Baccharis trimera Infusion Reduces Macrophages Activation and High-Fat Diet-Induced Metabolic Disorders in Mice
Abstract
:1. Introduction
2. Results
2.1. Chemical Profile of the BTi
2.2. Effect of the BTi on Food and Caloric Intake, Weight, Organ and Adipose Cushion Weights, and the Adiposity Index
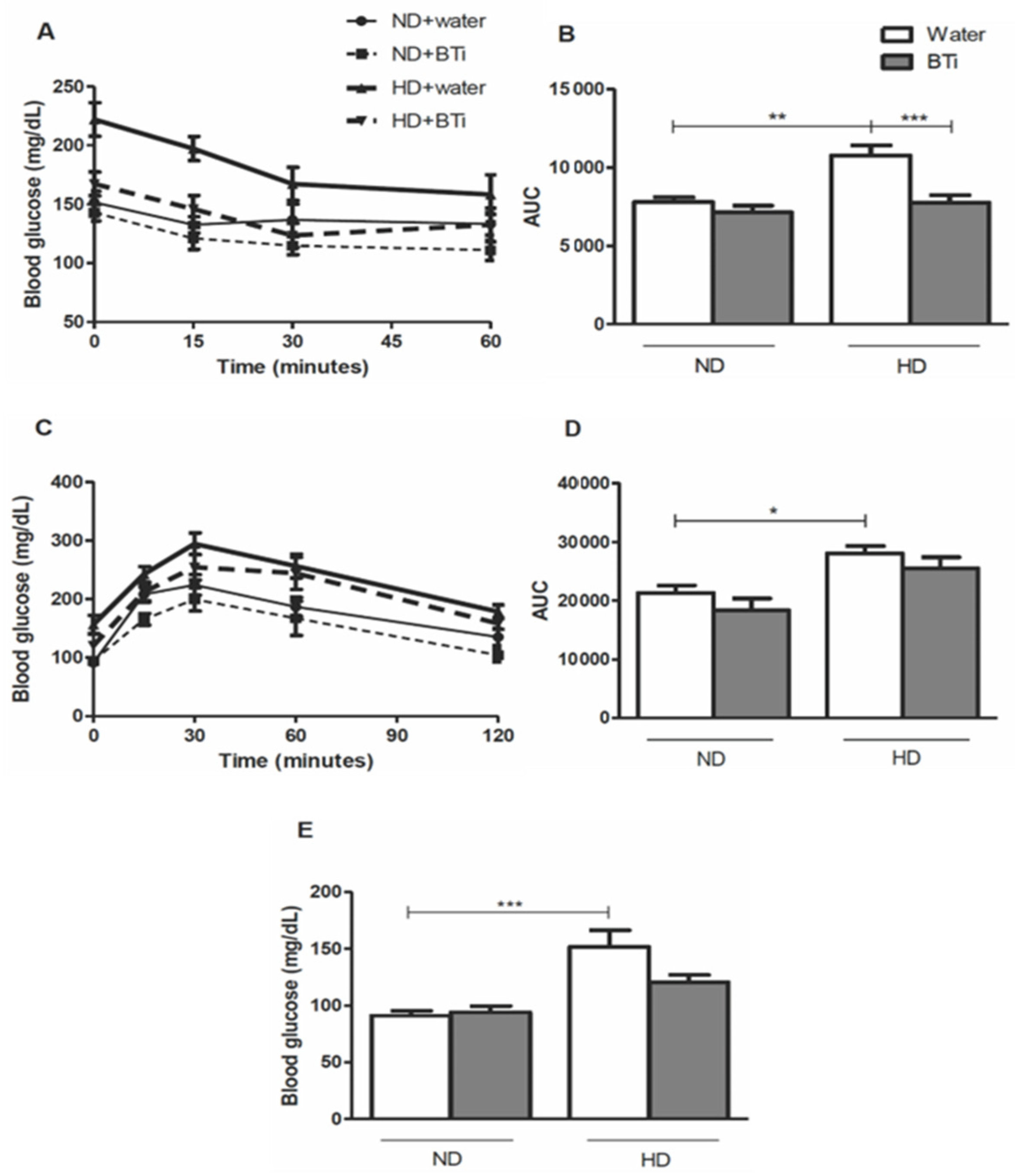
2.3. Effect of the BTi on the Glycemic Profile
2.4. Effect of the BTi on the Lipid Profile
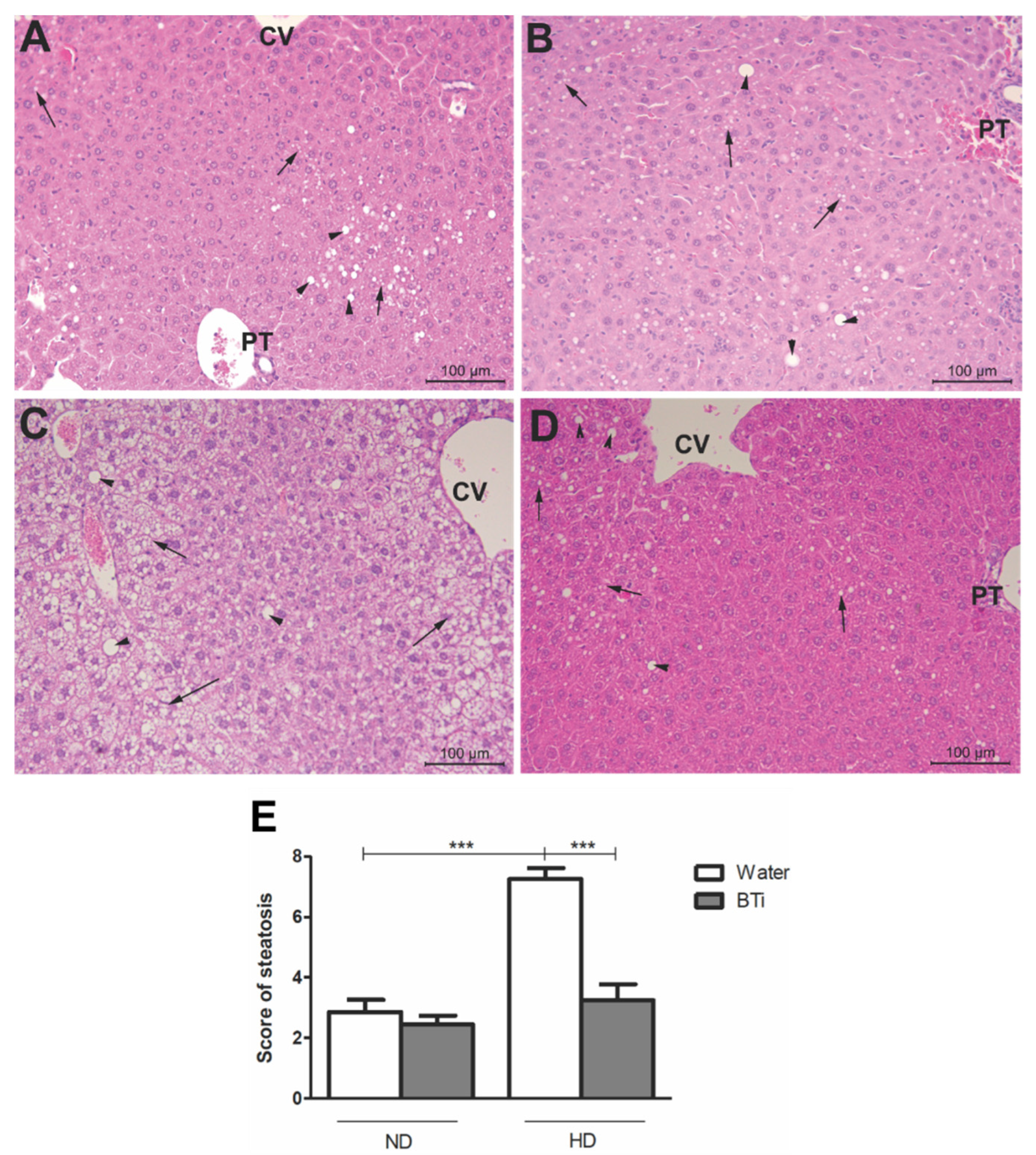
2.5. Effect of the BTi on Histological Parameters
2.6. Effect of the BTi on Leukocyte Infiltration, Formation of Crown-like Structures by Macrophages around Adipocytes and Macrophage Cellular Functions
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of the BTi
4.3. Analysis of the BTi by HPLC-DAD-MS/MS
4.4. Animals and Diet
4.5. Experimental Design
4.6. Measurement of Biochemical Parameters
4.7. Histological Analysis
4.8. Peritoneal Macrophages Harvesting and Counting
4.9. MTT Assay for Cell Viability
4.10. Nitric Oxide Production by Peritoneal Macrophages
4.11. Hydrogen Peroxide Production by Peritoneal Macrophages
4.12. Adhesion and Spreading of Peritoneal Macrophages
4.13. Phagocytic Activity of Peritoneal Macrophages
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Cienc. 2019, 91, e20190105. [Google Scholar] [CrossRef] [PubMed]
- BRASIL Agência Nacional de Vigilância Sanitária. Resolução—RDC N° 10, de 9 de Março de 2010; Ministério Da Saúde: Brasília, Brazil, 2010. [Google Scholar]
- Verdi, L.G.; Brighente, I.M.C.; Pizzolatti, M.G. Gênero Baccharis (Asteraceae): Aspectos Químicos, Econômicos e Biológicos. Quim. Nova 2005, 28, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Pádua, B.d.C.; Rossoni Júnior, J.V.; de Brito Magalhães, C.L.; Chaves, M.M.; Silva, M.E.; Pedrosa, M.L.; Souza, G.H.B.d.; Brandão, G.C.; Rodrigues, I.V.; Lima, W.G.; et al. Protective Effect of Baccharis Trimera Extract on Acute Hepatic Injury in a Model of Inflammation Induced by Acetaminophen. Mediat. Inflamm. 2014, 2014, 196598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, R.J.; Ratti da Silva, G.; Cola, I.M.; Kuchler, J.C.; Coelho, N.; Barboza, L.N.; Menetrier, J.V.; de Souza, R.; Zonta, F.N.; Froehlich, D.L.; et al. Promising Therapeutic Use of Baccharis Trimera (Less.) DC. as a Natural Hepatoprotective Agent against Hepatic Lesions That Are Caused by Multiple Risk Factors. J. Ethnopharmacol. 2020, 254, 112729. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Lívero, F.A.; da Silva, L.M.; Ferreira, D.M.; Galuppo, L.F.; Borato, D.G.; Prando, T.B.L.; Lourenço, E.L.B.; Strapasson, R.L.B.; Stefanello, M.É.A.; de Paula Werner, M.F.; et al. Hydroethanolic Extract of Baccharis Trimera Promotes Gastroprotection and Healing of Acute and Chronic Gastric Ulcers Induced by Ethanol and Acetic Acid. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 985–998. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, N.S.; Ferraz, A.d.B.F.; Pedron, C.; Correa, D.S.; Vieira, L.B.; Antunes, F.T.T.; de Souza, A.H. Baccharis Trimera Aqueous Extract Modulates Inflammation and Nociception in Mice. Clin. Phytosci. 2021, 7, 82. [Google Scholar] [CrossRef]
- de Souza Marinho do Nascimento, D.; Oliveira, R.; Camara, R.; Gomes, D.; Monte, J.; Costa, M.; Fernandes, J.; Langassner, S.; Rocha, H. Baccharis trimera (Less.) DC Exhibits an Anti-Adipogenic Effect by Inhibiting the Expression of Proteins Involved in Adipocyte Differentiation. Molecules 2017, 22, 972. [Google Scholar] [CrossRef] [Green Version]
- Silveira Rabelo, A.C.; Caldeira Costa, D. A Review of Biological and Pharmacological Activities of Baccharis Trimera. Chem. Biol. Interact. 2018, 296, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed Food and Chronic Noncommunicable Diseases: A Systematic Review and Meta-analysis of 43 Observational Studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Lipid Bilayer Stress in Obesity-Linked Inflammatory and Metabolic Disorders. Biochem. Pharmacol. 2018, 153, 168–183. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Harford, K.A.; Reynolds, C.M.; McGillicuddy, F.C.; Roche, H.M. Fats, Inflammation and Insulin Resistance: Insights to the Role of Macrophage and T-Cell Accumulation in Adipose Tissue. Proc. Nutr. Soc. 2011, 70, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capurso, C.; Capurso, A. From Excess Adiposity to Insulin Resistance: The Role of Free Fatty Acids. Vascul. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A. Gut Microbiome, Obesity, and Metabolic Dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MS n Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.R.; Rabelo, A.C.S.; Meira, J.S.; Rossoni-Júnior, J.V.; Castro-Borges, W.d.; Guerra-Sá, R.; Batista, M.A.; Silveira-Lemos, D.d.; Souza, G.H.B.d.; Brandão, G.C.; et al. Baccharis Trimera Inhibits Reactive Oxygen Species Production through PKC and Down-Regulation P47 Phox Phosphorylation of NADPH Oxidase in SK Hep-1 Cells. Exp. Biol. Med. 2017, 242, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Luo, J.-G.; Kong, L.-Y. Quality Evaluation of Desmodium Styracifolium Using High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionisation Tandem Mass Spectrometry. Phytochem. Anal. 2012, 23, 240–247. [Google Scholar] [CrossRef]
- Akaike, S.; Sumino, M.; Sekine, T.; Seo, S.; Kimura, N.; Ikegami, F. A New Ent-Clerodane Diterpene from the Aerial Parts of Baccharis Gaudichaudiana. Chem. Pharm. Bull. 2003, 51, 197–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, F.A.d.O.; Tanae, M.M.; Torres, L.M.B.; Lapa, A.J.; de Lima-Landman, M.T.R.; Souccar, C. A Comparative Study of Two Clerodane Diterpenes from Baccharis trimera (Less.) DC. on the Influx and Mobilization of Intracellular Calcium in Rat Cardiomyocytes. Phytomedicine 2014, 21, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal Models of Obesity and Diabetes Mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [Green Version]
- Souza, S.P.; Pereira, L.L.S.; Souza, A.A. Estudo Da Atividade Antiobesidade Do Extrato Metanólico de Baccharis trimera (Less.) DC. Brazilian J. Pharm. 2012, 93, 27–32. [Google Scholar]
- Taïlé, J.; Bringart, M.; Planesse, C.; Patché, J.; Rondeau, P.; Veeren, B.; Clerc, P.; Gauvin-Bialecki, A.; Bourane, S.; Meilhac, O.; et al. Antioxidant Polyphenols of Antirhea Borbonica Medicinal Plant and Caffeic Acid Reduce Cerebrovascular, Inflammatory and Metabolic Disorders Aggravated by High-Fat Diet-Induced Obesity in a Mouse Model of Stroke. Antioxidants 2022, 11, 858. [Google Scholar] [CrossRef]
- Zamani-Garmsiri, F.; Emamgholipour, S.; Rahmani Fard, S.; Ghasempour, G.; Jahangard Ahvazi, R.; Meshkani, R. Polyphenols: Potential Anti-inflammatory Agents for Treatment of Metabolic Disorders. Phyther. Res. 2022, 36, 415–432. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Cao, H. Chlorogenic Acid and Caffeic Acid from Sonchus Oleraceus Linn Synergistically Attenuate Insulin Resistance and Modulate Glucose Uptake in HepG2 Cells. Food Chem. Toxicol. 2019, 127, 182–187. [Google Scholar] [CrossRef]
- Oliveira, A.C.P.; Endringer, D.C.; Amorim, L.A.S.; Brandão, M.d.G.L.; Coelho, M.M. Effect of the Extracts and Fractions of Baccharis Trimera and Syzygium Cumini on Glycaemia of Diabetic and Non-Diabetic Mice. J. Ethnopharmacol. 2005, 102, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Niu, Y.; Gao, Y.; Wang, F.; Qin, W.; Lu, Y.; Hu, J.; Peng, L.; Liu, J.; Xiong, W. Borapetoside E, a Clerodane Diterpenoid Extracted from Tinospora Crispa, Improves Hyperglycemia and Hyperlipidemia in High-Fat-Diet-Induced Type 2 Diabetes Mice. J. Nat. Prod. 2017, 80, 2319–2327. [Google Scholar] [CrossRef]
- Bassoli, B.K.; Cassolla, P.; Borba-Murad, G.R.; Constantin, J.; Salgueiro-Pagadigorria, C.L.; Bazotte, R.B.; da Silva, R.S.d.S.F.; de Souza, H.M. Chlorogenic Acid Reduces the Plasma Glucose Peak in the Oral Glucose Tolerance Test: Effects on Hepatic Glucose Release and Glycaemia. Cell Biochem. Funct. 2008, 26, 320–328. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and Structural Characterization of α -Glucosidase Inhibitors from Hawthorn Leaf Flavonoids Extract by Ultrafiltration LC-DAD-MS n and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Cai, H.; Li, W.; Cai, B. Ultrafiltration Coupled with High-Performance Liquid Chromatography and Quadrupole-Time-of-Flight Mass Spectrometry for Screening Lipase Binders from Different Extracts of Dendrobium Officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, D.-S.; Kim, S.-H.; Kim, H.K. Anti-Obesity Activity, Acute Toxicity, and Chemical Constituents of Aqueous and Ethanol Viola Mandshurica Extracts. BMC Complement. Altern. Med. 2017, 17, 297. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.; Kim, J.; Lim, S. Anti-Obesity Effect of the Above-Ground Part of Valeriana Dageletiana Nakai Ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice. Nutrients 2017, 9, 689. [Google Scholar] [CrossRef] [Green Version]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, X.; Zhang, D.; Deng, B.; Tong, J.; Zhang, M.; Chen, L.; Duan, H.; Niu, W. Hypoxic Adipocytes Induce Macrophages to Release Inflammatory Cytokines That Render Skeletal Muscle Cells Insulin Resistant. Biochem. Biophys. Res. Commun. 2020, 521, 625–631. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective Increases of Bifidobacteria in Gut Microflora Improve High-Fat-Diet-Induced Diabetes in Mice through a Mechanism Associated with Endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.I.; Ijaz, M.U.; Hussain, M.; Haq, I.u.; Zhao, D.; Li, C. High-Fat Proteins Drive Dynamic Changes in Gut Microbiota, Hepatic Metabolome, and Endotoxemia-TLR-4-NFκB-Mediated Inflammation in Mice. J. Agric. Food Chem. 2020, 68, 11710–11725. [Google Scholar] [CrossRef]
- Torrisi, J.S.; Hespe, G.E.; Cuzzone, D.A.; Savetsky, I.L.; Nitti, M.D.; Gardenier, J.C.; García Nores, G.D.; Jowhar, D.; Kataru, R.P.; Mehrara, B.J. Inhibition of Inflammation and INOS Improves Lymphatic Function in Obesity. Sci. Rep. 2016, 6, 19817. [Google Scholar] [CrossRef] [Green Version]
- Katada, S.; Watanabe, T.; Mizuno, T.; Kobayashi, S.; Takeshita, M.; Osaki, N.; Kobayashi, S.; Katsuragi, Y. Effects of Chlorogenic Acid-Enriched and Hydroxyhydroquinone-Reduced Coffee on Postprandial Fat Oxidation and Antioxidative Capacity in Healthy Men: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2018, 10, 525. [Google Scholar] [CrossRef] [Green Version]
- Simões-Pires, C.A.; Queiroz, E.F.; Henriques, A.T.; Hostettmann, K. Isolation and on-line identification of anti-oxidant compounds from three Baccharis species by HPLC-UV-MS/MS with post-column derivatisation. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 307–314. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Paredes, J.D.; Villegas Gabutti, C.M.; Gamarra-Luques, C.; Bórquez, J.; Luna, L.; Wendel, G.H.; Maria, A.O.; et al. Antioxidant, gastroprotective, cytotoxic activities and UHPLC PDA-Q orbitrap mass spectrometry identification of metabolites in Baccharis grisebachii decoction. Molecules 2019, 24, 1085. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.M.Q.; Silva, G.R.D.; Cola, I.M.; Silva, A.O.; Schaedler, M.I.; Guarnier, L.P.; Palozi, R.A.C.; Barboza, L.N.; Menetrier, J.V.; Froelich, D.L.; et al. Baccharis trimera (Less.) DC: An innovative cardioprotective herbal medicine against multiple risk factors for cardiovascular disease. J. Med. Food 2020, 23, 676–684. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Erben, U.; Loddenkemper, C.; Doerfel, K.; Spieckermann, S.; Haller, D.; Heimesaat, M.M.; Zeitz, M.; Siegmund, B.; Kühl, A.A. A Guide to Histomorphological Evaluation of Intestinal Inflammation in Mouse Models. Int. J. Clin. Exp. Pathol. 2014, 7, 4557–4576. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of Reactive Nitrogen Intermediates and Reactive Oxygen Intermediates from Mouse Peritoneal Macrophages. Comparison of Activating Cytokines and Evidence for Independent Production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar]
- Pick, E.; Mizel, D. Rapid Microassays for the Measurement of Superoxide and Hydrogen Peroxide Production by Macrophages in Culture Using an Automatic Enzyme Immunoassay Reader. J. Immunol. Methods 1981, 46, 211–226. [Google Scholar] [CrossRef]
- Santos, E.W.; de Oliveira, D.C.; Hastreiter, A.; Beltran, J.S.d.O.; Rogero, M.M.; Fock, R.A.; Borelli, P. High-Fat Diet or Low-Protein Diet Changes Peritoneal Macrophages Function in Mice. Nutrire 2016, 41, 6. [Google Scholar] [CrossRef]
- Rabinovitch, M.; DeStefano, M.J. Macrophage Spreading in Vitro. Exp. Cell Res. 1973, 77, 323–334. [Google Scholar] [CrossRef]
- Costa Rosa, L.F.; Safi, D.A.; Curi, R. Effect of Thioglycollate and BCG Stimuli on Glucose and Glutamine Metabolism in Rat Macrophages. J. Leukoc. Biol. 1994, 56, 10–14. [Google Scholar] [CrossRef]
| Peak | Compound (CAS) | Ident. Level a | RT (min) | UV (nm) | [M + H]+ m/z | [M − H]− m/z | Molecular Formula | MS/MS (Negative Mode) m/z |
|---|---|---|---|---|---|---|---|---|
| 1 | quinic acid (77-95-2) | 1 | 1.2 | - | 193.0707 | 191.0561 | C7H12O6 | - |
| 2 | caffeoylquinic acid (1241-87-8) | 1 | 10.6 | 217–328 | 355.1024 | 353.0878 | C16H18O9 | 191(C7H11O6)− |
| 3 | 5-feruloyl-quinic acid | 2 | 11.0 | 215–322 | 369.1552 | 367.1035 | C17H20O9 | 191(C7H11O6)− |
| 4 | Vicenin 1 | 2 | 16.1 | 216–340 | 565.1552 | 563.1406 | C26H28O14 | 503(C24H23O12)−, 473(C23H21O11)−, 443(C22H19O10)−, 425(C22H17O9)−, 413(C25H17O6)−, 383(C20H15O8)−, 353(C19H13O7)−, 297(C17H13O5)− |
| 5 | isoschaftoside | 2 | 16.6 | 216–340 | 565.1552 | 563.1406 | C26H28O14 | 545(C26H25O13)−, 503(C24H23O12)−, 473(C23H21O11)−, 443(C22H19O10)−, 425(C22H17O9)−, 413(C21H17O9)−, 383(C20H15O8)−, 353(C19H13O7)−, 297(C17H13O5)− |
| 6 | schaftoside | 2 | 17.7 | 217–340 | 565.1552 | 563.14.06 | C26H28O14 | 473(C23H21O11)−, 443(C22H19O10)−, 425(C22H17O9)−, 413(C21H17O9)−, 383(C20H15O8)−, 353(C19H13O7)−, 297(C17H13O5)− |
| 7 | rutin (153-18-4) | 1 | 18.6 | 279–350 | 607.1607 | 609.1461 | C27H30O16 | 301(C15H9O7)−, 271(C14H7O6)−, 255(C14H7O5)− |
| 8 | 3,4-dicaffeoylquinic acid (57378-72-0) | 1 | 20.0 | 217–325 | 517.1341 | 515.1195 | C25H24O12 | 191(C7H11O6)−, 179(C9H7O4)−, 173(C7H9O5)−, 161(C9H7O4)− |
| 9 | 3,5-dicaffeoylquinic acid (89919-62-0) | 1 | 20.6 | 218–325 | 517.1341 | 515.1195 | C25H24O12 | 191(C7H11O6)−, 179(C9H7O4)−, 161(C9H7O4)− |
| 10 | 4,5-dicaffeoylquinic acid (89886-31-7) | 1 | 22.2 | 219–325 | 517.1341 | 515.11995 | C25H24O12 | 191(C7H11O6)−, 179(C9H7O4)−, 173(C7H9O5)− |
| 11 | putative clerodane diterpenoid | 3 | 28.5 | - | 347.1856 | 391.1762 * | C20H26O5 | 345(C20H25O5)−, 217(C14H17O2)− |
| 12 | putative clerodane diterpenoid | 3 | 29.9 | - | 349.2005 | 393.1919 * | C20H28O5 | - |
| 13 | Putative hexosyl-coumaroyl-triterpene | 3 | 30.8 | 310 | 843.4161 | 420.2009 ** | C45H62O15 | 679(C36H55O12)−, 599(C31H51O11)−, 179(C9H7O4)−, 161(C9H5O3)− |
| 14 | Unknown | 3 | 31.3 | 287–325 | - | 441.2041 ** | C47H64O16 | - |
| 15 | dihydroxy-trimethoxyflavone | 3 | 32.6 | 276–339 | 345.0969 | 343.0822 | C18H16O7 | 313(C16H9O7)−, 285(C15H9O6)− |
| Parameter | ND | HD | ||
|---|---|---|---|---|
| Water | BTi | Water | BTi | |
| Food intake (g) | 4.22 ± 0.03 | 3.75 ± 0.06 *** | 3.41 ± 0.05 *** | 3.04 ± 0.04 ### |
| Calorie intake (kj) | 67.07 ± 0.54 | 59.66 ± 0.92 *** | 73.05 ± 1.05 *** | 65.06 ± 0.75 ### |
| Initial body weight (g) | 32.62 ± 0.76 | 30.28 ± 0.84 | 30.89 ± 1.16 | 30.33 ± 1.07 |
| Final body weight (g) | 48.03 ± 0.90 | 41.14 ± 1.41 * | 55.71 ± 1.24 * | 47.22 ± 1.84 ## |
| Weight gain (g) | 14.76 ± 0.83 | 10.86 ± 0.86 * | 24.82 ± 1.19 *** | 16.89 ± 0.94 ### |
| Omental (g) | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 * |
| Retroperitoneal (g) | 1.26 ± 0.13 | 0.65 ± 0.07 | 1.76 ± 0.12 * | 1.13 ± 0.08 ### |
| Epididymal (g) | 1.98 ± 0.07 | 1.57 ± 0.09 | 2.89 ± 0.20 *** | 2.41 ± 0.13 |
| Mesenteric (g) | 1.08 ± 0.05 | 0.79 ± 0.05 | 2.21 ± 0.15 *** | 1.26 ± 0.11 ### |
| Perirenal (g) | 0.28 ± 0.02 | 0.23 ± 0.02 | 0.45 ± 0.06 * | 0.35 ± 0.04 |
| Visceral adipose tissue (g) | 4.15 ± 0.48 | 3.30 ± 0.19 | 7.39 ± 0.43 *** | 5.24 ± 0.22 ### |
| Adiposity index (%) | 9.67 ± 0.36 | 7.94 ± 0.26 * | 13.25 ± 0.62 *** | 11.09 ± 0.29 ## |
| Adipocyte area (µm2) | 580.1 ± 6.6 | 525.3 ± 5.4 *** | 740.3 ± 6.4 *** | 695.1 ± 7.8 ### |
| Liver (g) | 2.00 ± 0.12 | 1.79 ± 0.10 | 2.69 ± 0.20 * | 1.95 ± 0.15 ## |
| Parameter | ND | HD | ||
|---|---|---|---|---|
| Water | BTi | Water | BTi | |
| TG (mg/dL) | 149.7 ± 14.2 | 121.3 ± 11.9 | 142.4 ± 7.3 | 149.7 ± 8.5 |
| TC (mg/dL) | 160.0 ± 17.3 | 131.9 ± 10.0 | 218.6 ± 5.9 ** | 164.6 ± 9.9 ## |
| HDL-c (mg/dL) | 90.0 ± 9.7 | 80.9 ± 6.4 | 93.0 ± 3.4 | 95.3 ± 4.6 |
| LDL-c (mg/dL) | 40.0 ± 8.4 | 26.7 ± 3.5 | 97.1 ± 4.0 *** | 39.3 ± 5.8 ### |
| VLDL-c (mg/dL) | 30.0 ± 2.8 | 24.3 ± 2.4 | 28.5 ± 1.5 | 30.0 ± 1.7 |
| AI | 1.8 ±0.1 | 1.7 ± 0.0 | 2.4 ± 0.1 *** | 1.7 ± 0.0 ### |
| Parameter | ND | HD | ||
|---|---|---|---|---|
| Water | BTi | Water | BTi | |
| Leukocyte infiltrate (cell/mm3) | 1540 ± 95 | 1531 ± 132 | 2280 ± 127 ** | 1481 ± 81 ### |
| CLS/field | 0.82 ± 0.20 | 0.76 ± 0.12 *** | 4.20 ± 0.61 *** | 1.36 ± 0.30 ### |
| Cell viability (%) | 97.66 ± 1.59 | 89.38 ± 2.82 | 92.13 ± 3.01 | 93.42 ± 1.78 |
| Nitric oxide (µM NO2−) | 7.09 ± 1.61 | 10.41 ± 0.10 | 34.12 ± 4.34 *** | 8.36 ± 1.14 ### |
| Hydrogen peroxide (µM H2O2) | 28.16 ± 1.61 | 34.09 ± 2.60 | 28.60 ± 2.32 | 34.56 ± 2.21 |
| Spreading (%) | 10.47 ± 0.83 | 11.37 ± 0.69 | 25.00 ± 4.96 *** | 9.79 ± 0.96 ### |
| Cell adhesion (abs/1 × 105 cell) | 0.42 ± 0.02 | 0.28 ± 0.02 *** | 0.43 ± 0.03 | 0.25 ± 0.01 ### |
| Phagocytosis (%) | 48.00 ± 2.31 | 37.75 ± 1.32 | 55.00 ± 3.79 | 32.67 ± 2.33 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ximenes, T.V.N.; Carvalho, R.; Bonfá, I.S.; Santos, V.S.; Candeloro, L.; Alves, F.M.; Silva, D.B.; Carollo, C.A.; Gielow, K.d.C.F.; Silva-Filho, S.E.; et al. Baccharis trimera Infusion Reduces Macrophages Activation and High-Fat Diet-Induced Metabolic Disorders in Mice. Pharmaceuticals 2022, 15, 1258. https://doi.org/10.3390/ph15101258
Ximenes TVN, Carvalho R, Bonfá IS, Santos VS, Candeloro L, Alves FM, Silva DB, Carollo CA, Gielow KdCF, Silva-Filho SE, et al. Baccharis trimera Infusion Reduces Macrophages Activation and High-Fat Diet-Induced Metabolic Disorders in Mice. Pharmaceuticals. 2022; 15(10):1258. https://doi.org/10.3390/ph15101258
Chicago/Turabian StyleXimenes, Thalita Vieira Nascimento, Raquel Carvalho, Iluska Senna Bonfá, Vanessa Samúdio Santos, Luciane Candeloro, Flávio Macedo Alves, Denise Brentan Silva, Carlos Alexandre Carollo, Karine de Cássia Freitas Gielow, Saulo Euclides Silva-Filho, and et al. 2022. "Baccharis trimera Infusion Reduces Macrophages Activation and High-Fat Diet-Induced Metabolic Disorders in Mice" Pharmaceuticals 15, no. 10: 1258. https://doi.org/10.3390/ph15101258
APA StyleXimenes, T. V. N., Carvalho, R., Bonfá, I. S., Santos, V. S., Candeloro, L., Alves, F. M., Silva, D. B., Carollo, C. A., Gielow, K. d. C. F., Silva-Filho, S. E., & Toffoli-Kadri, M. C. (2022). Baccharis trimera Infusion Reduces Macrophages Activation and High-Fat Diet-Induced Metabolic Disorders in Mice. Pharmaceuticals, 15(10), 1258. https://doi.org/10.3390/ph15101258






