Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes
Abstract
1. Introduction
2. Results
2.1. Extraction, Characterization, and Purification of Twelve Compounds from GARCINIA Mangostana Fruit
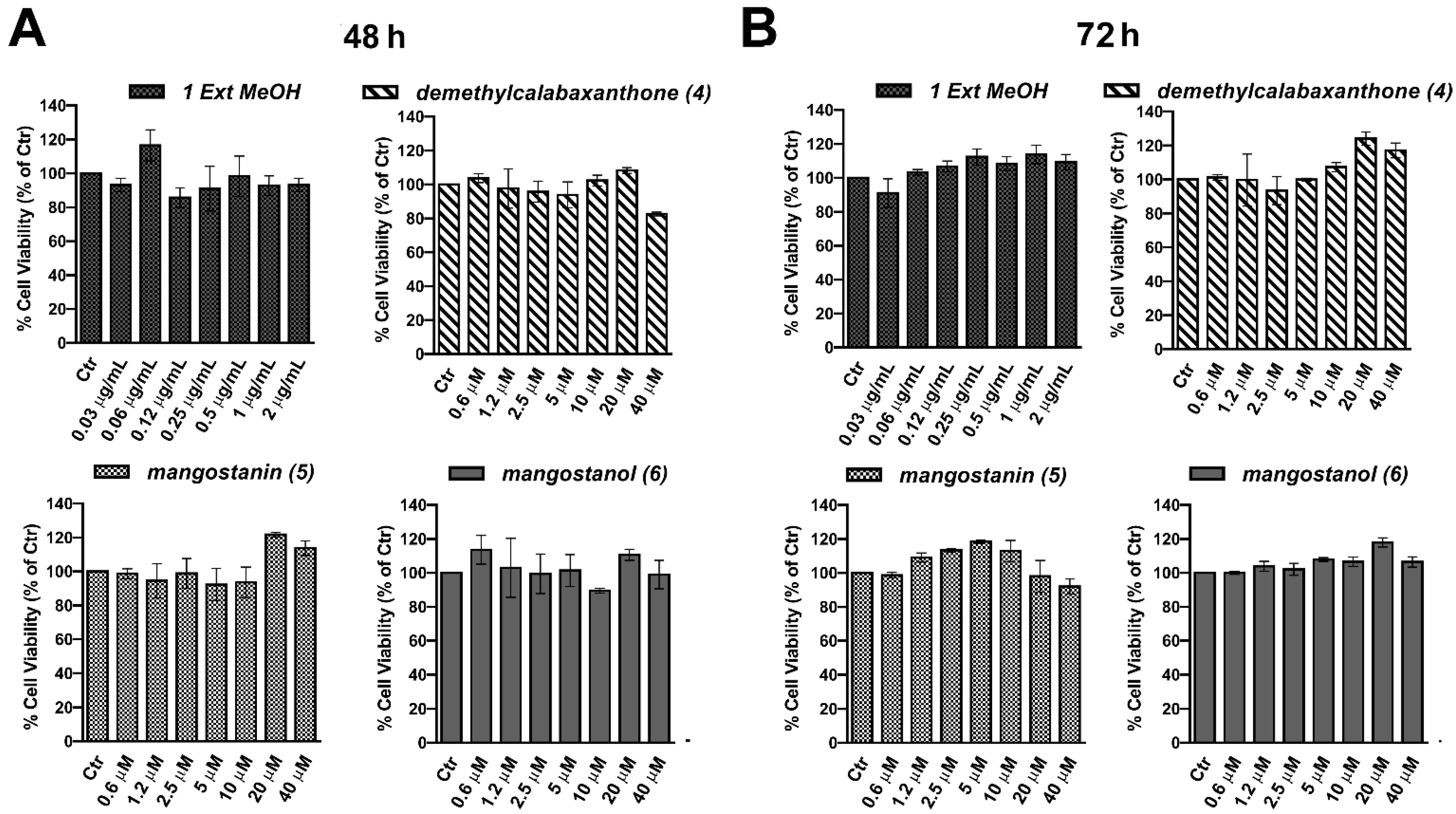
2.2. Evaluation of the Biological Effect of Garcinia mangostana Extracts and Isolated Compounds in Human Keratinocytes
2.3. Mangostanin Reverts and Prevents Cytotoxicity Induced by H2O2 in HaCaT Cells
2.4. Mangostanin Prevents the Generation of Intracellular Reactive Oxygen Species (ROS)
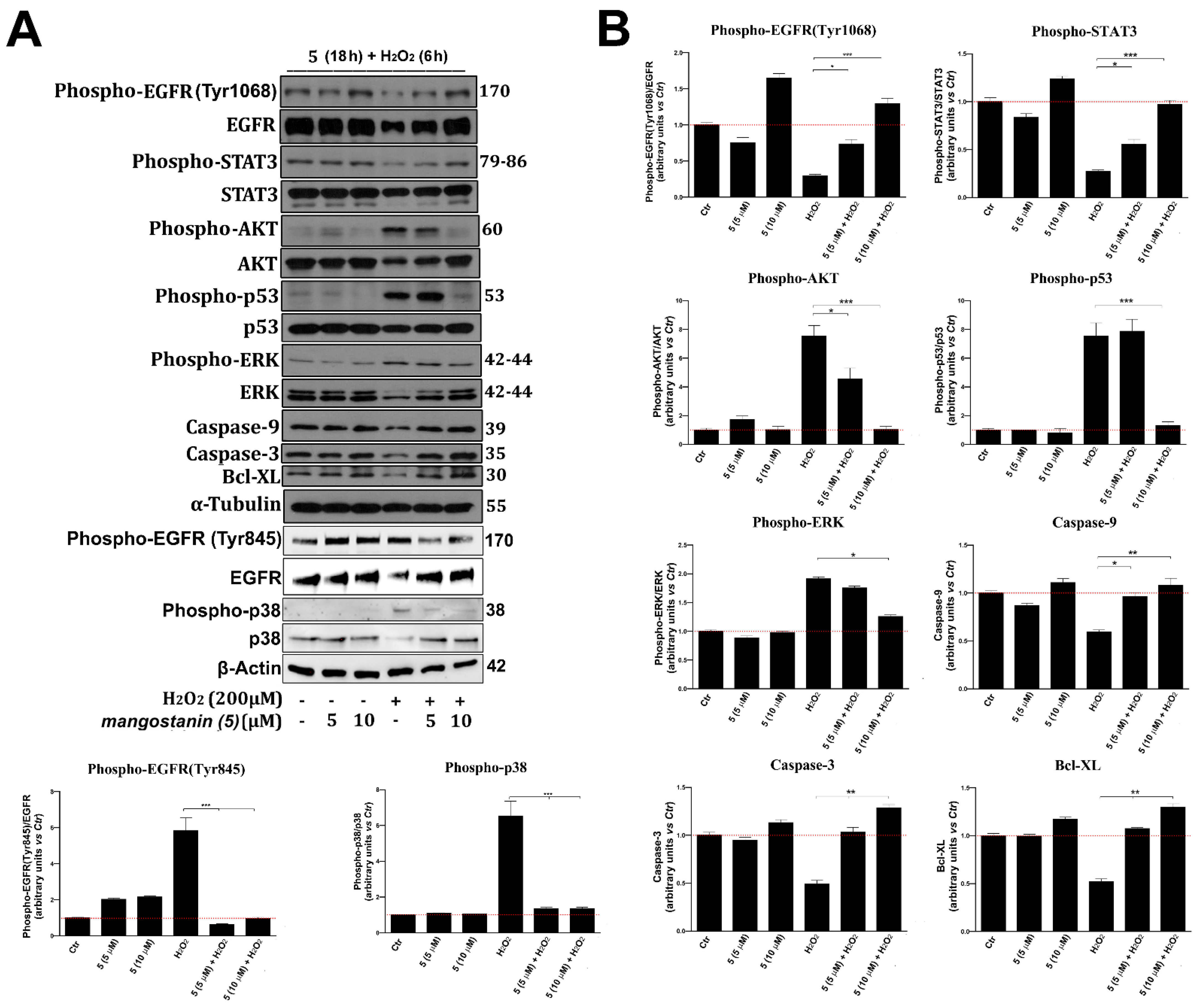
2.5. Mangostanin Prevents the Activation of Cell Death Molecular Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Plant Material
4.3. Extraction and Isolation Procedures of G. mangostana Arils and Shells
4.4. Quantitative Analysis of Mangostanin (5)
4.5. Cells
4.6. Determination of Cell Viability—MTT Assay
4.7. Determination of Reactive Oxygen Species (ROS)—Flow Cytometric Assay
4.8. Western Blot (WB) Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masullo, M.; Menegazzi, M.; Di Micco, S.; Beffy, P.; Bifulco, G.; Dal Bosco, M.; Novelli, M.; Pizza, C.; Masiello, P.; Piacente, S. Direct Interaction of Garcinol and Related Polyisoprenylated Benzophenones of Garcinia cambogia Fruits with the Transcription Factor STAT-1 as a Likely Mechanism of Their Inhibitory Effect on Cytokine Signaling Pathways. J. Nat. Prod. 2014, 77, 543–549. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109 Pt 1, 102–122. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Wang, X.; An, L.; Bao, J.; Zhang, J.; Cui, J.; Li, Y.; Jin, D.Q.; Tuerhong, M.; et al. Isolation, structural elucidation, and immunoregulation properties of an arabinofuranan from the rinds of Garcinia mangostana. Carbohydr. Polym. 2020, 246, 116567. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, V.T.; Le, H.T.; Nguyen, H.M.; Tran, T.H.; Do Thi, T.; Nguyen, X.C.; Ha, M.T. Garcinoxanthones SV, new xanthone derivatives from the pericarps of Garcinia mangostana together with their cytotoxic and antioxidant activities. Fitoterapia 2021, 151, 104880. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Siemak, J.; Klimowicz, A. Antioxidant activity and polyphenol content in extracts from various parts of fresh and frozen mangosteen. Acta Sci. Pol. Technol. Aliment. 2020, 19, 261–270. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Schrader, K.K. Antibacterial Activity of Constituents from Mangosteen Garcinia mangostana Fruit Pericarp against Several Channel Catfish Pathogens. J. Aquat. Anim. Health 2018, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food Funct. 2018, 9, 3860–3871. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Anti-inflammatory effect of Garcinia mangostana Linn. pericarp extract in methicillin-resistant Staphylococcus aureus-induced superficial skin infection in mice. Biomed. Pharmacother. 2019, 111, 705–713. [Google Scholar] [CrossRef]
- Cho, J.; Chin, Y.W. Neuroprotective and Memory-Enhancing Effects of The Water Extract from Pericarp of Mangosteen Fruit. Clin. Ther. 2016, 38, e31. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Zakaria, T.M.; Susanti, D.; Zakaria, Z.A. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Labban, R.S.M.; Alfawaz, H.A.; Almnaizel, A.T.; Al-Muammar, M.N.; Bhat, R.S.; El-Ansary, A. Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci. Rep. 2021, 11, 7278. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, Y.T.; Lin, L.G. New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct. 2015, 6, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Nafady, A.M.; Mohamed, G.A. Mangostanaxanthone VIII, a new xanthone from Garcinia mangostana and its cytotoxic activity. Nat. Prod. Res. 2019, 33, 258–265. [Google Scholar] [CrossRef]
- Li, K.; Wu, L.; Chen, Y.; Li, Y.; Wang, Q.; Li, M.; Hao, K.; Zhang, W.; Jiang, S.; Wang, Z. Cytotoxic and Antiproliferative Effects of β-Mangostin on Rat C6 Glioma Cells Depend on Oxidative Stress Induction via PI3K/AKT/mTOR Pathway Inhibition. Drug Des. Dev. Ther. 2020, 14, 5315–5324. [Google Scholar] [CrossRef]
- Benatrehina, P.A.; Pan, L.; Naman, C.B.; Li, J.; Kinghorn, A.D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, S.Y.; Kim, Y.J.; Kim, Y.M.; Chin, Y.W. Absorption, tissue distribution, tissue metabolism and safety of α-mangostin in mangosteen extract using mouse models. Food Chem. Toxicol. 2014, 66, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Kim, Y.M.; Chin, Y.W.; Chae, S. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int. J. Mol. Med. 2017, 40, 1941–1949. [Google Scholar] [CrossRef][Green Version]
- Ohno, R.; Moroishi, N.; Sugawa, H.; Maejima, K.; Saigusa, M.; Yamanaka, M.; Nagai, M.; Yoshimura, M.; Amakura, Y.; Nagai, R. Mangosteen pericarp extract inhibits the formation of pentosidine and ameliorates skin elasticity. J. Clin. Biochem. Nutr. 2015, 57, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, S.F.; Shaari, K.; Stanslas, J.; Lajis, N.H.; Dachriyanus. Cytotoxic xanthones from the stem bark of Garcinia cowa Roxb. J. Chem. Pharm. Res. 2015, 7, 227–236. [Google Scholar]
- Sriyatep, S.; Siridechakorn, I.; Maneerat, W.; Pansanit, A.; Ritthiwigrom, T.; Andersen, R.J.; Laphookhieo, S. Bioactive Prenylated Xanthones from the Young Fruits and Flowers of Garcinia cowa. J. Nat. Prod. 2015, 78, 265–271. [Google Scholar] [CrossRef]
- Masullo, M.; Bassarello, C.; Bifulco, G.; Piacente, S. Polyisoprenylated benzophenone derivatives from the fruits of Garcinia cambogia and their absolute configuration by quantum chemical circular dichroism calculations. Tetrahedron 2010, 66, 139–145. [Google Scholar] [CrossRef]
- Masullo, M.; Bassarello, C.; Suzuki, H.; Pizza, C.; Piacente, S. Polyisoprenylated Benzophenones and an Unusual Polyisoprenylated Tetracyclic Xanthone from the Fruits of Garcinia cambogia. J. Agric. Food Chem. 2008, 56, 5205–5210. [Google Scholar] [CrossRef]
- Abate, M.; Pepe, G.; Randino, R.; Pisanti, S.; Basilicata, M.G.; Covelli, V.; Bifulco, M.; Cabri, W.; D’Ursi, A.M.; Campiglia, P.; et al. Ganoderma lucidum Ethanol Extracts Enhance Re-Epithelialization and Prevent Keratinocytes from Free-Radical Injury. Pharmaceuticals 2020, 13, 224. [Google Scholar] [CrossRef]
- Turner, N.A.; Xia, F.; Azhar, G.; Zhang, X.; Liu, L.; Wei, J.Y. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J. Mol. Cell Cardiol. 1998, 30, 1789–1801. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.H.; Nie, X.T.; Jiang, W.R.; Zhang, Y.S. Sodium butyrate ameliorates lipopolysaccharide-induced cow mammary epithelial cells from oxidative stress damage and apoptosis. J. Cell Biochem. 2018, 120, 2370–2381. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hu, J.; Li, D.; Wang, X.; Zhang, R.; Zhang, H.; Shi, M.; Chen, H. Cigarette smoke extract induces EGFR-TKI resistance via promoting EGFR signaling pathway and ROS generation in NSCLC cell lines. Lung Cancer 2017, 109, 109–116. [Google Scholar] [CrossRef]
- Wang, J.; Qi, C.; Liu, L.; Zhao, L.; Cui, W.; Tian, Y.; Liu, B.; Li, J. Taurine Protects Primary Neonatal Cardiomyocytes Against Apoptosis Induced by Hydrogen Peroxide. Int. Heart J. 2018, 59, 190–196. [Google Scholar] [CrossRef]
- Jin, G.F.; Hurst, J.S.; Godley, B.F. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr. Eye Res. 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Xu, J.; Wang, Y.; Ma, F.; Li, Z.; Liu, N. Stat3 activation is critical for pluripotency maintenance. J. Cell Physiol. 2019, 234, 1044–1051. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Peerapattana, J.; Otsuka, K.; Otsuka, M. Application of NIR spectroscopy for the quality control of mangosteen pericarp powder: Quantitative analysis of alpha-mangostin in mangosteen pericarp powder and capsule. J. Nat. Med. 2013, 67, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.S.; Peerson, J.; Mishra, A.T.; Sadasiva Rao, M.V.; Rajeswari, K.P. Efficacy and tolerability of a novel herbal formulation for weight management. Obesity 2013, 21, 921–927. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Aizat, W.M.; Jamil, I.N.; Ahmad-Hashim, F.H.; Noor, N.M. Recent updates on metabolite composition and medicinal benefits of mangosteen plant. PeerJ 2019, 31, e6324. [Google Scholar] [CrossRef] [PubMed]
- Sadidi, M.; Lentz, S.I.; Feldman, E.L. Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation. Biochimie 2009, 91, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell. Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. P38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Vousden, K.H. p53, ROS and senescence in the control of aging. Aging 2010, 2, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Schnellmann, R.G. H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am. J. Physiol. Renal Physiol. 2004, 286, F858–F865. [Google Scholar]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. In vivo antibacterial activity of Garcinia mangostana pericarp extract against methicillin-resistant Staphylococcus aureus in a mouse superficial skin infection model. Pharm. Biol. 2016, 54, 2606–2615. [Google Scholar] [CrossRef]
- García, M.L.; Carrión, M.H.; Escobar, S.; Rodríguez, A.; Cortina, J.R. Optimization of the antioxidant capacity of mangosteen peels (Garcinia mangostana L.) extracts: Management of the drying extraction processes. Food Sci. Technol. Int. 2021, 27, 404–412. [Google Scholar] [CrossRef]
- Ngawhirunpat, T.; Opanasopi, P.; Sukma, M.; Sittisombut, C.; Kat, A.; Adachi, I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharm. Biol. 2010, 48, 55–62. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Abang Zaidel, D.N.; Muhamad, I.I.; Abdul Hamid, M.; Yaakob, H.; Mohd Jusoh, Y.M. Optimization of the antioxidant-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon 2019, 5, e02571. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Cui, J.; Hu, W.; Cai, Z.; Liu, Y.; Li, S.; Tao, W.; Xiang, H. New medicinal properties of mangostins: Analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol. Biochem. Behav. 2010, 95, 166–172. [Google Scholar] [CrossRef]
- Sukatta, U.; Takenaka, M.; Ono, H.; Okadome, H.; Sotome, I.; Nanayama, K.; Thanapase, W.; Isobe, S. Distribution of major xanthones in the pericarp, aril, and yellow gum of mangosteen (Garcinia mangostana linn.) fruit and their contribution to antioxidative activity. Biosci. Biotechnol. Biochem. 2013, 77, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Ethanolic Garcinia mangostana extract and α-mangostin improve dextran sulfate sodium-induced ulcerative colitis via the suppression of inflammatory and oxidative responses in ICR mice. J. Ethnopharmacol. 2021, 265, 113384. [Google Scholar] [CrossRef] [PubMed]
- Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Ginting, C.N.; Lister, I.N.E.; Girsang, E.; Amalia, A.; Wibowo, S.H.B.; Kusuma, H.S.W.; Rizal. Anti-aging Effects of Mangosteen Peel Extract and Its Phytochemical Compounds: Antioxidant Activity, Enzyme Inhibition and Molecular Docking Simulation. Trop. Life Sci. Res. 2020, 31, 127. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Pisanti, S.; Caputo, M.; Citro, M.; Vecchione, C.; Martinelli, R. 3-Hydroxytyrosol Promotes Angiogenesis In Vitro by Stimulating Endothelial Cell Migration. Int. J. Mol. Sci. 2020, 21, 3657. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, R. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef]
- Ciaglia, E.; Grimaldi, M.; Abate, M.; Scrima, M.; Rodriquez, M.; Laezza, C.; Ranieri, R.; Pisanti, S.; Ciuffreda, P.; Manera, C.; et al. The isoprenoid derivative N6 -benzyladenosine CM223 exerts antitumor effects in glioma patient-derived primary cells through the mevalonate pathway. Br. J. Pharmacol. 2017, 174, 2287–2301. [Google Scholar] [CrossRef]
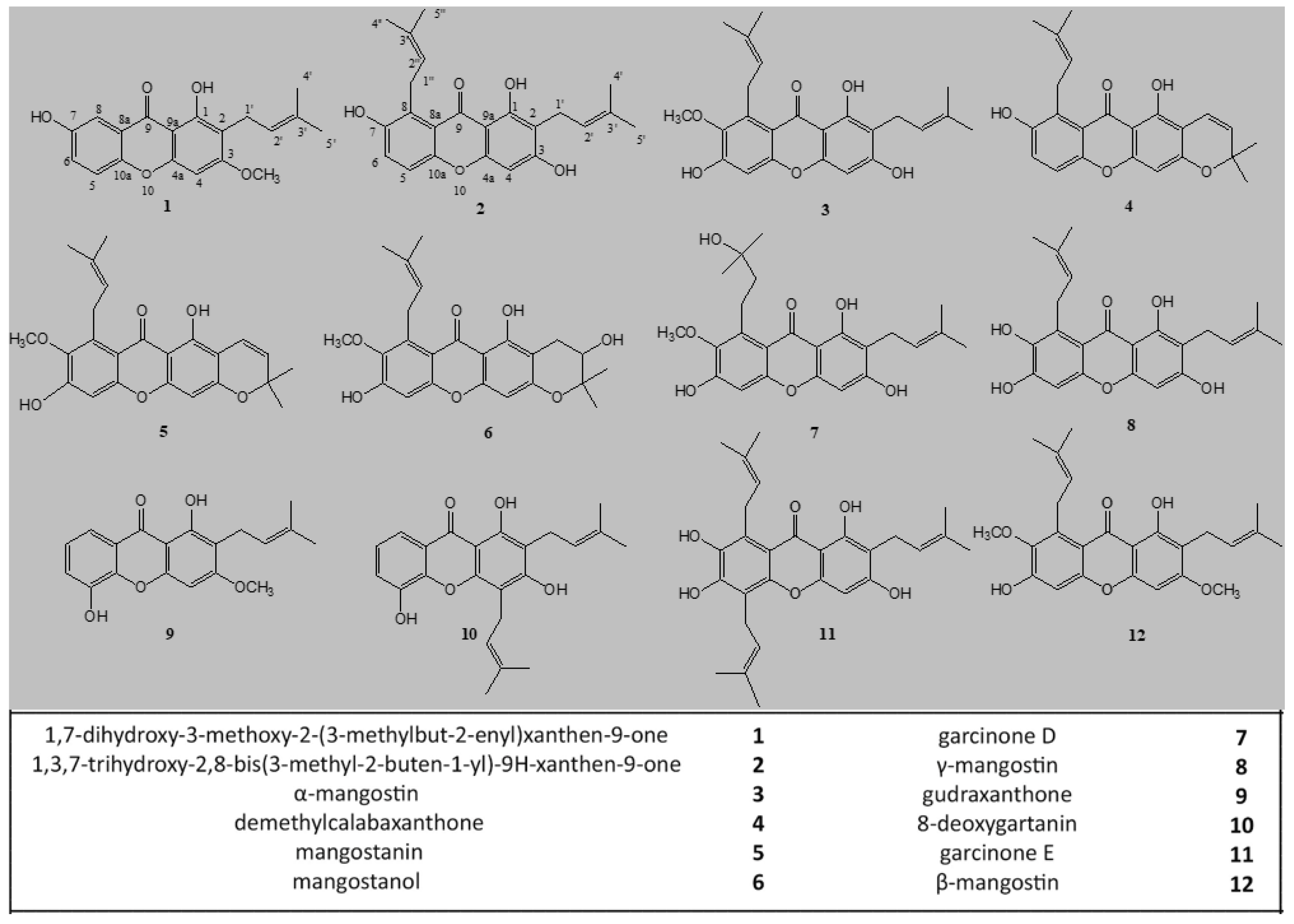
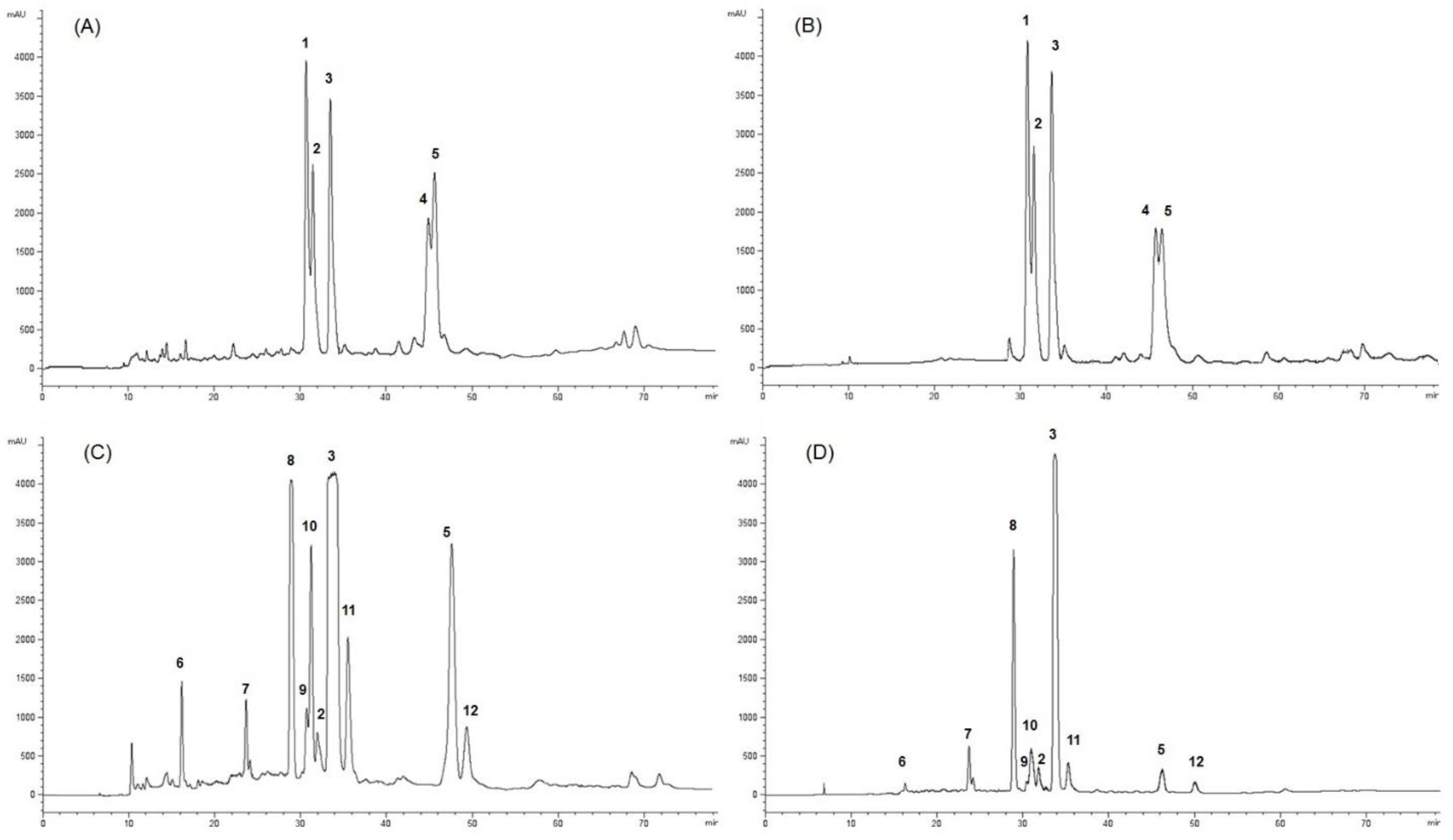



| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 159.9 | 161.0 | 161.8 | 153.4 | 150.4 | 156.4 | 161.0 | 161.5 | 157.0 | 157.0 | 161.8 | 161.4 |
| 2 | 112.3 | 110.8 | 111.4 | 104.7 | 105.3 | 105.0 | 111.3 | 110.8 | 112.3 | 112.3 | 110.6 | 111.0 |
| 3 | 165.8 | 163.7 | 163.8 | 160.9 | 160.3 | 162.4 | 163.8 | 163.0 | 165.5 | 165.5 | 163.8 | 163.8 |
| 4 | 90.4 | 92.5 | 92.9 | 94.4 | 94.5 | 94.4 | 92.8 | 92.4 | 90.8 | 90.8 | 92.8 | 92.9 |
| 5 | 119.4 | 116.4 | 102.5 | 123.4 | 102.7 | 102.1 | 102.3 | 100.6 | 147.8 | 147.8 | 114.3 | 102.5 |
| 5a | 157.7 | 156.0 | 156.2 | 157.7 | 157.7 | 158.6 | 156.1 | 155.9 | 157.7 | 157.7 | 155.7 | 156.1 |
| 6 | 124.8 | 123.4 | 156.6 | 116.5 | 157.0 | 155.9 | 156.4 | 154.2 | 121.2 | 121.2 | 148.6 | 156.4 |
| 7 | 155.3 | 151.9 | 144.8 | 152.5 | 144.2 | 144.4 | 144.8 | 141.8 | 124.6 | 124.6 | 141.4 | 144.8 |
| 8 | 108.9 | 129.6 | 138.6 | 119.0 | 138.6 | 137.7 | 139.0 | 129.4 | 115.3 | 115.3 | 128.6 | 138.8 |
| 8a | 121.8 | 119.6 | 112.0 | 129.0 | 111.3 | 115.1 | 111.5 | 111.8 | 122.3 | 122.3 | 114.0 | 112.0 |
| 9a | 104.5 | 103.4 | 103.8 | 104.5 | 104.5 | 107.0 | 104.0 | 103.5 | 104.3 | 104.3 | 102.8 | 103.8 |
| 9 | 183.8 | 184.4 | 183.6 | 184.4 | 183.6 | 179.0 | 180.9 | 182.7 | 181.9 | 182.5 | 183.0 | 183.5 |
| 10a | 150.0 | 151.8 | 158.3 | 152.7 | 157.7 | 156.8 | 157.2 | 154.0 | 146.5 | 146.5 | 152.2 | 157.9 |
| 1′ | 21.7 | 22.1 | 22.1 | 115.8 | 116.0 | 27.3 | 21.9 | 21.6 | 21.9 | 21.9 | 22.0 | 22.1 |
| 2′ | 123.5 | 123.4 | 123.8 | 128.1 | 128.3 | 69.3 | 123.6 | 123.6 | 123.0 | 123.0 | 124.0 | 123.9 |
| 3′ | 132.1 | 131.1 | 131.8 | 78.8 | 78.6 | 79.4 | 131.6 | 131.3 | 131.8 | 131.8 | 130.9 | 131.7 |
| 4′ | 17.5 | 17.2 | 17.6 | 28.1 | 27.9 | 19.9 | 17.7 | 17.4 | 17.5 | 17.5 | 17.4 | 17.8 |
| 5′ | 25.8 | 26.1 | 25.9 | 28.1 | 27.9 | 25.3 | 25.8 | 25.8 | 26.0 | 26.0 | 25.8 | 25.9 |
| 1″ | 26.5 | 26.9 | 26.5 | 26.3 | 26.9 | 23.0 | 26.0 | 26.7 | 26.5 | |||
| 2″ | 123.8 | 124.8 | 124.4 | 124.6 | 125.4 | 45.7 | 124.5 | 124.0 | 124.4 | |||
| 3″ | 131.0 | 132.0 | 131.4 | 131.5 | 131.8 | 71.6 | 131.2 | 131.2 | 132.0 | |||
| 4″ | 18.5 | 18.1 | 18.3 | 18.5 | 18.6 | 29.1 | 17.9 | 18.1 | 18.1 | |||
| 5″ | 26.1 | 25.9 | 26.2 | 25.9 | 26.3 | 29.1 | 25.5 | 25.8 | 25.9 | |||
| 1‴ | 23.3 | |||||||||||
| 2‴ | 124.0 | |||||||||||
| 3‴ | 131.8 | |||||||||||
| 4‴ | 18.3 | |||||||||||
| 5‴ | 28.0 | |||||||||||
| OCH3 | 56.1 | 56.0 | 56.0 | |||||||||
| OCH3 | 61.1 | 60.9 | 61.0 | 61.3 | 61.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, M.; Pagano, C.; Masullo, M.; Citro, M.; Pisanti, S.; Piacente, S.; Bifulco, M. Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes. Pharmaceuticals 2022, 15, 84. https://doi.org/10.3390/ph15010084
Abate M, Pagano C, Masullo M, Citro M, Pisanti S, Piacente S, Bifulco M. Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes. Pharmaceuticals. 2022; 15(1):84. https://doi.org/10.3390/ph15010084
Chicago/Turabian StyleAbate, Mario, Cristina Pagano, Milena Masullo, Marianna Citro, Simona Pisanti, Sonia Piacente, and Maurizio Bifulco. 2022. "Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes" Pharmaceuticals 15, no. 1: 84. https://doi.org/10.3390/ph15010084
APA StyleAbate, M., Pagano, C., Masullo, M., Citro, M., Pisanti, S., Piacente, S., & Bifulco, M. (2022). Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes. Pharmaceuticals, 15(1), 84. https://doi.org/10.3390/ph15010084










