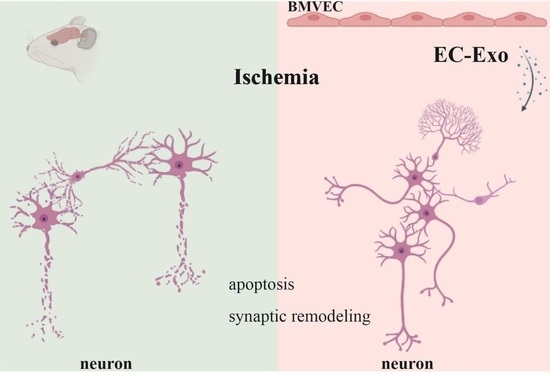
Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice
Abstract
:1. Introduction
2. Results
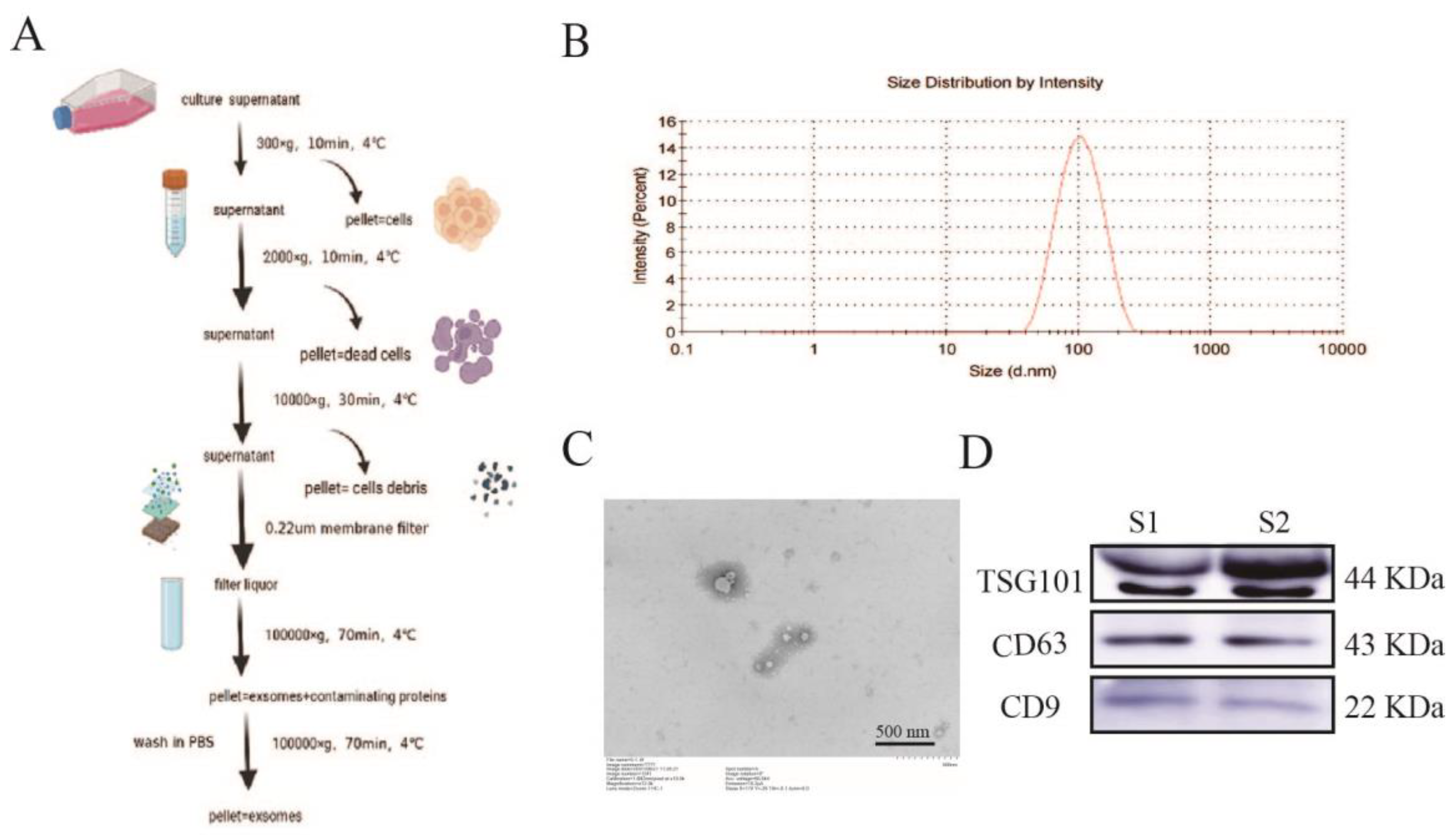
2.1. Isolation and Identification of Exosomes
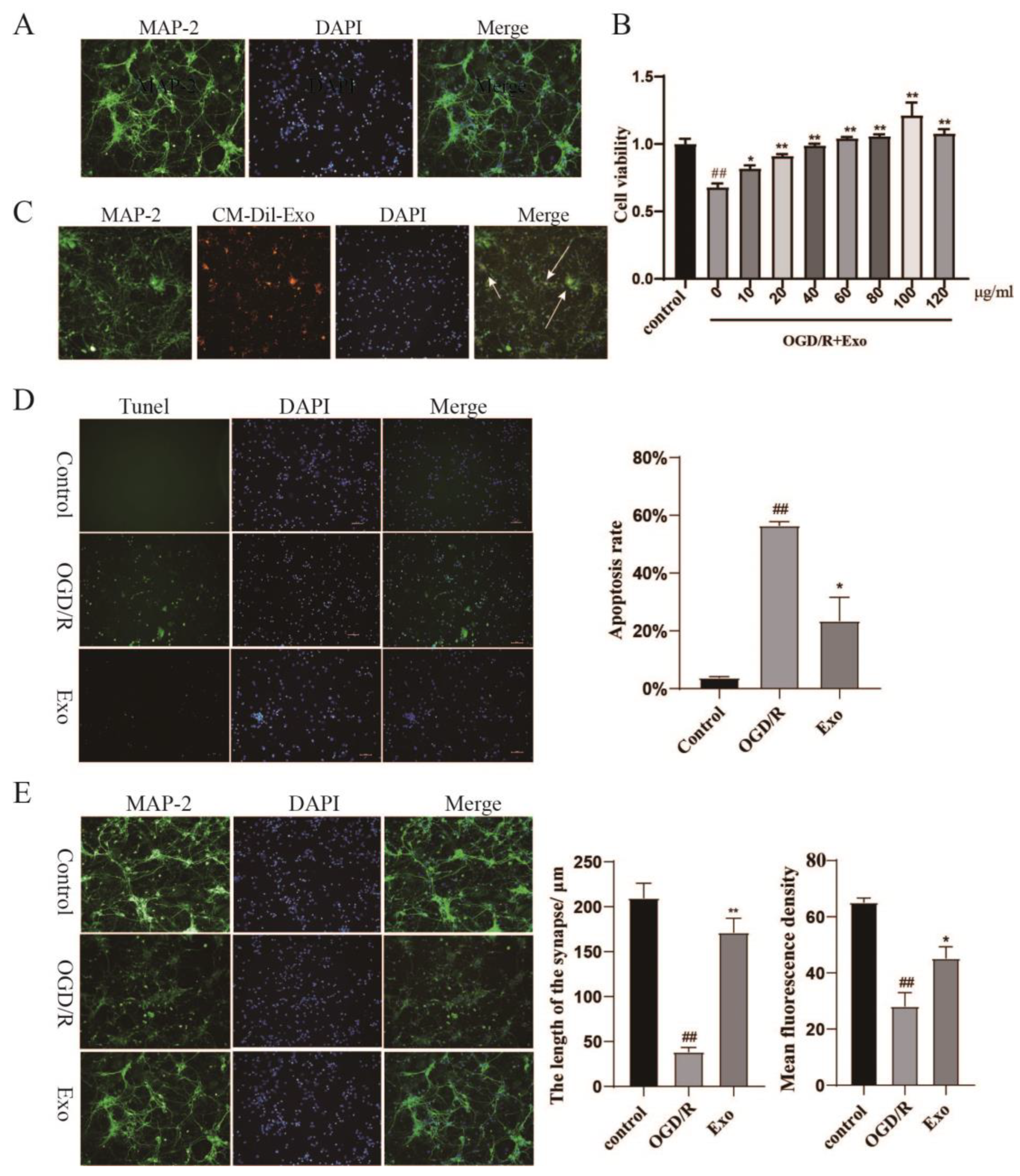
2.2. Identification of Primary Neurons and EC-Exo Protects Neurons from OGD/R Damage
2.3. EC-Exo Increases the Neuronal Synaptic Length and Inhibits Apoptosis In Vitro
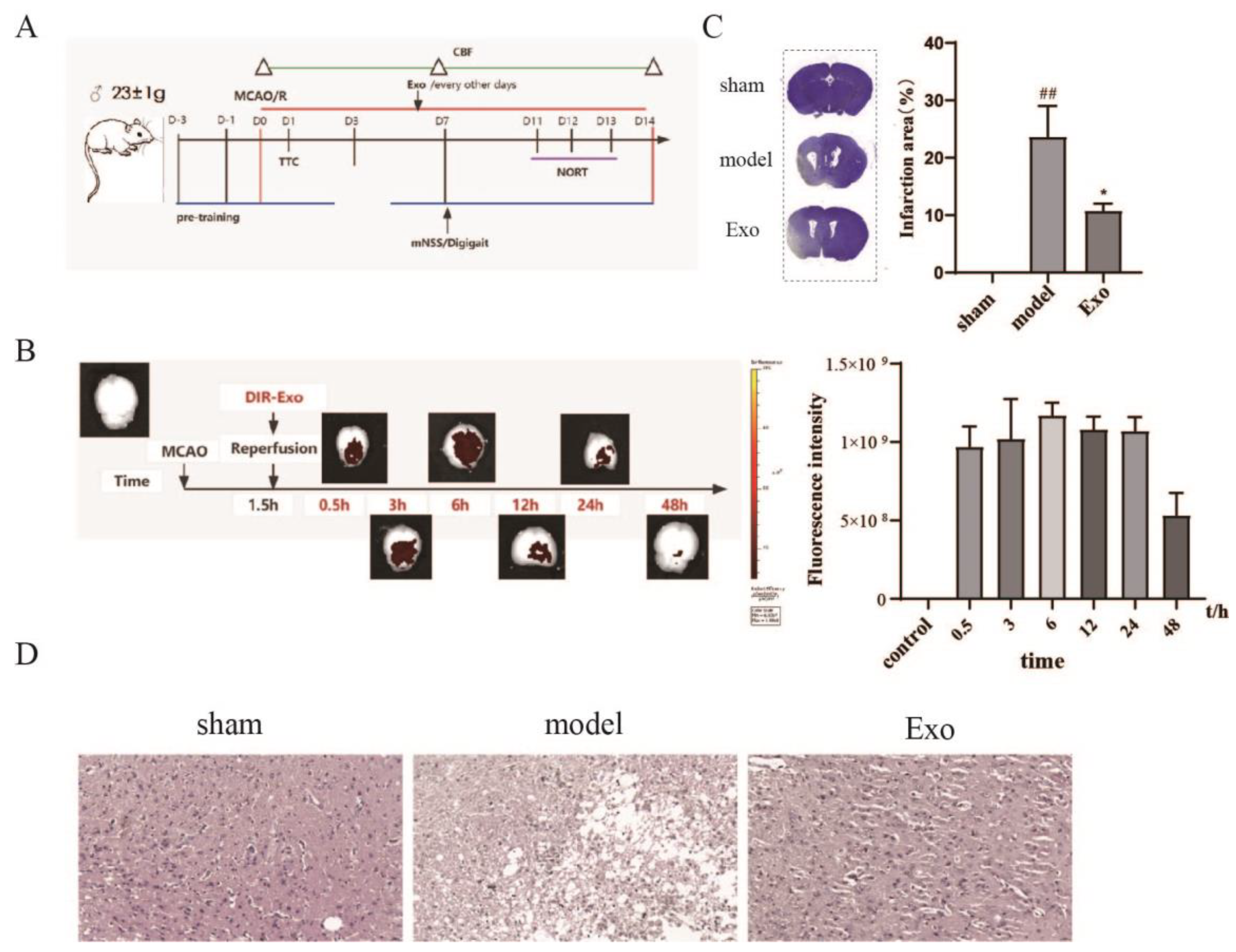
2.4. EC-Exo Treatment Improves Pathology in MCAO/R Mice
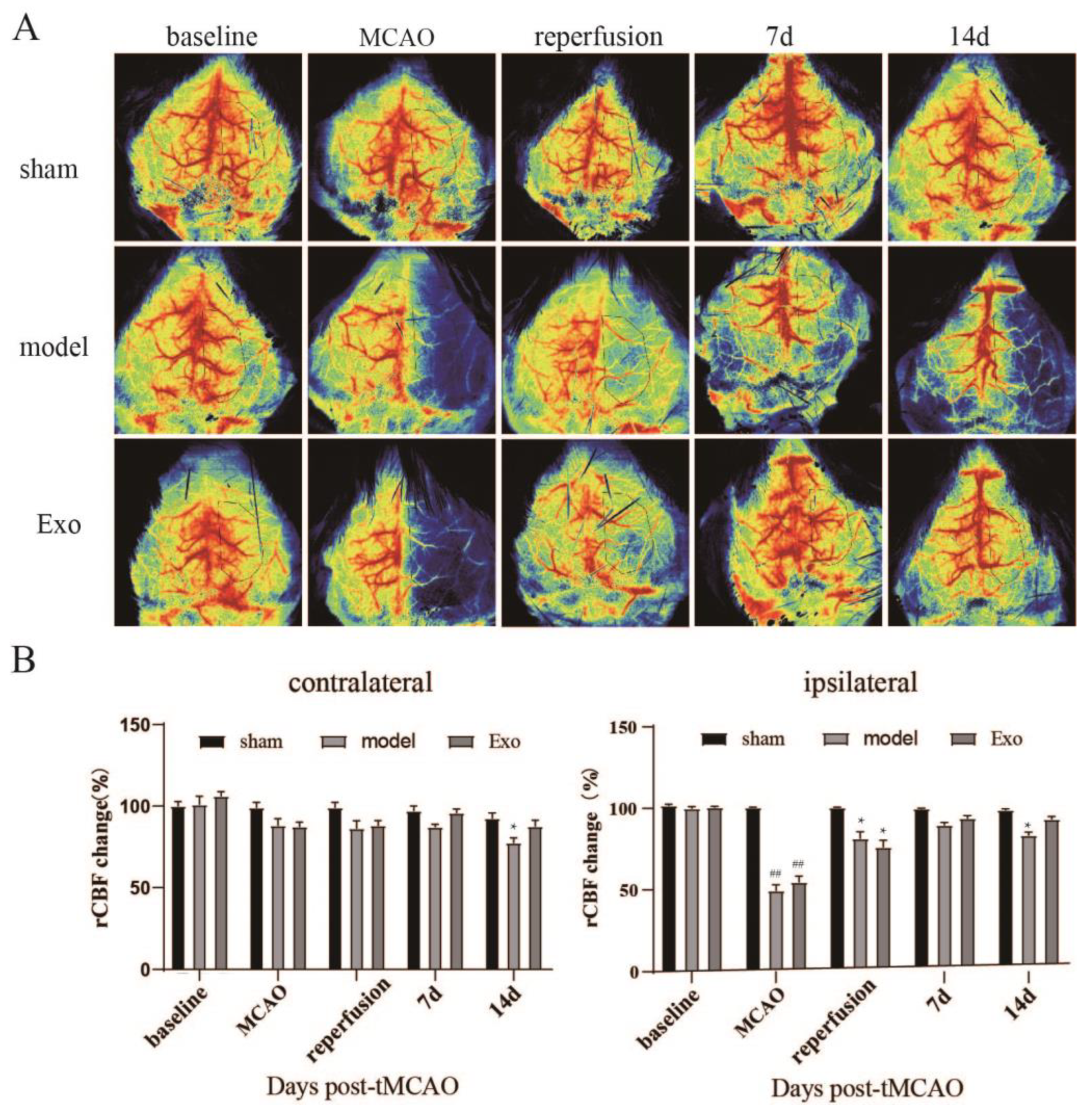
2.5. EC-Exo Increases rCBF in MCAO/R Mice
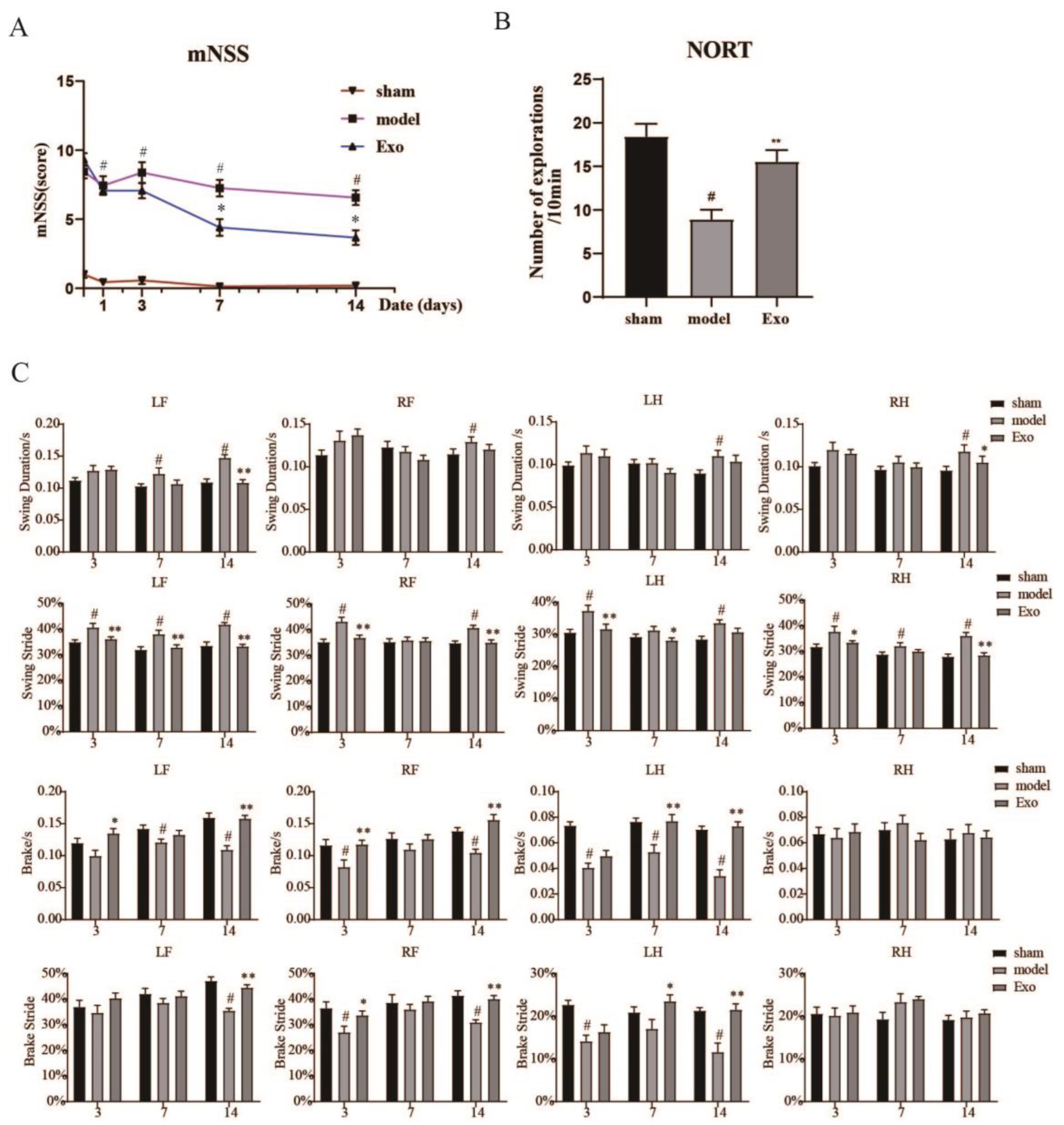
2.6. EC-Exo Improves Neurobehavioral Outcomes in MCAO/R Mice
2.7. EC-Exo Treatment Improves Exploratory Behavioral Outcomes in MCAO/R Mice
2.8. EC-Exo Treatment Improves Gait Outcomes in MCAO/R Mice
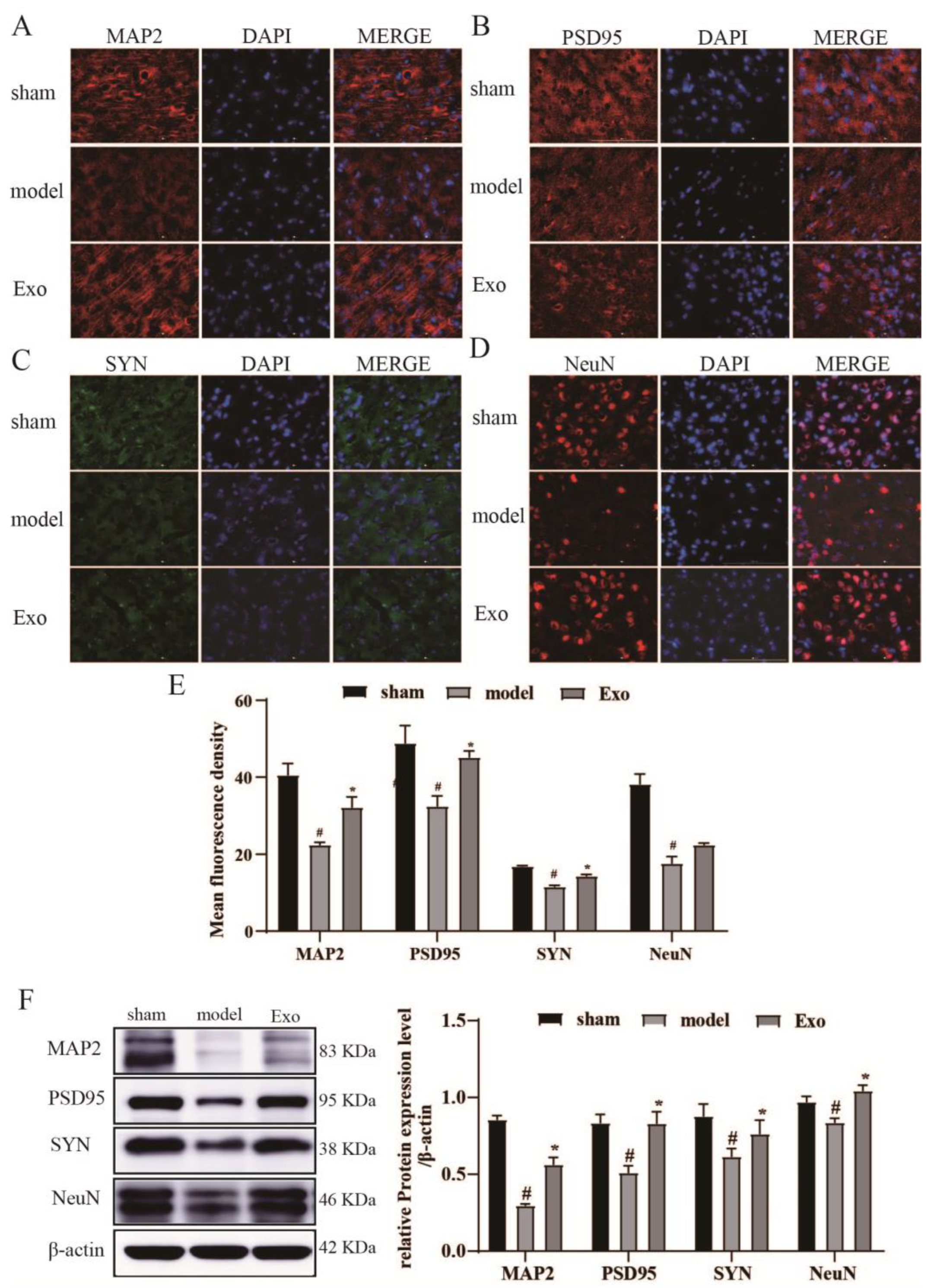
2.9. EC-Exo Promotes Expression of Synaptic Plasticity Proteins in MCAO/R Mouse
2.10. EC-Exo Inhibits Apoptosis in the Brain of MCAO/R Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Exosome Extraction and Characterization
4.3. Primary Mouse Neuronal Cultures
4.4. CM-DiI Labeling of Exosome and Cell Uptake of Exosomes
4.5. OGD/R Model and Exosome Treatment
4.6. Cell Viability Assays
4.7. MCAO/R Model and Exosome Treatment
4.8. Fluorescently Labeled Exosomes and In Vivo Tracking Studies
4.9. Measurement of rCBF
4.10. Examination of Neurological Functions
4.11. Gait Analysis
4.12. NORT
4.13. Crystalline Violet Staining
4.14. HE
4.15. TUNEL Assay
4.16. Western Blot Analysis
4.17. Immunostaining
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weldegwergs, K.; Westerman, R.; Wijeratne, T.; Winkler, A.S.; Xuan, B.T.; Yonemoto, N.; Feigin, V.L.; Vos, T.; Murray, C.J. GBD 2016 Neurology Collaborators, Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Bae, H.J.; Lim, J.S.; Godefroy, O.; Roussel, M.; Kang, Y.; Jahng, S.; Köhler, S.; et al. Long-Term Cognitive Decline After Stroke: An Individual Participant Data Meta-Analysis. Stroke 2022, 53, 1318–1327. [Google Scholar] [CrossRef]
- Fisher, M.; Savitz, S.I. Pharmacological brain cytoprotection in acute ischemic stroke—Renewed hope in the reperfusion era. Nat. Rev. Neurol. 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Lyden, P.D. Cerebroprotection for Acute Ischemic Stroke: Looking Ahead. Stroke 2021, 52, 3033–3044. [Google Scholar] [CrossRef]
- Lyden, P.; Buchan, A.; Boltze, J.; Fisher, M.; STAIR XI Consortium*. Top Priorities for Cerebroprotective Studies—A Paradigm Shift: Report From STAIR XI. Stroke 2021, 52, 3063–3071. [Google Scholar] [CrossRef]
- Savitz, S.I.; Baron, J.C.; Fisher, M.; STAIR X Consortium. Stroke Treatment Academic Industry Roundtable X: Brain Cytoprotection Therapies in the Reperfusion Era. Stroke 2019, 50, 1026–1031. [Google Scholar] [CrossRef]
- Michiels, C.; Arnould, T.; Remacle, J. Endothelial cell responses to hypoxia: Initiation of a cascade of cellular interactions. Biochim. Biophys. Acta 2000, 1497, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Van, N.G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Martínez, M.C.; Tesse, A.; Zobairi, F.; Andriantsitohaina, R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1004-9. [Google Scholar] [CrossRef] [PubMed]
- Sharghi-Namini, S.; Tan, E.; Ong, L.L.; Ge, R.; Asada, H.H. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci. Rep. 2014, 4, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Chai, Y.; Lv, S.; Ye, M.; Wu, M.; Xie, L.; Fan, Y.; Zhu, X.; Gao, Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 2017, 40, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Venkat, P.; Cui, C.; Chopp, M.; Zacharek, A.; Wang, F.; Landschoot-Ward, J.; Shen, Y.; Chen, J. MiR-126 Mediates Brain Endothelial Cell Exosome Treatment-Induced Neurorestorative Effects After Stroke in Type 2 Diabetes Mellitus Mice. Stroke 2019, 50, 2865–2874. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, B.X.; Du, M.M.; Wang, X.Y.; Li, W. Chinese preparation Xuesaitong promotes the mobilization of bone marrow mesenchymal stem cells in rats with cerebral infarction. Neural Regen Res. 2016, 11, 292–297. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, X.; Wang, M.; Li, Z.; Qiao, M.; Hu, H.; Chen, D. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. J. Mater. Chem. B. 2019, 7, 2421–2433. [Google Scholar] [CrossRef]
- Gardiner, C.; Di, V.D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Judicone, C.; Poncelet, P.; Robert, S.; Arnaud, L.; Sampol, J.; Dignat-George, F. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost. 2012, 10, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Parsons, M.W.; Levi, C.R.; Lin, L.; Aviv, R.I.; Spratt, N.J.; Butcher, K.S.; Lou, M.; Kleinig, T.J.; Bivard, A. Influence of occlusion site and baseline ischemic core on outcome in patients with ischemic stroke. Neurology 2019, 92, e2626–e2643. [Google Scholar] [CrossRef] [PubMed]
- Cotrina, M.L.; Lou, N.; Tome-Garcia, J.; Goldman, J.; Nedergaard, M. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience 2017, 343, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, S.G.; Kontos, C.K.; Pappa, V.; Thomadaki, H.; Kontsioti, F.; Dervenoulas, J.; Papageorgiou, E.; Economopoulos, T.; Scorilas, A. The novel member of the BCL2 gene family, BCL2L12, is substantially elevated in chronic lymphocytic leukemia patients, supporting its value as a significant biomarker. Oncologist 2011, 16, 1280–1291. [Google Scholar] [CrossRef] [Green Version]
- Zaric, M.; Drakulic, D.; Stojanovic, I.G.; Mitrovic, N.; Grkovic, I.; Martinovic, J. Regional-specific effects of cerebral ischemia/reperfusion and dehydroepiandrosterone on synaptic NMDAR/PSD-95 complex in male Wistar rats. Brain Res. 2018, 1688, 73–80. [Google Scholar] [CrossRef]
- Osimo, E.F.; Beck, K.; Reis, M.T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef]
- Wi, S.; Yu, J.H.; Kim, M.; Cho, S.R. In Vivo Expression of Reprogramming Factors Increases Hippocampal Neurogenesis and Synaptic Plasticity in Chronic Hypoxic-Ischemic Brain Injury. Neural Plast. 2016, 2016, 2580837. [Google Scholar] [CrossRef] [Green Version]
- Tavosanis, G. Dendritic structural plasticity. Dev. Neurobiol. 2015, 72, 73–86. [Google Scholar] [CrossRef]
- Wiweko, B.; Muna, N.; Gunawarti, D.P.; Nasution, R.U.; Zesario, A. High bax-bcl-2 ratio expression on granulosa cells from endometriosis patients. J. Comput. Theor. Nanosci. 2017, 23, 6720–6722. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Pu, J.; Chen, Y.; Mao, Y.; Guo, Z.; Pan, H.; Zhang, L.; Zhang, H.; Sun, B.; Zhang, B. Plasma Exosomes Spread and Cluster Around β-Amyloid Plaques in an Animal Model of Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Gao, B.; Sun, C.; Bai, Y.; Cheng, D.; Zhang, Y.; Li, X.; Zhao, J.; Xu, D. Vascular Endothelial Cell-derived Exosomes Protect Neural Stem Cells Against Ischemia/reperfusion Injury. Neuroscience 2020, 441, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Engel, O.; Kolodziej, S.; Dirnagl, U.; Prinz, V. Modeling Stroke in Mice-Middle Cerebral Artery Occlusion with the Filament Model. J. Vis. Exp. 2011, 47, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996, 27, 1616–1622. [Google Scholar] [CrossRef]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Yang, G.Y. Therapeutic application of exosomes in ischaemic stroke. Stroke Vasc. Neurol 2021, 6, 483–495. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Yuan, Q.; Guo, L.; Xiao, G.; Zhang, T.; Liang, B.; Yao, R.; Zhu, Y.; Li, Y.; Hu, L. Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice. Pharmaceuticals 2022, 15, 1287. https://doi.org/10.3390/ph15101287
Sun J, Yuan Q, Guo L, Xiao G, Zhang T, Liang B, Yao R, Zhu Y, Li Y, Hu L. Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice. Pharmaceuticals. 2022; 15(10):1287. https://doi.org/10.3390/ph15101287
Chicago/Turabian StyleSun, Jin, Qing Yuan, Lichen Guo, Guangxu Xiao, Tong Zhang, Bing Liang, Rongmei Yao, Yan Zhu, Yue Li, and Limin Hu. 2022. "Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice" Pharmaceuticals 15, no. 10: 1287. https://doi.org/10.3390/ph15101287
APA StyleSun, J., Yuan, Q., Guo, L., Xiao, G., Zhang, T., Liang, B., Yao, R., Zhu, Y., Li, Y., & Hu, L. (2022). Brain Microvascular Endothelial Cell-Derived Exosomes Protect Neurons from Ischemia–Reperfusion Injury in Mice. Pharmaceuticals, 15(10), 1287. https://doi.org/10.3390/ph15101287