weight disorders: A preclinical and clinical review of the therapeutic potential of past and present CB1 drugs. Biomolecules 2020, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Ann. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Zanfirescu, A.; Ungurianu, A.; Mihai, D.P.; Radulescu, D.; Nitulescu, G.M. Targeting monoacylglycerol lipase in pursuit of therapies for neurological and neurodegenerative diseases. Molecules 2021, 26, 5668. [Google Scholar] [CrossRef] [PubMed]
- Flannery, L.E.; Kerr, D.M.; Finn, D.P.; Roche, M. FAAH inhibition attenuates TLR3-mediated hyperthermia, nociceptive- and anxiety-like behaviour in female rats. Behav. Brain Res. 2018, 353, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Kinsey, S.G.; Lichtman, A.H. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009, 11, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.J. The cannabinoid system and its pharmacological manipulation—A review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam. Clin. Pharmacol. 2006, 20, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Cardoso-Cruz, H.; Galhardo, V. Animal models of congenital hypoalgesia: Untapped potential for assessing pain-related plasticity. Neurosci. Lett. 2019, 702, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.; Okorokov, A.L.; Hill, M.N.; Bras, J.T.; Lee, M.-C.; Li, S.; Gossage, S.J.; van Drimmelen, M.; Morena, M.; Houlden, H.; et al. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br. J. Anaesth. 2019, 123, e249–e253. [Google Scholar] [CrossRef] [Green Version]
- Vacondio, F.; Silva, C.; Lodola, A.; Fioni, A.; Rivara, S.; Duranti, A.; Tontini, A.; Sanchini, S.; Clapper, J.R.; Piomelli, D.; et al. Structure-property relationships of a class of carbamate-based fatty acid amide hydrolase (FAAH) inhibitors: Chemical and biological stability. ChemMedChem 2009, 4, 1495–1504. [Google Scholar] [CrossRef] [Green Version]
- Mor, M.; Lodola, A.; Rivara, S.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Piersanti, G.; Clapper, J.R.; King, A.R.; et al. Synthesis and quantitative structure−activity relationship of fatty acid amide hydrolase inhibitors: Modulation at the N-portion of biphenyl-3-yl alkylcarbamates. J. Med. Chem. 2008, 51, 3487–3498. [Google Scholar] [CrossRef] [Green Version]
- Otrubova, K.; Ezzili, C.; Boger, D.L. The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH). Bioorg. Med. Chem. Lett. 2011, 21, 4674–4685. [Google Scholar] [CrossRef] [Green Version]
- Seierstad, M.; Breitenbucher, J.G. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J. Med. Chem. 2008, 51, 7327–7343. [Google Scholar] [CrossRef]
- Palermo, G.; Rothlisberger, U.; Cavalli, A.; De Vivo, M. Computational insights into function and inhibition of fatty acid amide hydrolase. Eur. J. Med. Chem. 2015, 91, 15–26. [Google Scholar] [CrossRef]
- Bowman, A.L.; Makriyannis, A. Approximating protein flexibility through dynamic pharmacophore models: Application to fatty acid amide hydrolase (FAAH). J. Chem. Inf. Model. 2011, 51, 3247–3253. [Google Scholar] [CrossRef] [Green Version]
- Mileni, M.; Johnson, D.S.; Wang, Z.; Everdeen, D.S.; Liimatta, M.; Pabst, B.; Bhattacharya, K.; Nugent, R.A.; Kamtekar, S.; Cravatt, B.F.; et al. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc. Natl. Acad. Sci. USA 2008, 105, 12820–12824. [Google Scholar] [CrossRef] [Green Version]
- Haller, J.; Goldberg, S.R.; Pelczer, K.G.; Aliczki, M.; Panlilio, L.V. The effects of anandamide signaling enhanced by the FAAH inhibitor URB597 on coping styles in rats. Psychopharmacology 2013, 230, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Li, G.L.; Winter, H.; Arends, R.; Jay, G.W.; Le, V.; Young, T.; Huggins, J.P. Assessment of the pharmacology and tolerability of PF-04457845, an irreversible inhibitor of fatty acid amide hydrolase-1, in healthy subjects. Br. J. Clin. Pharmacol. 2012, 73, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Smith, S.E.; Liimatta, M.B.; Beidler, D.; Sadagopan, N.; Dudley, D.T.; Young, T.; Wren, P.; Zhang, Y.; Swaney, S.; et al. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective fatty acid amide hydrolase Inhibitor that reduces inflammatory and noninflammatory pain. J. Pharmacol. Exp. Ther. 2011, 338, 114–124. [Google Scholar] [CrossRef]
- Sagar, D.R.; Jhaveri, M.; Chapman, V. Targeting the Cannabinoid System to Produce Analgesia. In Behavioral Neurobiology of the Endocannabinoid System; Springer: Berlin/Heidelberg, Germany, 2009; Volume 1, pp. 275–287. [Google Scholar]
- Sagar, D.R.; Kendall, D.A.; Chapman, V. Inhibition of fatty acid amide hydrolase produces PPAR-α-mediated analgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 2008, 155, 1297–1306. [Google Scholar] [CrossRef] [Green Version]
- Schuelert, N.; Johnson, M.P.; Oskins, J.L.; Jassal, K.; Chambers, M.G.; McDougall, J.J. Local application of the endocannabinoid hydrolysis inhibitor URB597 reduces nociception in spontaneous and chemically induced models of osteoarthritis. Pain 2011, 152, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-X.; Ke, B.-W.; Liu, J.; Ma, G.; Hai, K.-R.; Gong, D.-Y.; Yang, Z.; Zhou, C. Inhibition of fatty acid amide hydrolase improves depressive-like behaviors independent of its peripheral antinociceptive effects in a rat model of neuropathic pain. Anesth. Analg. 2019, 129, 587–597. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Liebowitz, M.R.; Stein, M.B.; Grunfeld, J.; Van Hove, I.; Simmons, W.K.; Van Der Ark, P.; Palmer, J.A.; Saad, Z.S.; Pemberton, D.J.; et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: A double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology 2020, 46, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, R.; Mercolini, L. Discontinued anxiolytic drugs (2009–2014). Expert Opin. Investig. Drugs 2015, 24, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Pawsey, S.; Wood, M.; Browne, H.; Donaldson, K.; Christie, M.; Warrington, S. Safety, tolerability and pharmacokinetics of FAAH inhibitor V158866: A double-blind, randomised, placebo-controlled phase I study in healthy volunteers. Drugs R&D 2016, 16, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Bhuniya, D.; Kharul, R.K.; Hajare, A.; Shaikh, N.; Bhosale, S.; Balwe, S.; Begum, F.; De, S.; Athavankar, S.; Joshi, D.; et al. Discovery and evaluation of novel FAAH inhibitors in neuropathic pain model. Bioorg. Med. Chem. Lett. 2018, 29, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, A.; Ferré, J.-C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.-Y.; Le Tulzo, Y.; et al. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, D.P.; Nitulescu, G.M.; Ion, G.N.D.; Ciotu, C.I.; Chirita, C.; Negres, S. Computational drug repurposing algorithm targeting TRPA1 calcium channel as a potential therapeutic solution for multiple sclerosis. Pharmaceutics 2019, 11, 446. [Google Scholar] [CrossRef] [Green Version]
- Mendez, D.; Gaulton, A.; Bento, A.P.; Chambers, J.; de Veij, M.; Félix, E.; Magariños, M.P.; Mosquera, J.F.; Mutowo, P.; Nowotka, M.; et al. ChEMBL: Towards direct deposition of bioassay data. Nucleic Acids Res. 2019, 47, D930–D940. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Shoichet, B.K.; Irwin, J.J. Benchmarking sets for molecular docking. J. Med. Chem. 2006, 49, 6789–6801. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Bemis, G.W.; Murcko, M.A. The properties of known drugs. 1. Molecular frameworks. J. Med. Chem. 1996, 39, 2887–2893. [Google Scholar] [CrossRef]
- Warren, G.L.; Andrews, C.W.; Capelli, A.-M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A critical assessment of docking programs and scoring functions. J. Med. Chem. 2005, 49, 5912–5931. [Google Scholar] [CrossRef]
- Min, X.; Thibault, S.T.; Porter, A.C.; Gustin, D.J.; Carlson, T.J.; Xu, H.; Lindstrom, M.; Xu, G.; Uyeda, C.; Ma, Z.; et al. Discovery and molecular basis of potent noncovalent inhibitors of fatty acid amide hydrolase (FAAH). Proc. Natl. Acad. Sci. USA 2011, 108, 7379–7384. [Google Scholar] [CrossRef] [Green Version]
- Manzanares, J.; Julian, M.D.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain activity of anandamide: A rewarding bliss? Acta Pharmacol. Sin. 2019, 40, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Bluett, R.J.; Gamble-George, J.C.; Hermanson, D.J.; Hartley, N.D.; Marnett, L.J.; Patel, S. Central anandamide deficiency predicts stress-induced anxiety: Behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry 2014, 4, e408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt-Schreiner, K.; Valek, L.; Kang, J.S.; Khlebtovsky, A.; Trautmann, S.; Hahnefeld, L.; Schreiber, Y.; Gurke, R.; Thomas, D.; Wilken-Schmitz, A.; et al. High glucosylceramides and low anandamide contribute to sensory loss and pain in Parkinson’s disease. Mov. Disord. 2020, 35, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Lorca, M.; Valdes, Y.; Chung, H.; Romero-Parra, J.; Pessoa-Mahana, D.; Mella, J.; Parra, R.; Mahana, P. Three-dimensional quantitative structure-activity relationships (3D-QSAR) on a series of piperazine-carboxamides fatty acid amide hydrolase (FAAH) inhibitors as a useful tool for the design of new cannabinoid ligands. Int. J. Mol. Sci. 2019, 20, 2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabrizi, M.A.; Baraldi, P.G.; Ruggiero, E.; Saponaro, G.; Baraldi, S.; Romagnoli, R.; Martinelli, A.; Tuccinardi, T. Pyrazole phenylcyclohexylcarbamates as inhibitors of human fatty acid amide hydrolases (FAAH). Eur. J. Med. Chem. 2015, 97, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, Y.; Li, Y.; Ren, J. Discovery of uracil derivatives as potent inhibitors of fatty acid amide hydrolase. Molecules 2016, 21, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sarris, K.; Kage, K.; Zhang, D.; Brown, S.P.; Kolasa, T.; Surowy, C.; El Kouhen, O.F.; Muchmore, S.W.; Brioni, J.D.; et al. Synthesis and evaluation of benzothiazole-based analogues as novel, potent, and selective fatty acid amide hydrolase inhibitors. J. Med. Chem. 2008, 52, 170–180. [Google Scholar] [CrossRef]
- Zięba, A.; Laitinen, T.; Patel, J.; Poso, A.; Kaczor, A. Docking-based 3D-QSAR studies for 1,3,4-oxadiazol-2-one derivatives as FAAH inhibitors. Int. J. Mol. Sci. 2021, 22, 6108. [Google Scholar] [CrossRef]
- Mileni, M.; Garfunkle, J.; Ezzili, C.; Cravatt, B.F.; Stevens, R.C.; Boger, D.L. Fluoride-mediated capture of a noncovalent bound state of a reversible covalent enzyme inhibitor: X-ray crystallographic analysis of an exceptionally potent α-ketoheterocycle inhibitor of fatty acid amide hydrolase. J. Am. Chem. Soc. 2011, 133, 4092–4100. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanzadeh, B.; Mansouri, M.T.; Sahraei, H.; Alboghobeish, S. Involvement of opioid receptors in the systemic and peripheral antinociceptive actions of montelukast in the animal models of pain. Eur. J. Pharmacol. 2016, 779, 38–45. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, X.; Huang, H.; Zhu, Y.; Wu, Y. Montelukast attenuates neuropathic pain through inhibiting p38 mitogen-activated protein kinase and nuclear factor-kappa B in a rat model of chronic constriction injury. Anesth. Analg. 2014, 118, 1090–1096. [Google Scholar] [CrossRef]
- Gizzo, S.; Saccardi, C.; Patrelli, T.S.; Berretta, R.; Capobianco, G.; Di Gangi, S.; Vacilotto, A.; Bertocco, A.; Noventa, M.; Ancona, E.; et al. Update on raloxifene: Mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstet. Gynecol. Surv. 2013, 68, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Fujii, Y.; Munezane, H.; Ohue, M.; Takagi, Y. Analgesic effect of raloxifene on back and knee pain in postmenopausal women with osteoporosis and/or osteoarthritis. J. Bone Miner. Metab. 2010, 28, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Scharla, S.; Oertel, H.; Helsberg, K.; Kessler, F.; Langer, F.; Nickelsen, T. Skeletal pain in postmenopausal women with osteoporosis: Prevalence and course during raloxifene treatment in a prospective observational study of 6 months duration. Curr. Med. Res. Opin. 2006, 22, 2393–2402. [Google Scholar] [CrossRef]
- Sadreddini, S.; Molaeefard, M.; Noshad, H.; Ardalan, M.; Asadi, A. Efficacy of raloxifene in treatment of fibromyalgia in menopausal women. Eur. J. Intern. Med. 2008, 19, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, J. Revefenacin for the treatment of chronic obstructive pulmonary disease. Expert Rev. Clin. Pharmacol. 2019, 12, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J. Mechanism of action of a new class of insulin secretagogues. Exp. Clin. Endocrinol. Diabetes 1999, 107 (Suppl. 4), S140–S143. [Google Scholar] [CrossRef]
- Rico, S.; Antonijoan, R.; Barbanoj, M. Ebastine in the light of CONGA recommendations for the development of third-generation antihistamines. J. Asthma Allergy 2009, 2, 73–92. [Google Scholar]
- Tracey, W.D., Jr. Nociception. Curr. Biol. 2017, 27, R129–R133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, H.-G. Peripheral and central mechanisms of pain generation. Analgesia 2007, 177, 3–28. [Google Scholar] [CrossRef]
- Sapunar, D.; Kostic, S.; Banozic, A.; Puljak, L. Dorsal root ganglion—A potential new therapeutic target for neuropathic pain. J. Pain Res. 2012, 5, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.M.; Blanton, H.L.; Pietrzak, A.; Little, W.; Sherfey, C.; Guindon, J. Front and hind paw differential analgesic effects of amitriptyline, gabapentin, ibuprofen, and URB937 on mechanical and cold sensitivity in cisplatin-induced neuropathy. Mol. Pain 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, R.; Bandiera, T.; Mangione, A.; DeMartini, C.; Siani, F.; Nappi, G.; Sandrini, G.; Guijarro, A.; Armirotti, A.; Piomelli, D.; et al. Effects of peripheral FAAH blockade on NTG-induced hyperalgesia-evaluation of URB937 in an animal model of migraine. Cephalalgia 2015, 35, 1065–1076. [Google Scholar] [CrossRef] [Green Version]
- Kinsey, S.G.; Naidu, P.S.; Cravatt, B.F.; Dudley, D.T.; Lichtman, A.H. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol. Biochem. Behav. 2011, 99, 718–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wang, L.; Van Berkel, G.J.; Kertesz, V.; Gan, J. Quantitation of repaglinide and metabolites in mouse whole-body thin tissue sections using droplet-based liquid microjunction surface sampling-high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2016, 1439, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, Y.; Boström, M.; Sandelius, Å.; Blennow, K.; Zetterberg, H.; Kuhn, G.; Kalm, M. The anti-asthmatic drug, montelukast, modifies the neurogenic potential in the young healthy and irradiated brain. Cell Death Dis. 2018, 9, 775. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, G.A.; Al-Badr, A.A. Buclizine. Profiles Drug Subst. Excip. Relat. Methodol. 2011, 36, 1–33. [Google Scholar]
- Veenman, L. Raloxifene as treatment for various types of brain injuries and neurodegenerative diseases: A good start. Int. J. Mol. Sci. 2020, 21, 7586. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminfo. 2011, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.W. PaDEL-descriptor: An open-source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. In Protein Engineering; Humana Press: New York, NY, USA, 2017; pp. 43–67. [Google Scholar]
- Mileni, M.; Kamtekar, S.; Wood, D.C.; Benson, T.E.; Cravatt, B.F.; Stevens, R.C. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: Discovery of a deacylating water molecule and insight into enzyme inactivation. J. Mol. Biol. 2010, 400, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Bertolacci, L.; Romeo, E.; Veronesi, M.; Magotti, P.; Albani, C.; Dionisi, M.; Lambruschini, C.; Scarpelli, R.; Cavalli, A.; De Vivo, M.; et al. A binding site for nonsteroidal anti-inflammatory drugs in fatty acid amide hydrolase. J. Am. Chem. Soc. 2012, 135, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Saha, A.; Shih, A.Y.; Mirzadegan, T.; Seierstad, M. Predicting the binding of fatty acid amide hydrolase inhibitors by free energy perturbation. J. Chem. Theory Comput. 2018, 14, 5815–5822. [Google Scholar] [CrossRef]
- Krieger, E.; Dunbrack, R.L.; Hooft, R.W.W.; Krieger, B. Assignment of protonation states in proteins and ligands: Combining pKa prediction with hydrogen bonding network optimization. In Computational Drug Discovery and Design; Springer: New York, NY, USA, 2011; pp. 405–421. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins Struct. Funct. Bioinform. 2006, 65, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.; Gao, J.; York, D.M. An efficient linear-scaling Ewald method for long-range electrostatic interactions in combined QM/MM calculations. J. Chem. Theory Comput. 2004, 1, 2–13. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef]










| Set | nF | nCl | nBr | nI | nX | nOH | nCOOH | nCONH2 | nNH2 | nNO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| FI | 0–8 | 0–3 | 0–2 | 0–1 | 0–8 | 0–2 | 0–1 | 0–1 | 0–1 | 0–2 |
| DCY | 0–10 | 0–6 | 0–5 | 0–3 | 0–10 | 0–4 | 0–3 | 0–1 | 0–3 | 0–2 |
| Code | Class | Type | Cutoff | Descriptor’s Mathematical Representation |
|---|---|---|---|---|
| CrippenLogP | 2D | Crippen | >4.582 | Crippen’s LogP |
| SpMAD_D | 2D | Topological | >12.569 | spectral mean absolute deviation from the topological distance matrix |
| SpMax5_Bhi | 2D | Burden modified | >3.333 | largest absolute eigenvalue of Burden modified matrix—n 5/weighted by relative first ionization potential |
| Au | 3D | WHIM | >98.038 | a total size index—unweighted |
| Ae | 3D | WHIM | >96.826 | a total size index—weighted by relative Sanderson electronegativities |
| Ai | 3D | WHIM | >100.252 | a total size index- weighted by relative first ionization potential |
| As | 3D | WHIM | >97.668 | a total size index—weighted by relative I-state |
| Av | 3D | WHIM | >81.277 | a total size index—weighted by relative van der Waals volumes |
| RDF85m | 3D | RDF | >5.699 | radial distribution function—085/weighted by relative mass |
| WPSA-1 | 3D | CPSA | >382.111 | partial positive surface area (PPSA-1) × total molecular surface area/1000 |
| WPSA-2 | 3D | CPSA | >767.505 | partial positive surface area × total positive charge on the molecule (PPSA-2) × total molecular surface area/1000 |
| Code | Name | RpS | Category |
|---|---|---|---|
| DB06442 | Avasimibe | 15.38 | investigational |
| DB08078 | {4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy}acetic acid | 15.38 | experimental |
| DB12390 | MBX-8025 | 15.38 | investigational |
| DB07142 | 5-[(3R)-3-(5-methoxy-3′,5′-dimethylbiphenyl-3-yl)but-1-yn-1-yl]-6-methylpyrimidine-2,4-diamine | 14.19 | experimental |
| DB07144 | 5-[(3R)-3-(5-methoxy-2′,6′-dimethylbiphenyl-3-yl)but-1-yn-1-yl]-6-methylpyrimidine-2,4-diamine | 14.19 | experimental |
| DB11718 | Encorafenib | 13.82 | approved |
| DB12170 | Veledimex | 13.32 | investigational |
| DB12226 | Terameprocol | 13.32 | investigational |
| DB08896 | Regorafenib | 13.25 | approved |
| DB12524 | BI-671800 | 13.22 | investigational |
| DB09171 | β-Methylfentanyl | 13.10 | illicit |
| DB09174 | Lofentanil | 13.10 | illicit |
| DB09179 | R-30490 | 13.10 | experimental |
| DB13016 | LY-2300559 | 13.02 | investigational |
| DB15052 | Ansofaxine | 13.02 | investigational |
| DB13232 | Suxibuzone | 11.33 | experimental |
| DB04741 | Myxothiazol | 8.61 | experimental |
| DB01347 | Saprisartan | 7.54 | experimental |
| DB07238 | Nesbuvir | 7.52 | investigational |
| DB16169 | Fonadelpar | 7.51 | investigational |
| Code | Name | RpS | ΔG (kcal/mol) | LE | No. of Contacts | H-Bonding Residues | H-Bond Length (Å) | Category |
|---|---|---|---|---|---|---|---|---|
| DB00481 | Raloxifene | 7.23 | −10.464 | 0.3078 | 21 | Lys142 | 2.51 | approved |
| Cys269 | 1.84 | investigational | ||||||
| Val270 | 2.28 | |||||||
| Leu278 | 2.57 | |||||||
| DB00471 | Montelukast | 7.06 | −10.298 | 0.2512 | 27 | Lys142 | 2.69 | approved |
| Lys142 | 2.53 | |||||||
| Ser217 | 2.17 | |||||||
| Ser241 | 2.80 | |||||||
| Gly272 | 2.55 | |||||||
| Gln273 | 1.94 | |||||||
| DB11855 | Revefenacin | 6.91 | −12.020 | 0.2732 | 27 | Ile238 | 1.81 | approved |
| (noncovalent) | Gly239 | 2.60 | investigational | |||||
| Gly240 | 3.00 | |||||||
| Sert241 | 2.33 | |||||||
| Trp531 | 1.85 | |||||||
| Met191 | 2.65 | |||||||
| Revefenacin | −5.710 | 0.3807 | 14 | Ser217 | 2.16 | |||
| (covalent) | Thr236 | 2.72 | ||||||
| DB00912 | Repaglinide | 6.89 | −9.306 | 0.2820 | 21 | Ser193 | 2.04 | approved |
| Thr488 | 2.13 | investigational | ||||||
| DB00354 | Buclizine | 6.87 | −9.808 | 0.3164 | 21 | Thr488 | 1.70 | approved |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanfirescu, A.; Nitulescu, G.; Mihai, D.P.; Nitulescu, G.M. Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals 2022, 15, 38. https://doi.org/10.3390/ph15010038
Zanfirescu A, Nitulescu G, Mihai DP, Nitulescu GM. Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals. 2022; 15(1):38. https://doi.org/10.3390/ph15010038
Chicago/Turabian StyleZanfirescu, Anca, Georgiana Nitulescu, Dragos Paul Mihai, and George Mihai Nitulescu. 2022. "Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing" Pharmaceuticals 15, no. 1: 38. https://doi.org/10.3390/ph15010038
APA StyleZanfirescu, A., Nitulescu, G., Mihai, D. P., & Nitulescu, G. M. (2022). Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals, 15(1), 38. https://doi.org/10.3390/ph15010038







