Development of a Silicone-Based Polymer Matrix as a Suitable Transdermal Therapeutic System for Diallyl Disulfide
Abstract
1. Introduction
2. Results
2.1. Solubility of DADS
2.2. Results of Membrane Diffusion Measurement
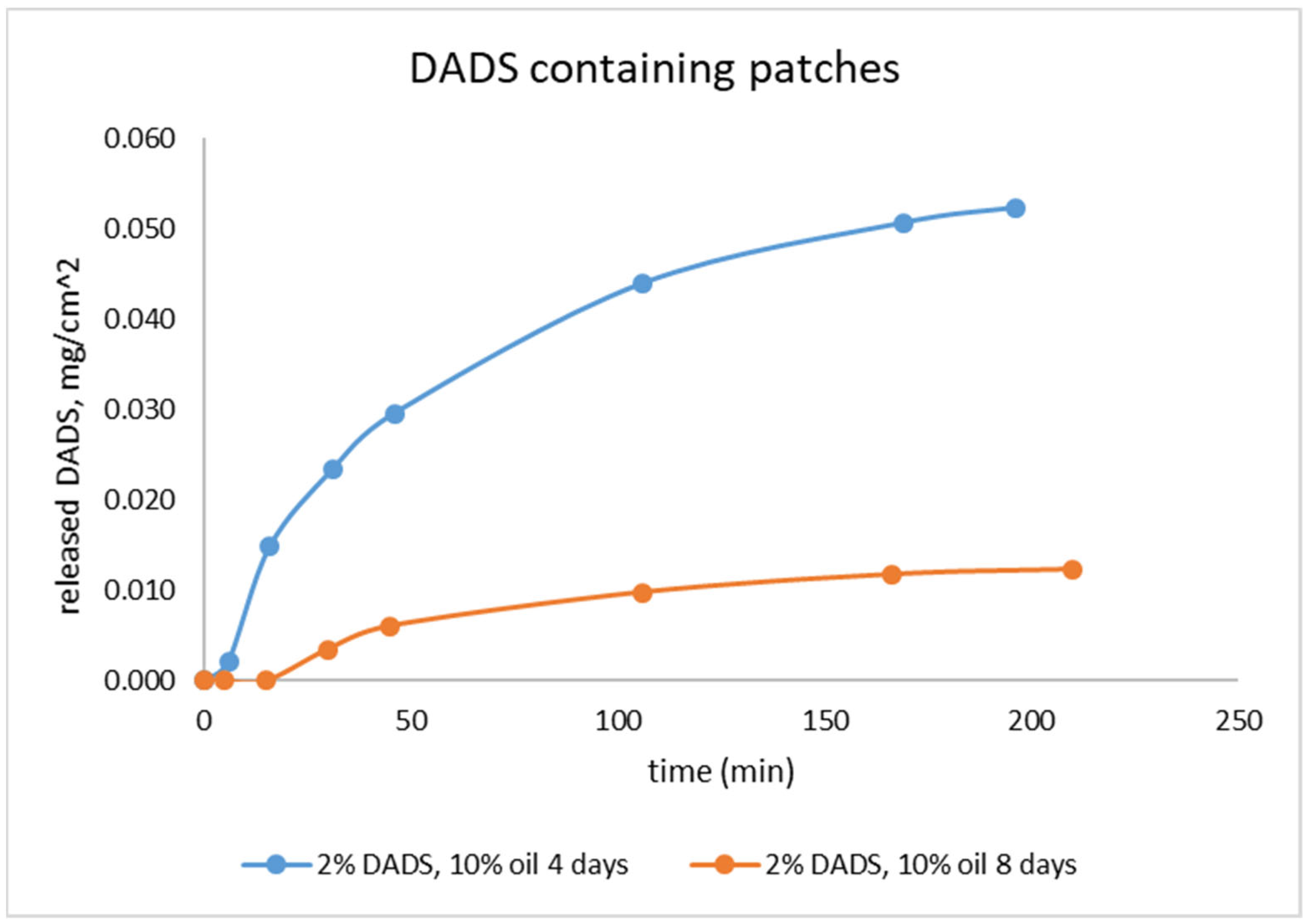
2.3. Investigation of Stability of the Drug Release with Franz Diffusion Cell
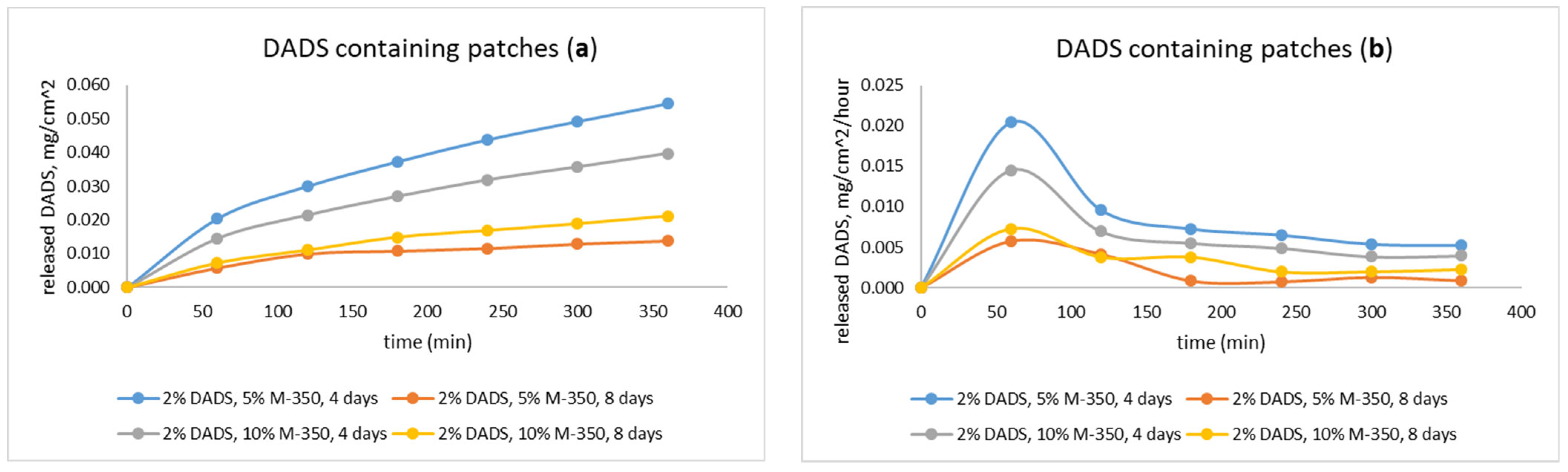
2.4. Investigation of Drug Release with Flow-Through Cell
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumental
4.3. Production of Diallyl Disulfide
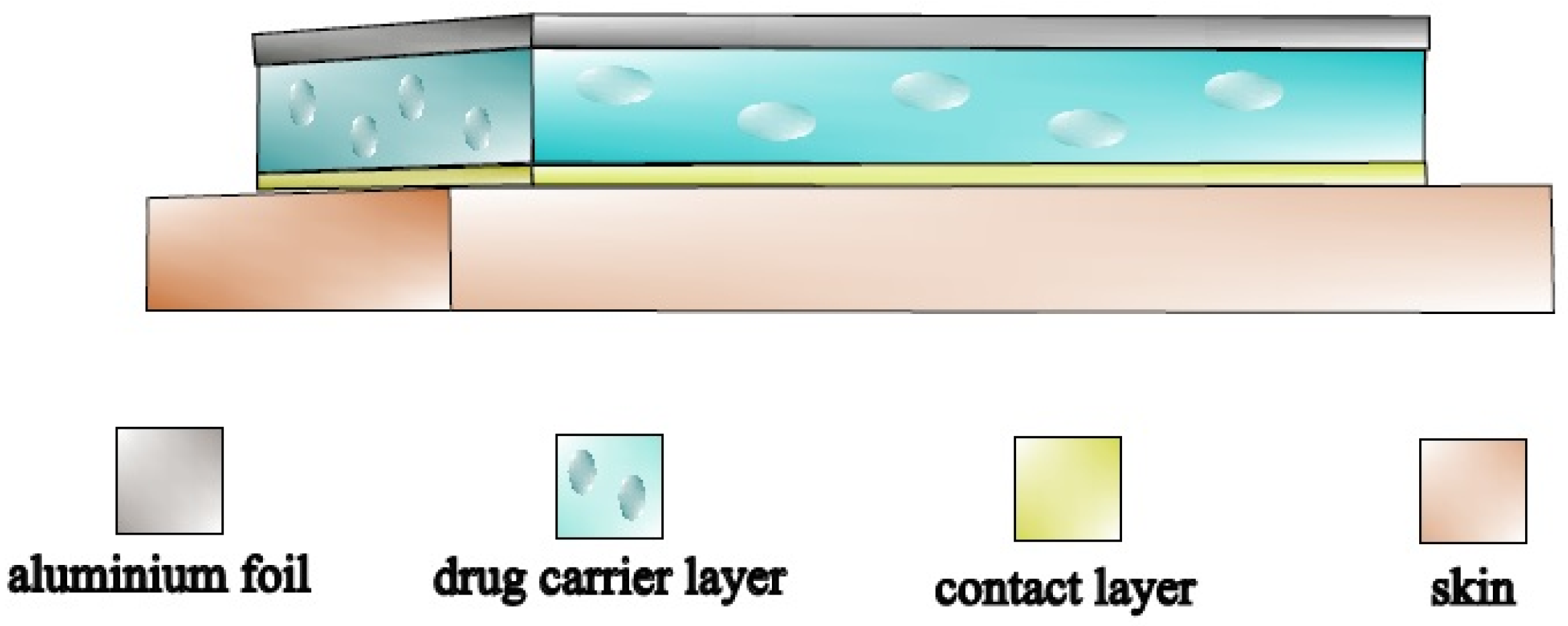
4.4. Production of Silicone Membranes
4.5. Production of Diallyl Disulfide Containing Transdermal Patches
4.6. Measurement of Membrane Diffusion
4.7. Measurement of the In Vitro Release of DADS-Containing Transdermal Patches
4.7.1. Measurement in the Franz Cell
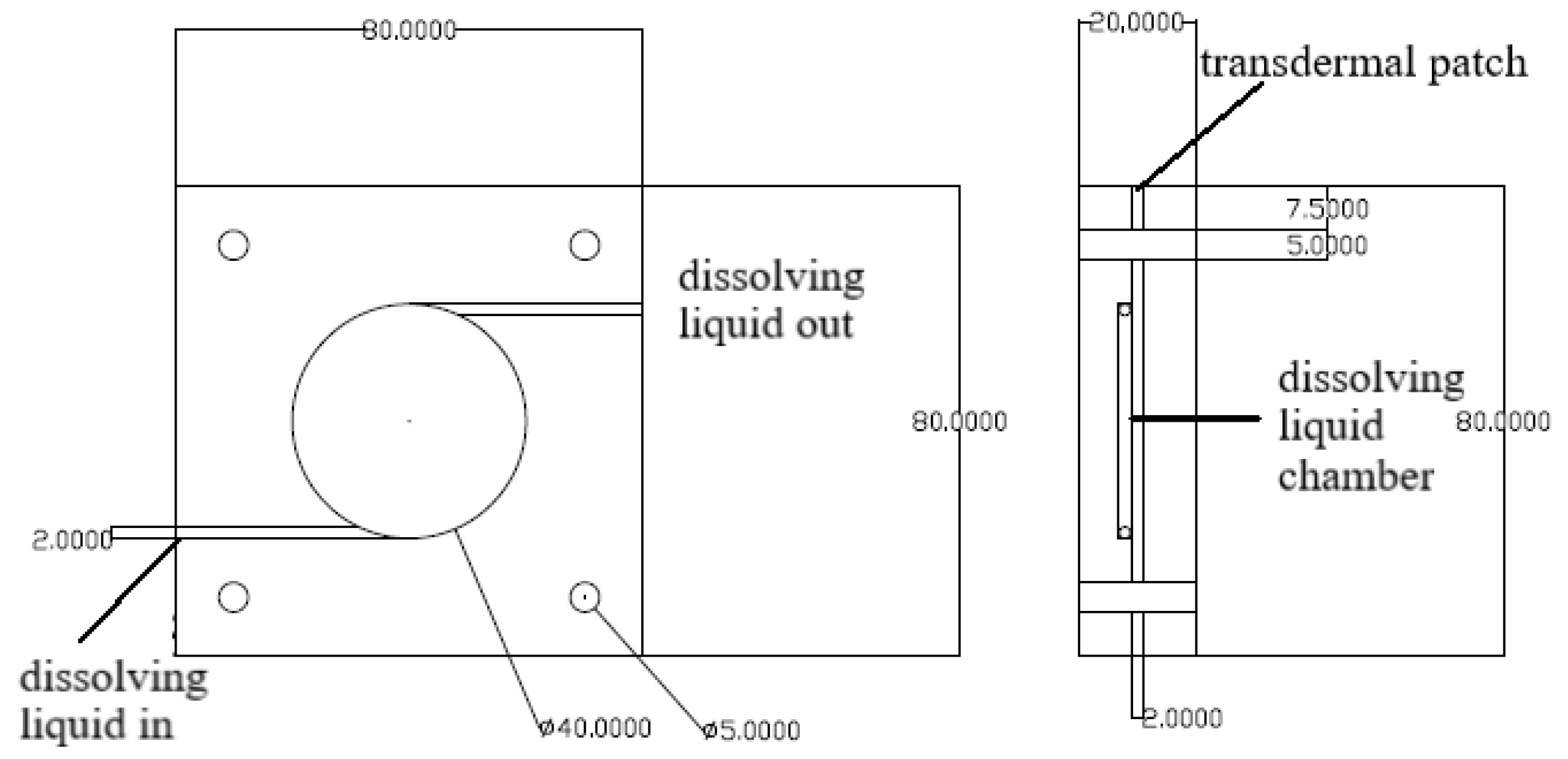
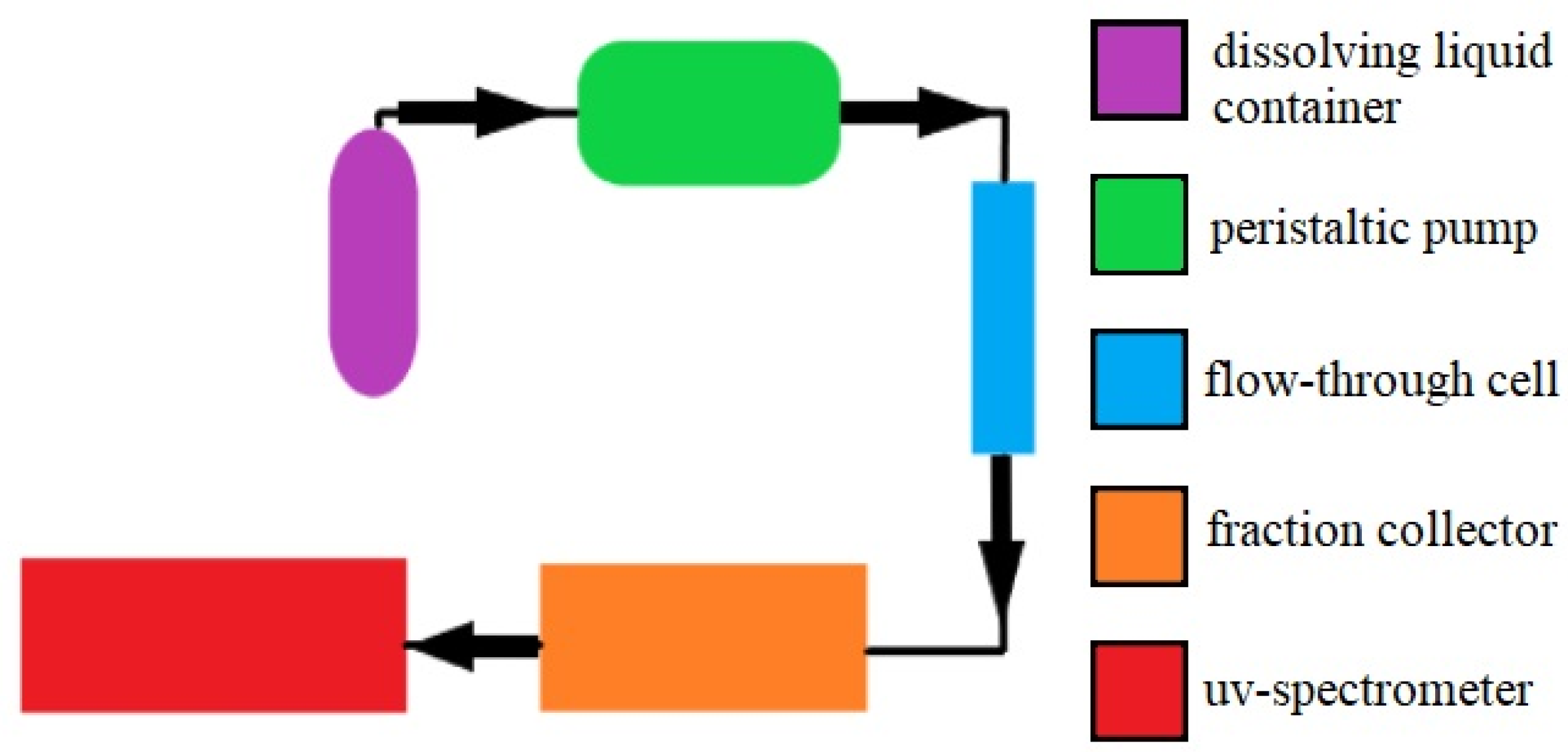
4.7.2. Measurement in the Flow-Through Cell
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Sá, K.N.; Moreira, L.; Baptista, A.F.; Yeng, L.T.; Teixeira, M.J.; Galhardoni, R.; de Andrade, D.C. Prevalence of Chronic Pain in Developing Countries: Systematic Review and Meta-Analysis. Pain Rep. 2019, 4, e779. [Google Scholar] [CrossRef] [PubMed]
- Dineen, J.; Freeman, R. Autonomic Neuropathy. Semin. Neurol. 2015, 35, 458–468. [Google Scholar] [CrossRef]
- Murnion, B.P. Neuropathic Pain: Current Definition and Review of Drug Treatment. Aust. Prescr. 2018, 41, 60–63. [Google Scholar] [CrossRef]
- Seidel, M.F.; Hügle, T.; Morlion, B.; Koltzenburg, M.; Chapman, V.; MaassenVanDenBrink, A.; Lane, N.E.; Perrot, S.; Zieglgänsberger, W. Neurogenic Inflammation as a Novel Treatment Target for Chronic Pain Syndromes. Exp. Neurol. 2022, 356, 114108. [Google Scholar] [CrossRef] [PubMed]
- Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues and Organs|Physiological Reviews. Available online: https://journals.physiology.org/doi/abs/10.1152/physrev.00028.2021 (accessed on 12 August 2022).
- Guo, J.; Li, G.; Yang, L. Role of H2S in Pain: Growing Evidences of Mystification. Eur. J. Pharmacol. 2020, 883, 173322. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Perry, A.; Zhou, Z.; Bucci, M.; Papapetropoulos, A.; Cirino, G.; Wood, M.E. Phosphinodithioate and Phosphoramidodithioate Hydrogen Sulfide Donors. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Handbook of Experimental Pharmacology; Moore, P.K., Whiteman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 337–363. ISBN 978-3-319-18144-8. [Google Scholar]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen Sulfide (H2S) Releasing Agents: Chemistry and Biological Applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, L.; Ma, T.; Osama, A.; Shen, T.; He, Y.; Fang, J. Progress and Perspective on Hydrogen Sulfide Donors and Their Biomedical Applications. Med. Res. Rev. 2022, 42, 1930–1977. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Hogestatt, E.D.; Julius, D.; Jordt, S.-E.; Zygmunt, P.M. Pungent Products from Garlic Activate the Sensory Ion Channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H 2 S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cruz, E.Y.; Silva-Islas, C.A.; Maldonado, P.D.; Pedraza-Chaverri, J.; Carballo-Villalobos, A.I. Antinociceptive Effect of Garlic Garlic Preparations and Derivative compounds. Eur. J. Pain 2022, 26, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yue, Z.; Nie, L.; Zhao, P.; Zhu, K.; Wang, Q. Biological Functions of Diallyl Disulfide, a Garlic-Derived Natural Organic Sulfur Compound. Evid. Based Complementary Altern. Med. 2021, 2021, 5103626. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Y.; Wang, C.; Huang, J.; Wang, X.; Liu, Y.; Wang, H. Exploring the Role and Mechanisms of Diallyl Trisulfide and Diallyl Disulfide in Chronic Constriction-Induced Neuropathic Pain in Rats. Korean J Pain 2020, 33, 216–225. [Google Scholar] [CrossRef]
- Porta, A.; Rodríguez, L.; Bai, X.; Batallé, G.; Roch, G.; Pouso-Vázquez, E.; Balboni, G.; Pol, O. Hydrogen Sulfide Inhibits Inflammatory Pain and Enhances the Analgesic Properties of Delta Opioid Receptors. Antioxidants 2021, 10, 1977. [Google Scholar] [CrossRef]
- Gîtin, L.; Dinică, R.; Neagu, C.; Dumitrascu, L. Sulfur Compounds Identification and Quantification from Allium spp. Fresh Leaves. J. Food Drug Anal. 2014, 22, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, X.; Jiang, X.; Nie, Y. Synthesis, Characterization and Bioactivity Evaluation of Diallyl Disulfide. J Cent. South Univ. Technol. 2006, 13, 515–518. [Google Scholar] [CrossRef]
- Mashak, A.; Rahimi, A. Silicone Polymers in Controlled Drug Delivery Systems: A Review. Iran. Polym. J. 2009, 18, 279–295. [Google Scholar]
- Sabbagh, F.; Kim, B.S. Recent Advances in Polymeric Transdermal Drug Delivery Systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Tanner, T.; Marks, R. Delivering Drugs by the Transdermal Route: Review and Comment. Skin Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Hoffman, A.S. The Origins and Evolution of “Controlled” Drug Delivery Systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal Patches: History, Development and Pharmacology. Br. J. Pharm. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Wagner, Ö. Development of a New Silicon-Based Transdermal System. I. Study of Silicone Elastomers and Effect of Liquid Ingredients. Drug Dev. Ind. Pharm. 1998, 24, 243–252. [Google Scholar] [CrossRef]
- Wagner, Ö.; Hencsei, P.; Liptay, G. Development of a New Silicone Base Transdermal Therapeutic System. Silicon Chem. 2002, 1, 223–227. [Google Scholar] [CrossRef]
- Wagner, Ö. Development of a New Silicone-base Transdermal Therapeutic System II Study of Study of trifunctional ingredients. J. Appl. Polym. Sci. 2001, 82, 1187–1194. [Google Scholar] [CrossRef]
- Wagner, Ö. A New Transdermal System I. Period. Polytech. Chem. Eng. 1991, 35, 169–176. [Google Scholar]
- Tojo, K.; Sun, Y.; Ghannam, M.; Chien, Y.W. Simple Evaluation Method of Intrinsic Diffusivity for Membrane-Moderated Controlled Release. Drug Dev. Ind. Pharm. 1985, 11, 1363–1371. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a Static Franz Diffusion Cell System for in Vitro Permeation Studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef]
- Shelke, S.; Pathan, I.; Shinde, G.; Agrawal, G.; Damale, M.; Chouthe, R.; Panzade, P.; Kulkarni, D. Poloxamer-Based In Situ Nasal Gel of Naratriptan Hydrochloride Deformable Vesicles for Brain Targeting. BioNanoScience 2020, 10, 633–648. [Google Scholar] [CrossRef]
- Shinde, G.; Desai, P.; Shelke, S.; Patel, R.; Bangale, G.; Kulkarni, D. Mometasone Furoate-Loaded Aspasomal Gel for Topical Treatment of Psoriasis: Formulation, Optimization, in Vitro and in Vivo Performance. J. Dermatol. Treat. 2022, 33, 885–896. [Google Scholar] [CrossRef]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a Therapeutic Target for Nociceptive Pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Manolache, A.; Babes, A.; Madalina Babes, R. Mini-Review: The Nociceptive Sensory Functions of the Polymodal Receptor Transient Receptor Potential Ankyrin Type 1 (TRPA1). Neurosci. Lett. 2021, 764, 136286. [Google Scholar] [CrossRef]
- Pozsgai, G.; Bátai, I.Z.; Pintér, E. Effects of Sulfide and Polysulfides Transmitted by Direct or Signal Transduction-Mediated Activation of TRPA1 Channels: TRPA1-Mediated Effects of Sulfide and Polysulfides. Br. J. Pharmacol. 2019, 176, 628–645. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Wang, Z.; Zhang, Y.; Wang, K. Hydrogen Sulfide Attenuates Diabetic Neuropathic Pain through NO/CGMP/PKG Pathway and μ-Opioid Receptor. Exp. Biol. Med. 2020, 245, 823–834. [Google Scholar] [CrossRef]
- Dombi, Á.; Sánta, C.; Bátai, I.Z.; Kormos, V.; Kecskés, A.; Tékus, V.; Pohóczky, K.; Bölcskei, K.; Pintér, E.; Pozsgai, G. Dimethyl Trisulfide Diminishes Traumatic Neuropathic Pain Acting on TRPA1 Receptors in Mice. Int. J. Mol. Sci. 2021, 22, 3363. [Google Scholar] [CrossRef]
- Blair, H.A. Capsaicin 8% Dermal Patch: A Review in Peripheral Neuropathic Pain. Drugs 2018, 78, 1489–1500. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137): New Insights Into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Jia, J.; Xiao, Y.; Wang, W.; Qing, L.; Xu, Y.; Song, H.; Zhen, X.; Ao, G.; Alkayed, N.J.; Cheng, J. Differential Mechanisms Underlying Neuroprotection of Hydrogen Sulfide Donors against Oxidative Stress. Neurochem. Int. 2013, 62, 1072–1078. [Google Scholar] [CrossRef]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a Novel Mitochondria-Targeted Hydrogen Sulfide Donor, Stimulates Cellular Bioenergetics, Exerts Cytoprotective Effects and Protects against the Loss of Mitochondrial DNA Integrity in Oxidatively Stressed Endothelial Cells in Vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni-Chitnis, M.; Njie-Mbye, Y.F.; Mitchell, L.; Robinson, J.; Whiteman, M.; Wood, M.E.; Opere, C.A.; Ohia, S.E. Inhibitory Action of Novel Hydrogen Sulfide Donors on Bovine Isolated Posterior Ciliary Arteries. Exp. Eye Res. 2015, 134, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The Novel Mitochondria-Targeted Hydrogen Sulfide (H2S) Donors AP123 and AP39 Protect against Hyperglycemic Injury in Microvascular Endothelial Cells in Vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef]
- Huang, C.W.; Feng, W.; Peh, M.T.; Peh, K.; Dymock, B.W.; Moore, P.K. A Novel Slow-Releasing Hydrogen Sulfide Donor, FW1256, Exerts Anti-Inflammatory Effects in Mouse Macrophages and in Vivo. Pharmacol. Res. 2016, 113, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Luo, S.; Liu, J.; Xie, S.; Liu, Y.; Xu, J.; Zhu, Z.; Xu, S. Controllable Thioester-Based Hydrogen Sulfide Slow-Releasing Donors as Cardioprotective Agents. Chem. Commun. 2019, 55, 6193–6196. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, U.; van der Vlies, A.J. Design and Synthesis of Polymeric Hydrogen Sulfide Donors. Bioconjug. Chem. 2014, 25, 1290–1300. [Google Scholar] [CrossRef]
- Marwah, M.K.; Shokr, H.; Sanchez-Aranguren, L.; Badhan, R.K.S.; Wang, K.; Ahmad, S. Transdermal Delivery of a Hydrogen Sulphide Donor, ADT-OH Using Aqueous Gel Formulations for the Treatment of Impaired Vascular Function: An Ex Vivo Study. Pharm. Res. 2022, 39, 341–352. [Google Scholar] [CrossRef]
- Rong, F.; Wang, T.; Zhou, Q.; Peng, H.; Yang, J.; Fan, Q.; Li, P. Intelligent Polymeric Hydrogen Sulfide Delivery Systems for Therapeutic Applications. Bioact. Mater. 2023, 19, 198–216. [Google Scholar] [CrossRef]
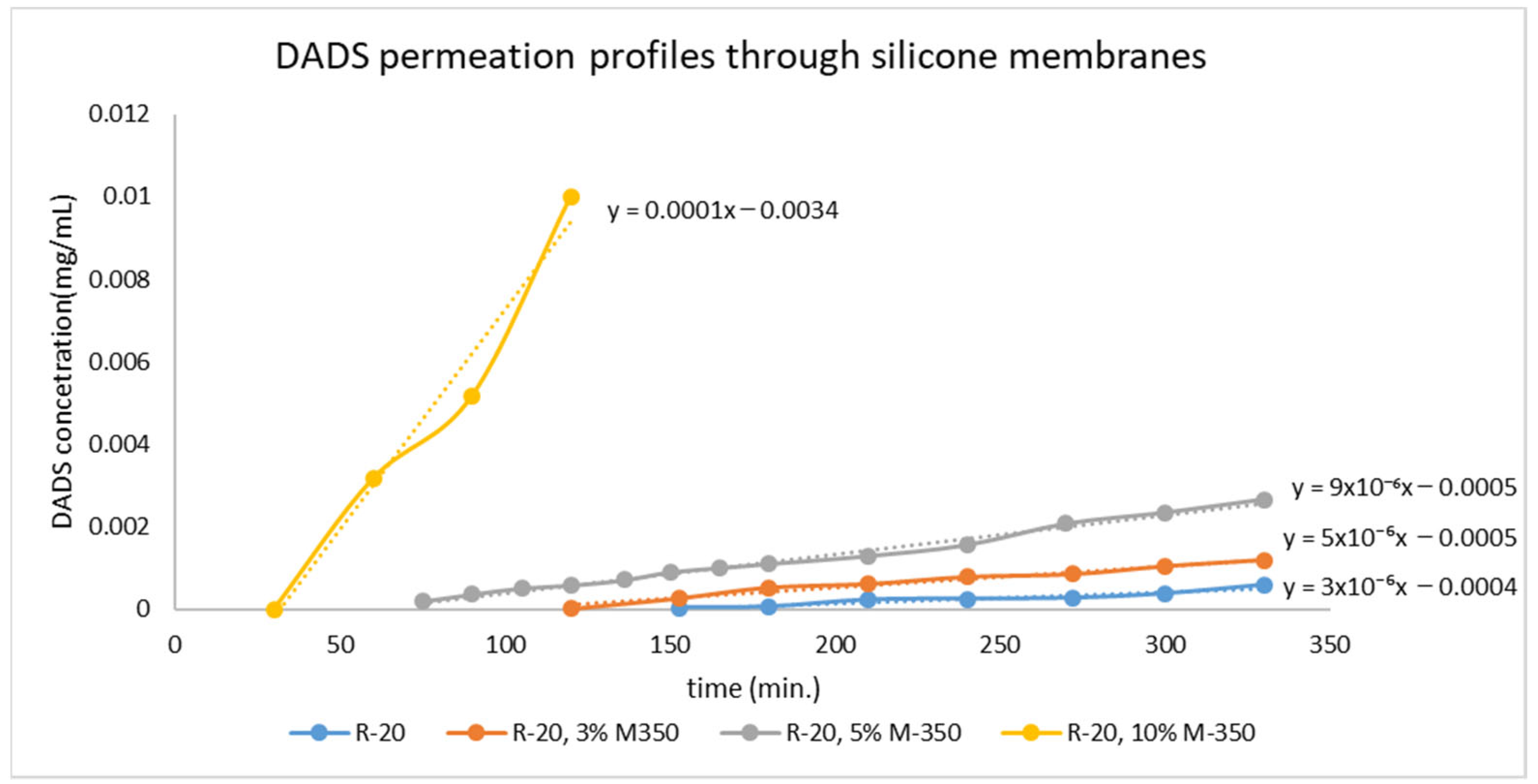
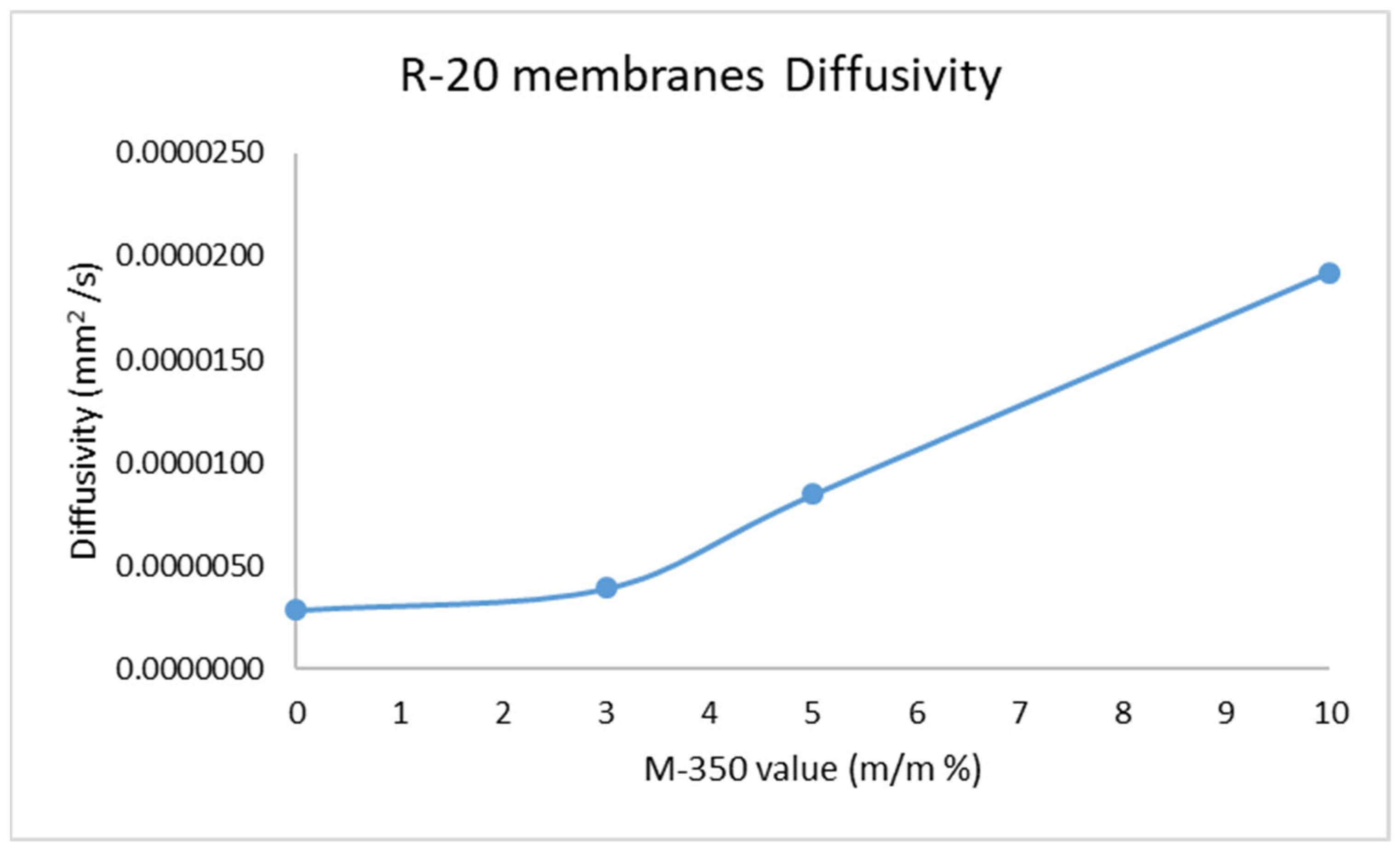
| Sample | l (mm) | t0 (s) | D (mm2/s) |
|---|---|---|---|
| R-20 | 0.37 | 8000 | 2.85 × 10−6 |
| R-20, 3% M-350 | 0.375 | 6000 | 3.91 × 10−6 |
| R-20, 5% M-350 | 0.39 | 3000 | 8.45 × 10−6 |
| R-20, 10% M-350 | 0.485 | 2040 | 1.92 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
László, S.; Hajna, Z.; Egyed, A.; Pintér, E.; Wagner, Ö. Development of a Silicone-Based Polymer Matrix as a Suitable Transdermal Therapeutic System for Diallyl Disulfide. Pharmaceuticals 2022, 15, 1182. https://doi.org/10.3390/ph15101182
László S, Hajna Z, Egyed A, Pintér E, Wagner Ö. Development of a Silicone-Based Polymer Matrix as a Suitable Transdermal Therapeutic System for Diallyl Disulfide. Pharmaceuticals. 2022; 15(10):1182. https://doi.org/10.3390/ph15101182
Chicago/Turabian StyleLászló, Szabolcs, Zsófia Hajna, Attila Egyed, Erika Pintér, and Ödön Wagner. 2022. "Development of a Silicone-Based Polymer Matrix as a Suitable Transdermal Therapeutic System for Diallyl Disulfide" Pharmaceuticals 15, no. 10: 1182. https://doi.org/10.3390/ph15101182
APA StyleLászló, S., Hajna, Z., Egyed, A., Pintér, E., & Wagner, Ö. (2022). Development of a Silicone-Based Polymer Matrix as a Suitable Transdermal Therapeutic System for Diallyl Disulfide. Pharmaceuticals, 15(10), 1182. https://doi.org/10.3390/ph15101182








