In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights
Abstract
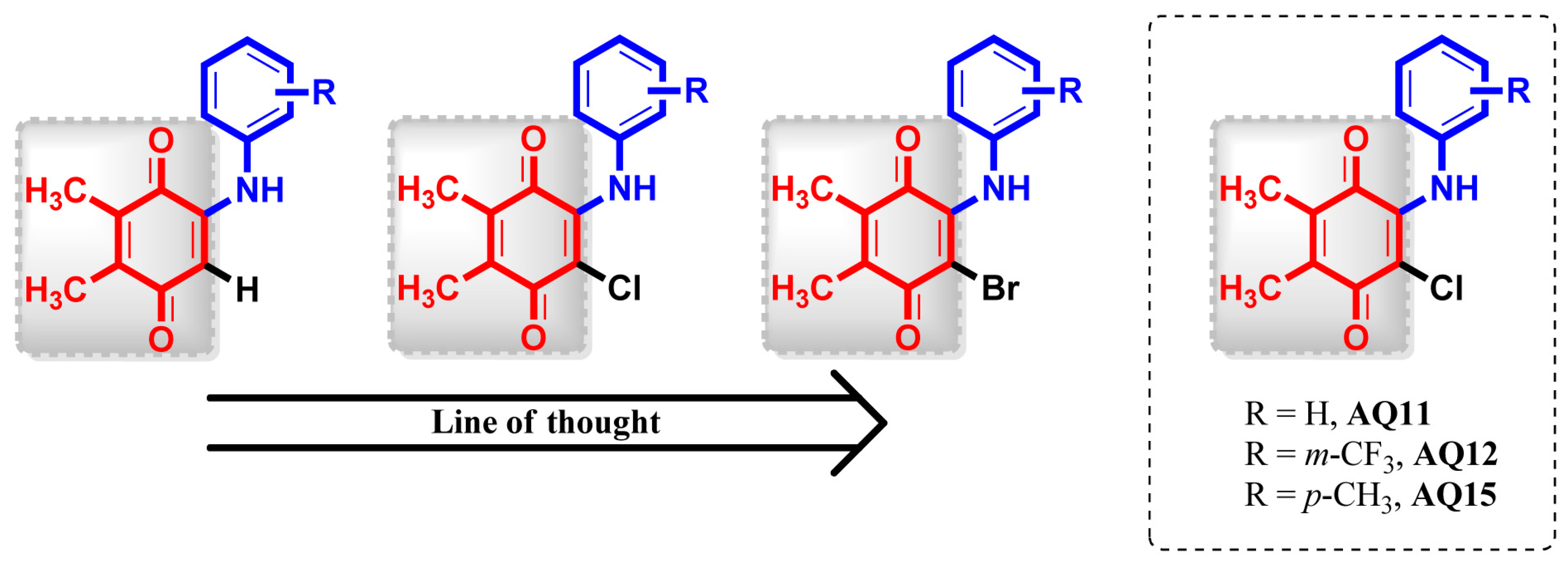
:1. Introduction
2. Results
2.1. Anticancer Activity Assessment
2.1.1. In Vitro Screening of Tumor Cell Growth Inhibition at One Dose
2.1.2. In Vitro Full-Panel Five-Dose 60-Cell Lines Assay
2.1.3. Cell Viability Assay on CRC and Breast Cancer Cells
2.1.4. Cell Death Investigation
2.2. In Silico Studies
2.2.1. Molecular Docking
2.2.2. Estimation of Pharmacokinetic Parameters
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Anticancer Activity Studies
4.2.1. In Vitro Single-Dose Anticancer Screening by NCI
4.2.2. In Vitro Five-Dose Anticancer Screening by NCI
4.2.3. Cell Culture, Drug Treatment, and MTT Assay
4.2.4. Cell Death Analysis
4.2.5. Statistical Analyses
4.3. In Silico Studies
4.3.1. Molecular Docking
4.3.2. ADME Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, R.J.; Miller, R.; Coleman, N. Colorectal cancer screening: Prospects for molecular stool analysis. Nat. Rev. Cancer 2005, 5, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Dalal, N.; Jalandra, R.; Sharma, M.; Prakash, H.; Makharia, G.K.; Solanki, P.R.; Singh, R.; Kumar, A. Omics technologies for improved diagnosis and treatment of colorectal cancer: Technical advancement and major perspectives. Biomed. Pharmacother. 2020, 131, 110648. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef]
- Jin, K.; Ren, C.; Liu, Y.; Lan, H.; Wang, Z. An update on colorectal cancer microenvironment, epigenetic and immunotherapy. Int. Immunopharmacol. 2020, 89 (Pt A), 107041. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, M.A. The association between colorectal cancer and breast cancer. J. Chronic Dis. 1976, 29, 243–261. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Topham, A.; Herzberg, T.; Weiner, S.; Weinberg, D.S. Risk of colorectal cancer after breast cancer. Lancet 2001, 357, 837–840. [Google Scholar] [CrossRef]
- Kmet, L.M.; Cook, L.S.; Weiss, N.S.; Schwartz, S.M.; White, E. Risk factors for colorectal cancer following breast cancer. Breast Cancer Res. Treat. 2003, 79, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.A.; O’Malley, J.P.; Gough, A.; Buckley, D.I.; Wallace, J.; Fagnan, L.J.; Morris, C.; Mori, M.; Heintzman, J.D.; Lieberman, D. Association between documented family history of cancer and screening for breast and colorectal cancer. Prev. Med. 2013, 57, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Segelman, J.; Nordgren, A.; Lindström, L.; Frisell, J.; Martling, A. Increased risk of colorectal cancer in patients diagnosed with breast cancer in women. Cancer Epidemiol. 2016, 41, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef]
- Lau, K.H.; Tan, A.M.; Shi, Y. New and Emerging Targeted Therapies for Advanced Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2288. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Improving drug candidates by design: A focus on physicochemical properties as a means of improving compound disposition and safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef] [PubMed]
- Guha, R. On exploring structure-activity relationships. Methods Mol. Biol. 2013, 993, 81–94. [Google Scholar] [PubMed] [Green Version]
- Shrestha, J.P.; Subedi, Y.P.; Chen, L.H.; Chang, C.W.T. A mode of action study of cationic anthraquinone analogues: A new class of highly potent anticancer agents. MedChemComm 2015, 6, 2012–2022. [Google Scholar] [CrossRef]
- Gholampour, M.; Seradj, H.; Pirhadi, S.; Khoshneviszadeh, M. Novel 2-amino-1,4-naphthoquinone hybrids: Design, synthesis, cytotoxicity evaluation and in silico studies. Bioorg. Med. Chem. 2020, 28, 115718. [Google Scholar] [CrossRef] [PubMed]
- Kruschel, R.D.; Buzid, A.; Khandavilli, U.B.R.; Lawrence, S.E.; Glennon, J.D.; McCarthy, F.O. Isoquinolinequinone N-oxides as anticancer agents effective against drug resistant cell lines. Org. Biomol. Chem. 2020, 18, 557–568. [Google Scholar] [CrossRef]
- Eyong, K.O.; Ketsemen, H.L.; Zhao, Z.; Du, L.Q.; Ingels, A.; Mathieu, V.; Kornienko, A.; Hull, K.G.; Folefoc, G.N.; Baskaran, S.; et al. Antiproliferative activity of naphthoquinones and indane carboxylic acids from lapachol against a panel of human cancer cell lines. Med. Chem. Res. 2020, 29, 1058–1066. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Subramaniyan, S.A.; Kim, S.Y.; Kim, J.S.; Park, B.H.; Shim, K.S.; Yoo, D.J. Synthesis and Anticancer Evaluation of 1,4-Naphthoquinone Derivatives Containing a Phenylaminosulfanyl Moiety. ChemMedChem 2019, 14, 532–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defant, A.; Mancini, I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules. Molecules 2019, 24, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayrak, N.; Yıldırım, H.; Yıldız, M.; Radwan, M.O.; Otsuka, M.; Fujita, M.; Ciftci, H.I.; Tuyun, A.F. A novel series of chlorinated plastoquinone analogues: Design, synthesis, and evaluation of anticancer activity. Chem. Biol. Drug Des. 2020, 95, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, N.; Yildirim, H.; Yildiz, M.; Radwan, M.O.; Otsuka, M.; Fujita, M.; Tuyun, A.F.; Ciftci, H.I. Design, synthesis, and biological activity of Plastoquinone analogues as a new class of anticancer agents. Bioorg. Chem. 2019, 92, 103255. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Bayrak, N.; Yildirim, H.; Yildiz, M.; Radwan, M.O.; Otsuka, M.; Fujita, M.; Tuyun, A.F. Discovery and structure-activity relationship of plastoquinone analogues as anticancer agents against chronic myelogenous leukemia cells. Arch. Pharm. 2019, 352, 1900170. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, N.; Ciftci, H.I.; Yildiz, M.; Yildirim, H.; Sever, B.; Tateishi, H.; Otsuka, M.; Fujita, M.; Tuyun, A.F. Structure based design, synthesis, and evaluation of anti-CML activity of the quinolinequinones as LY83583 analogues. Chem. Biol. Interact. 2021, 345, 109555. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Sever, B.; Tateishi, H.; Otsuka, M.; Fujita, M.; Tuyun, A.F. Design, synthesis and investigation of the mechanism of action underlying anti-leukemic effects of the quinolinequinones as LY83583 analogues. Bioorg. Chem. 2021, 114, 105160. [Google Scholar] [CrossRef] [PubMed]
- Jannuzzi, A.T.; Yıldız, M.; Bayrak, N.; Yıldırım, H.; Shilkar, D.; Jayaprakash, V.; Tuyun, A.F. Anticancer agents based on Plastoquinone analogues with N-phenylpiperazine: Structure-activity relationship and mechanism of action in breast cancer cells. Chem. Biol. Interact. 2021, 349, 109673. [Google Scholar] [CrossRef] [PubMed]
- DTP Developmental Therapeutics Program. Available online: https://dtp.cancer.gov/databases_tools/docs/compare/compare_methodology.htm#perform (accessed on 25 September 2022).
- Kazakova, O.; Mioc, A.; Smirnova, I.; Baikova, I.; Voicu, A.; Vlaia, L.; Macașoi, I.; Mioc, M.; Drăghici, G.; Avram, Ş.; et al. Novel Synthesized N-Ethyl-Piperazinyl-Amides of C2-Substituted Oleanonic and Ursonic Acids Exhibit Cytotoxic Effects through Apoptotic Cell Death Regulation. Int. J. Mol. Sci. 2021, 22, 10967. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Pauli, K.D. Some practical considerations and applications of the National-Cancer-Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Cuartas, V.; Aragón-Muriel, A.; Liscano, Y.; Polo-Cerón, D.; Crespo-Ortiz, M.; Quiroga, J.; Abonia, R.; Insuasty, B. Anticancer activity of pyrimidodiazepines based on 2-chloro-4-anilinoquinazoline: Synthesis, DNA binding and molecular docking. RSC Adv. 2021, 11, 23310–23329. [Google Scholar] [CrossRef] [PubMed]
- Rostom, S.A.; Shalaby, M.A.; El-Demellawy, M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogues and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003, 38, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigrowolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Loehrer, P.J., Sr.; Roth, B.J.; Einhorn, L.H. Cisplatin as first-line therapy for metastatic breast cancer. J. Clin. Oncol. 1988, 6, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, R.; Bisagni, G.; Cocconi, G.; Boni, C.; Di Blasio, B.; Ceci, G. Cisplatin and etoposide in advanced colorectal carcinoma. Ann. Oncol. 1991, 2, 687–688. [Google Scholar] [CrossRef]
- Haller, D.G. Recent updates in the clinical use of platinum compounds for the treatment of gastrointestinal cancers. Semin. Oncol. 2004, 31, 10–16. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Hassiotou, F.; Blancafort, P.; Filgueira, L. Cisplatin induces differentiation of breast cancer cells. Front. Oncol. 2013, 3, 134. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Duan, W.M.; Wu, M.Y.; Wang, W.J.; Liu, L.; Xu, M.D.; Zhu, J.; Li, D.M.; Gui, Q.; Lian, L.; et al. Participation of autophagy in the cytotoxicity against breast cancer cells by cisplatin. Oncol. Rep. 2015, 34, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, C.; Huang, Z.; Zhou, D.; Xiang, S.; Qi, Q.; Chen, X.; Arbely, E.; Liu, C.Y.; Du, P.; et al. Cisplatin inhibits SIRT3-deacetylation MTHFD2 to disturb cellular redox balance in colorectal cancer cell. Cell Death Dis. 2020, 11, 649. [Google Scholar] [CrossRef]
- Lynce, F.; Nunes, R. Role of Platinums in Triple-Negative Breast Cancer. Curr. Oncol. Rep. 2021, 23, 50. [Google Scholar] [CrossRef]
- Ciftci, H.; Sever, B.; Ocak, F.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; DeMirci, H.; Tateishi, H.; Otsuka, M.; Fujita, M.; et al. In Vitro and In Silico Study of Analogues of Plant Product Plastoquinone to Be Effective in Colorectal Cancer Treatment. Molecules 2022, 27, 693. [Google Scholar] [CrossRef] [PubMed]
- Brogden, A.L.; Hopcroft, N.H.; Searcey, M.; Cardin, C.J. Ligand bridging of the DNA Holliday junction: Molecular recognition of a stacked-X four-way junction by a small molecule. Angew. Chem. Int. Ed. Eng. 2007, 46, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2016-2; Schrödinger, LLC: New York, NY, USA, 2016.
- Schrödinger Release 2016-2: QikProp; Schrödinger, LLC: New York, NY, USA, 2016.
- SwissADME. Available online: http://www.swissadme.ch (accessed on 29 August 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Tukulula, M.; Little, S.; Gut, J.; Rosenthal, P.J.; Wan, B.; Franzblau, S.G.; Chibale, K. The design, synthesis, in silico ADME profiling, antiplasmodial and antimycobacterial evaluation of new arylamino quinoline derivatives. Eur. J. Med. Chem. 2012, 57, 259–267. [Google Scholar] [CrossRef]
- Rasal, N.K.; Sonawane, R.B.; Jagtap, S.V. Potential 2,4-dimethyl-1H-pyrrole-3-carboxamide bearing benzimidazole template: Design, synthesis, in vitro anticancer and in silico ADME study. Bioorg. Chem. 2020, 97, 103660. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Vigiani, E.; Vincenzi, F.; Pasquini, S.; Varani, K.; Colotta, V. Piperazine- and Piperidine-Containing Thiazolo[5,4-d]pyrimidine Derivatives as New Potent and Selective Adenosine A2A Receptor Inverse Agonists. Pharmaceuticals 2020, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Neurath, M.F. The molecular therapy of colorectal cancer. Mol. Aspects Med. 2010, 31, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Tandon, V.K.; Kumar, S. Recent development on naphthoquinone derivatives and their therapeutic applications as anticancer agents. Expert Opin. Ther. Pat. 2013, 23, 1087–1108. [Google Scholar] [CrossRef]
- Yilmaz Goler, A.M.; Jannuzzi, A.T.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Otsuka, M.; Fujita, M.; Radwan, M.O.; TuYuN, A.F. In Vitro and In Silico Study to Assess Toxic Mechanisms of Hybrid Molecules of Quinone-Benzocaine as Plastoquinone Analogues in Breast Cancer Cells. ACS Omega 2022, 7, 30250–30264. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.E.; Hahm, H.A.; Armstrong, D.K. Apoptosis and Breast Cancer. In Apoptosis and Cancer Chemotherapy. Cancer Drug Discovery and Development; Hickman, J.A., Dive, C., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 291–303. [Google Scholar]
- Parton, M.; Dowsett, M.; Smith, I. Studies of apoptosis in breast cancer. BMJ 2001, 322, 1528–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Sales, K.M.; Fuller, B.; Seifalian, A.M.; Winslet, M.C. Apoptosis and colorectal cancer: Implications for therapy. Trends Mol. Med. 2009, 15, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J.M. Recent advances in basic science apoptosis and colorectal cancer. Gut 2004, 53, 1701–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, F.; Hashida, M. In silico approaches for predicting ADME properties of drugs. Drug Metab. Pharm. 2004, 19, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Honório, K.M.; Moda, T.L.; Andricopulo, A.D. Pharmacokinetic properties and in silico ADME modeling in drug discovery. Med. Chem. 2013, 9, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Durán-Iturbide, N.A.; Díaz-Eufracio, B.I.; Medina-Franco, J.L. In Silico ADME/Tox Profiling of Natural Products: A Focus on BIOFACQUIM. ACS Omega 2020, 5, 16076–16084. [Google Scholar] [CrossRef]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National-Cancer-Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Can, M.; Ellakwa, D.E.; Suner, S.C.; Ibrahim, M.A.; Oral, A.; Sekeroglu, N.; Özalp, B.; Otsuka, M.; Fujita, M.; et al. Anticancer activity of Turkish marine extracts: A purple sponge extract induces apoptosis with multitarget kinase inhibition activity. Investig. New Drugs 2020, 38, 1326–1333. [Google Scholar] [CrossRef] [PubMed]










| Compound | Substitution Groups | Cell Type (IC50, μM) | ||
|---|---|---|---|---|
| R | K562 a | PBMC a | SI c | |
| AQ-11 | H | 0.75 ± 0.05 | 5.14 ± 1.76 | 6.85 |
| AQ-12 | m-CF3 | 0.88 ± 0.06 | 3.00 ± 1.22 | 3.41 |
| AQ-15 | p-CH3 | 0.76 ± 0.04 | 7.64 ± 1.58 | 10.05 |
| Imatinib b | 5.58 ± 1.83 | 33.92 ± 4.19 | 6.08 | |
| Panel/Cancer Cell Line | Compound | ||
|---|---|---|---|
| AQ-11 | AQ-12 | AQ-15 | |
| Growth Percentage of Cell Lines in NCI 60 | |||
| Leukemia | |||
| CCRF-CEM | 5.28 | 5.90 | 47.98 |
| HL-60(TB) | 45.64 | 38.46 | 73.23 |
| K-562 | 15.93 | 3.78 | 54.03 |
| MOLT-4 | 12.72 | 23.53 | 66.29 |
| RPMI-8226 | 53.63 | −3.12 | 87.67 |
| SR | 62.86 | 24.97 | 79.88 |
| Non-Small Cell Lung Cancer | |||
| A549/ATCC | 99.81 | 96.78 | 101.90 |
| EKVX | 55.24 | 49.44 | 93.61 |
| HOP-62 | 92.60 | 90.98 | 89.82 |
| HOP-92 | 46.02 | 48.86 | 95.70 |
| NCI-H226 | 96.94 | 90.87 | 101.59 |
| NCI-H23 | ND | ND | ND |
| NCI-H322M | 99.29 | 100.88 | 104.93 |
| NCI-H460 | 95.74 | 94.85 | 100.91 |
| NCI-H522 | 83.46 | 17.73 | 93.90 |
| CRC | |||
| COLO 205 | 107.07 | 101.80 | 111.35 |
| HCC-2998 | ND | ND | ND |
| HCT-116 | 79.11 | 33.86 | 95.79 |
| HCT-15 | 94.19 | 65.71 | 101.63 |
| HT29 | 99.13 | 103.04 | 104.47 |
| KM12 | 78.34 | 67.20 | 100.25 |
| SW-620 | 96.49 | 17.07 | 102.49 |
| CNS Cancer | |||
| SF-268 | 95.39 | 94.34 | 95.65 |
| SF-295 | 102.14 | 96.20 | 107.03 |
| SF-539 | 95.42 | 93.40 | 97.15 |
| SNB-19 | 88.79 | 92.41 | 96.00 |
| SNB-75 | 65.44 | 66.47 | 65.44 |
| U251 | 84.30 | 78.99 | 99.94 |
| Melanoma | |||
| LOX IMVI | ND | ND | ND |
| MALME-3M | 107.98 | 94.35 | 102.03 |
| M14 | 95.87 | 85.23 | 102.50 |
| MDA-MB-435 | 102.30 | 98.46 | 107.59 |
| SK-MEL-2 | 85.48 | 85.77 | 95.31 |
| SK-MEL-28 | 106.11 | 98.26 | 104.07 |
| SK-MEL-5 | ND | ND | ND |
| UACC-257 | 90.27 | 78.53 | 106.17 |
| UACC-62 | 90.28 | 80.22 | 96.80 |
| Ovarian Cancer | |||
| IGROV1 | 9.69 | −7.20 | 80.83 |
| OVCAR-3 | 101.79 | 70.40 | 105.64 |
| OVCAR-4 | −97.92 | −80.41 | 99.38 |
| OVCAR-5 | 103.60 | 100.01 | 100.88 |
| OVCAR-8 | 94.89 | 87.14 | 104.55 |
| NCI/ADR-RES | ND | ND | ND |
| SK-OV-3 | ND | ND | ND |
| Renal Cancer | |||
| 786-0 | 99.18 | 101.74 | 104.29 |
| A498 | 91.24 | 55.17 | 72.19 |
| ACHN | 100.46 | −37.69 | 96.49 |
| CAKI-1 | 96.08 | 92.74 | 91.53 |
| RXF 393 | 96.80 | 97.82 | 108.41 |
| SN12C | 91.13 | 87.62 | 96.37 |
| TK-10 | 128.28 | 183.73 | 142.33 |
| UO-31 | 98.81 | 77.33 | 92.50 |
| Prostate Cancer | |||
| PC-3 | 73.87 | 64.35 | 86.95 |
| DU-145 | 101.26 | 99.72 | 107.98 |
| Breast Cancer | |||
| MCF7 | 90.72 | 35.36 | 96.29 |
| MDA-MB-231/ATCC | 15.35 | 18.96 | 94.55 |
| HS 578T | 100.91 | 87.57 | 90.46 |
| BT-549 | 113.97 | 120.60 | 119.94 |
| T-47D | −38.88 | −38.96 | 84.68 |
| MDA-MB-468 | −76.01 | −65.55 | 52.82 |
| Panel/Cell Line | GI50 | TGI | LC50 |
|---|---|---|---|
| Leukemia | |||
| CCRF-CEM | 1.93 | >100 | >100 |
| HL-60(TB) | 2.34 | 6.54 | >100 |
| K-562 | 2.40 | >100 | >100 |
| MOLT-4 | 2.22 | >100 | >100 |
| RPMI-8226 | 1.32 | 7.32 | >100 |
| SR | 2.59 | >100 | |
| Non-Small Cell Lung Cancer | |||
| A549/ATCC | 13.30 | >100 | >100 |
| EKVX | 1.49 | 3.73 | 9.31 |
| HOP-62 | 11.50 | 27.60 | 66.50 |
| HOP-92 | 1.51 | 3.04 | 6.14 |
| NCI-H226 | 10.80 | 34.90 | >100 |
| NCI-H23 | 6.87 | 24.00 | 65.40 |
| NCI-H322M | 22.70 | >100 | >100 |
| NCI-H460 | 11.60 | 32.20 | 89.30 |
| NCI-H522 | 2.24 | 5.66 | >100 |
| CRC | |||
| COLO 205 | 13.00 | 29.30 | 66.00 |
| HCC-2998 | 5.88 | 18.40 | 46.00 |
| HCT-116 | 1.93 | 3.99 | 8.22 |
| HCT-15 | 2.20 | 5.05 | 16.20 |
| HT29 | 7.29 | 51.80 | >100 |
| KM12 | 3.58 | 25.70 | >100 |
| SW-620 | 2.09 | 4.73 | >100 |
| CNS Cancer | |||
| SF-268 | 4.99 | 28.60 | >100 |
| SF-295 | 6.55 | 24.00 | 74.10 |
| SF-539 | 2.40 | 6.61 | 24.80 |
| SNB-19 | 5.83 | 23.40 | 77.40 |
| SNB-75 | 1.16 | 13.60 | 91.00 |
| U251 | 4.45 | 17.90 | 58.20 |
| Melanoma | |||
| LOX IMVI | 1.69 | 3.32 | 6.51 |
| MALME-3M | 1.98 | 6.04 | 30.90 |
| M14 | 9.96 | 32.70 | >100 |
| MDA-MB-435 | 5.98 | 19.50 | 49.20 |
| SK-MEL-2 | 8.26 | 21.70 | 50.90 |
| SK-MEL-28 | 3.36 | 10.80 | 36.00 |
| SK-MEL-5 | 4.14 | 15.70 | 43.80 |
| UACC-257 | 2.61 | 6.72 | 26.90 |
| UACC-62 | 3.46 | 16.10 | 56.00 |
| Ovarian Cancer | |||
| IGROV1 | 1.42 | 3.19 | 7.15 |
| OVCAR-3 | 2.31 | 5.58 | >100 |
| OVCAR-4 | 1.58 | 2.97 | 5.58 |
| OVCAR-5 | 1.98 | 4.13 | 8.61 |
| OVCAR-8 | 2.68 | 6.89 | >100 |
| NCI/ADR-RES | 3.16 | 42.30 | >100 |
| SK-OV-3 | 1.30 | 50.70 | >100 |
| Renal Cancer | |||
| 786-0 | 10.60 | 24.30 | 55.70 |
| A498 | 13.70 | 45.20 | >100 |
| ACHN | 1.73 | 3.19 | 5.87 |
| CAKI-1 | 1.94 | 9.11 | 32.40 |
| RXF 393 | 1.49 | 2.85 | 5.44 |
| SN12C | 2.25 | 23.40 | >100 |
| TK-10 | 19.00 | 37.00 | 71.90 |
| UO-31 | 1.46 | 4.18 | 14.20 |
| Prostate Cancer | |||
| PC-3 | 2.67 | 12.80 | >100 |
| DU-145 | 15.60 | 34.80 | 77.70 |
| Breast Cancer | |||
| MCF7 | 1.71 | 3.79 | 8.39 |
| MDA-MB-231/ATCC | 1.59 | 3.28 | 6.77 |
| HS 578T | 6.00 | 64.40 | >100 |
| BT-549 | 8.56 | 22.20 | 51.70 |
| T-47D | 1.17 | 3.60 | >100 |
| MDA-MB-468 | 1.24 | 2.94 | 7.00 |
| Compound | IC50 Value (µM) | |
|---|---|---|
| MCF-7 Cells | HCT-116 Cells | |
| AQ-12 | 6.06 ± 3.09 | 5.11 ± 2.14 |
| Cisplatin | 19.67 ± 5.94 | 23.68 ± 6.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciftci, H.; Sever, B.; Bayrak, N.; Yıldız, M.; Yıldırım, H.; Tateishi, H.; Otsuka, M.; Fujita, M.; TuYuN, A.F. In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights. Pharmaceuticals 2022, 15, 1266. https://doi.org/10.3390/ph15101266
Ciftci H, Sever B, Bayrak N, Yıldız M, Yıldırım H, Tateishi H, Otsuka M, Fujita M, TuYuN AF. In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights. Pharmaceuticals. 2022; 15(10):1266. https://doi.org/10.3390/ph15101266
Chicago/Turabian StyleCiftci, Halilibrahim, Belgin Sever, Nilüfer Bayrak, Mahmut Yıldız, Hatice Yıldırım, Hiroshi Tateishi, Masami Otsuka, Mikako Fujita, and Amaç Fatih TuYuN. 2022. "In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights" Pharmaceuticals 15, no. 10: 1266. https://doi.org/10.3390/ph15101266
APA StyleCiftci, H., Sever, B., Bayrak, N., Yıldız, M., Yıldırım, H., Tateishi, H., Otsuka, M., Fujita, M., & TuYuN, A. F. (2022). In Vitro Cytotoxicity Evaluation of Plastoquinone Analogues against Colorectal and Breast Cancers along with In Silico Insights. Pharmaceuticals, 15(10), 1266. https://doi.org/10.3390/ph15101266









