Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models
Abstract
:1. Introduction
2. Results
2.1. Effect of Sponge Extract on In Vitro Model of Diabetic Neuropathy
2.1.1. High Glucose (HG)-Induced Decreases in DRG Neuronal Viability and Neurite Length
2.1.2. Effect of X. testudinaria Extract on HG-Induced Oxidative Stress in DRG Culture
2.2. Evaluation of Acute Toxicity of X. testudinaria Extract in Mice
2.3. Effect of X. testudinaria Extract on In Vivo Model of Diabetic Neuropathy
2.3.1. Effect of X. testudinaria Extract on STZ-Induced Hot Hyperalgesia
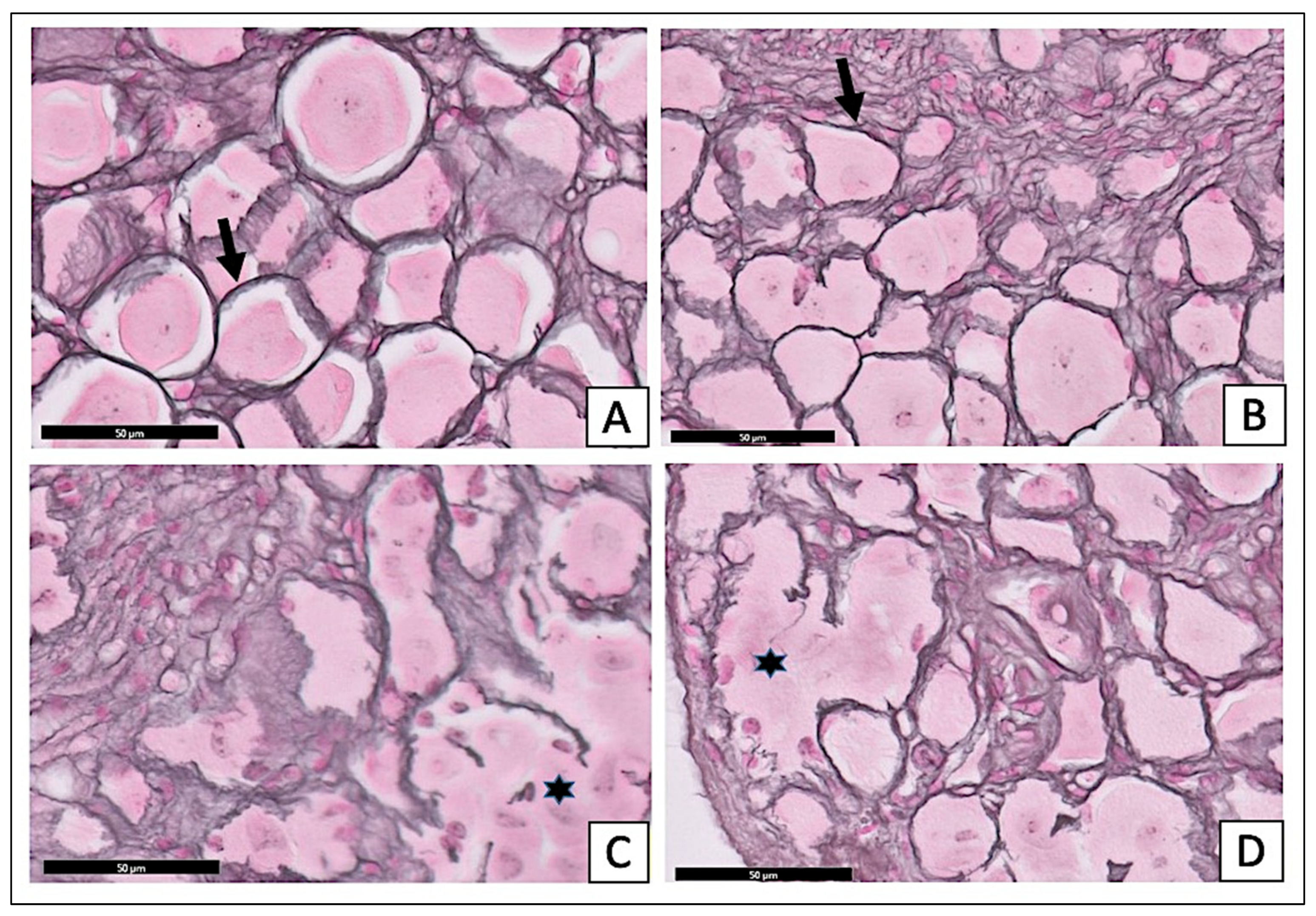
2.3.2. Effect of X. testudinaria Extract on DRG Histology
2.3.3. Effect of X. testudinaria Extract on Glucose Level
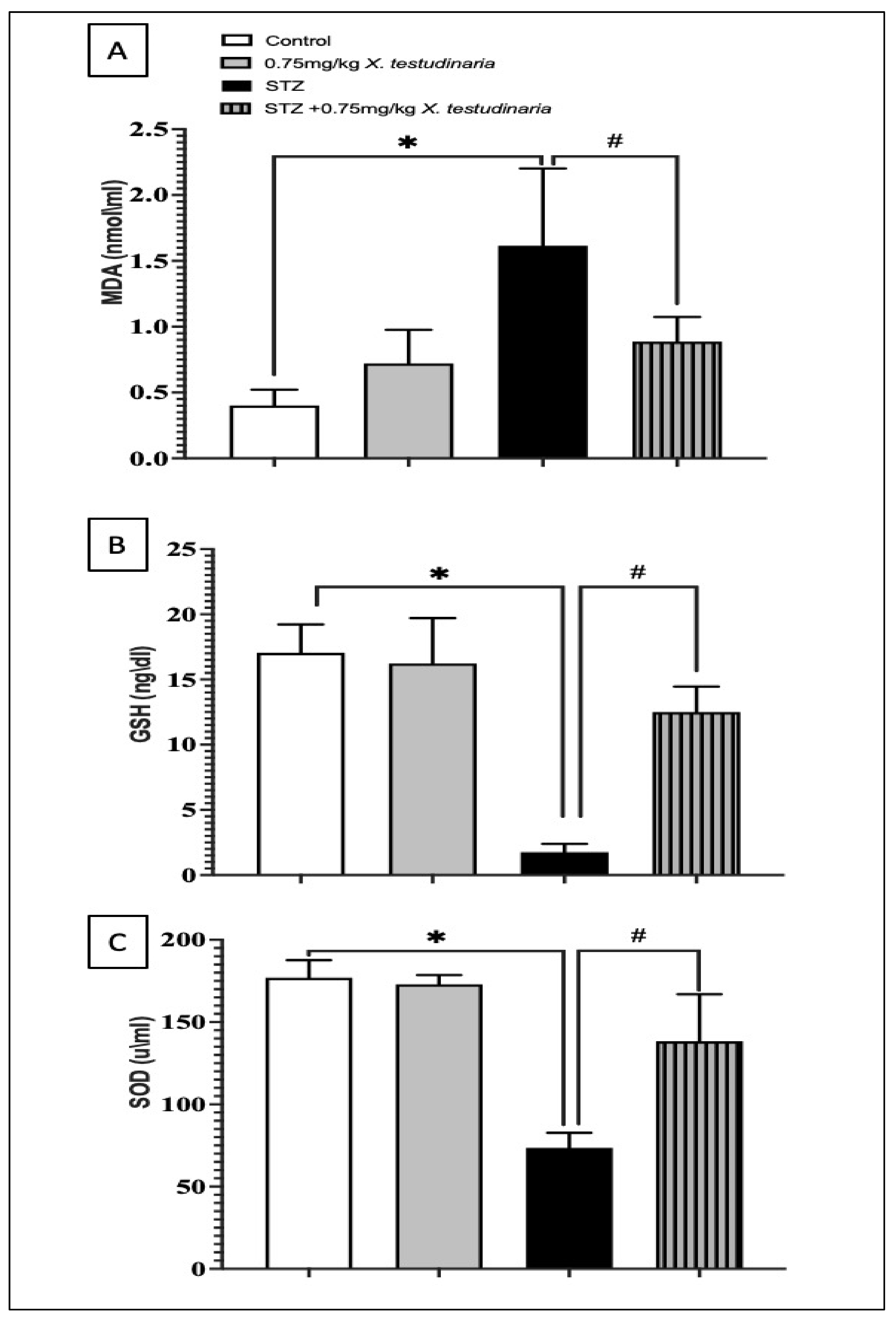
2.3.4. Effect of X. testudinaria Extract on STZ-Induced Oxidative Stress in a Mice Model
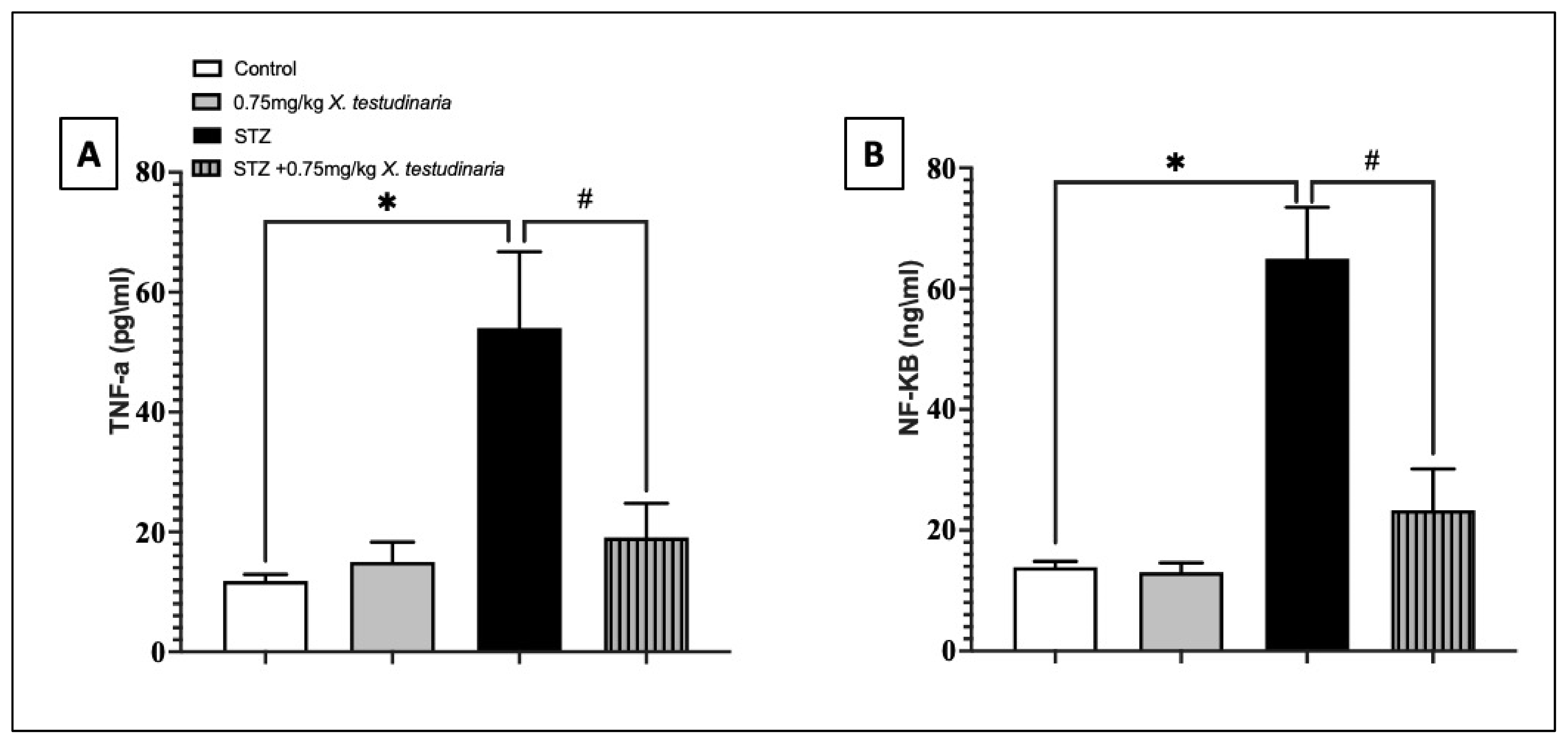
2.3.5. Effect of X. testudinaria Extract on Pro-Inflammatory Biomarkers in DRG of Diabetic Neuropathy Mice (TNF-α, NF-κB)
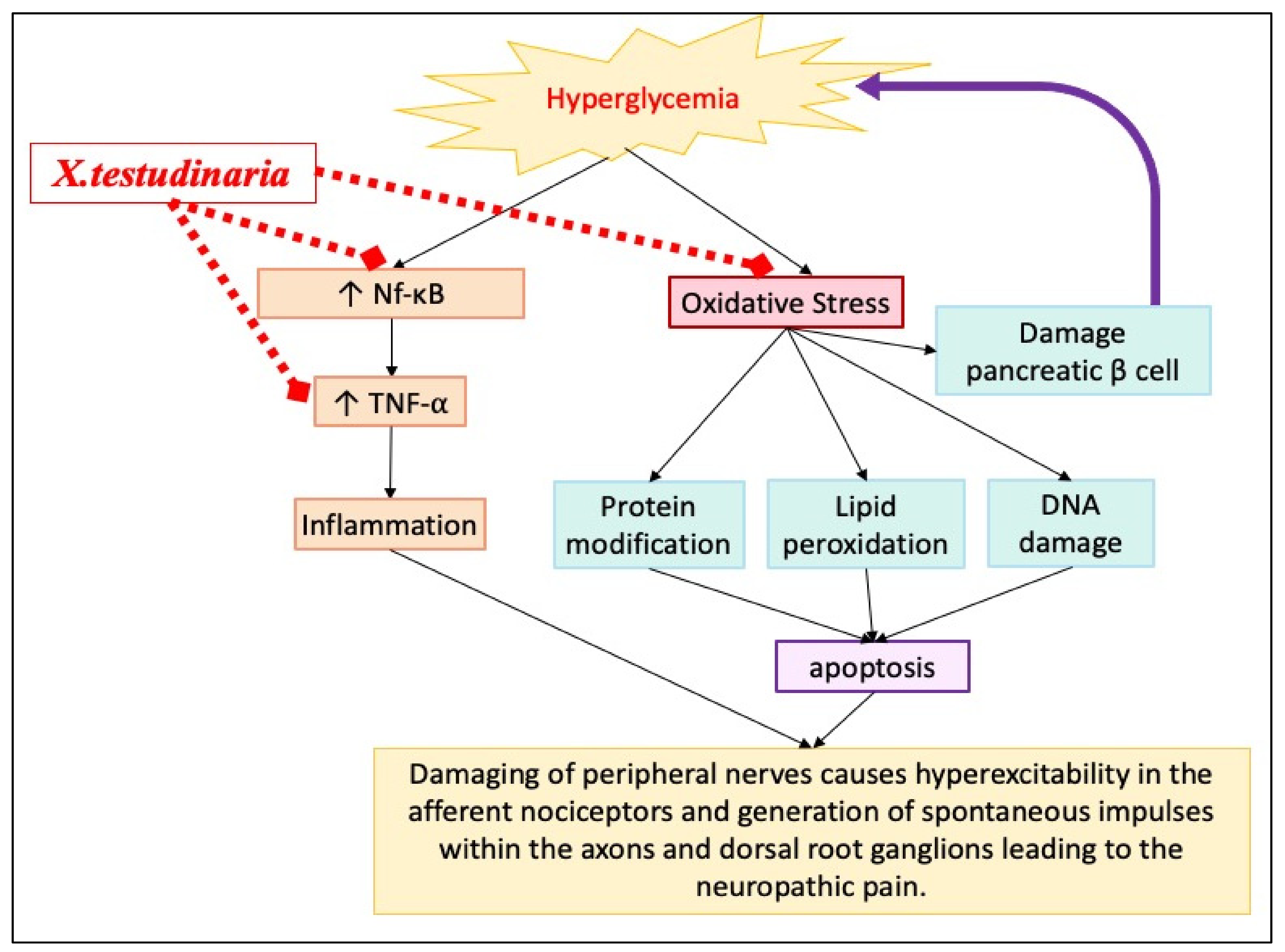
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Xestospongia Testudinaria
4.1.2. Animals
4.2. Ethical Approval
4.3. Methods
4.3.1. Extraction of the Sponge and Preparation of the Extract Was Carried Out as Follows
4.3.2. DRG Isolation and Culture
4.3.3. In Vitro and in Vivo Induction of Diabetic Peripheral Neuropathy Model
- In vitro induction:
- In vivo induction:
4.3.4. In Vitro and In Vivo Experimental Design
- In vitro design:
- In vivo design:
4.3.5. In vitro Studies to Evaluate the Neuroprotective Effect of Sponge Extract Using Viability Assay
4.3.6. Immunocytochemistry
4.3.7. In Vivo Studies to Evaluate the Neuroprotective Effect of Sponge Extract by Evaluating Hot Hyperalgesia
4.3.8. Evaluation of Oxidative Stress and Pro-Inflammatory Biomarkers
4.3.9. Histological Examination
- (1)
- Hematoxylin and Eosin (H & E) stain for all specimens for a routine histological examination.
- (2)
- Masson’s Trichrome stain for the liver and pancreas to show the collagen fibers [17].
- (3)
- Reticulin stain for DRG demonstration of reticulin fibers [39].
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algeffari, M.A. Painful Diabetic Peripheral Neuropathy among Saudi Diabetic Patients is Common but Under-recognized: Multicenter Cross-sectional study at primary health care setting. J. Fam. Community Med. 2018, 25, 43–47. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [Green Version]
- Skapare, E.; Konrade, I.; Liepinsh, E.; Strele, I.; Makrecka, M.; Bierhaus, A.; Lejnieks, A.; Pirags, V.; Dambrova, M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J. Diabetes Complicat. 2013, 27, 262–267. [Google Scholar] [CrossRef]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef] [Green Version]
- Leinninger, G.M.; Backus, C.; Sastry, A.M.; Yi, Y.B.; Wang, C.W.; Feldman, E.L. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol. Dis. 2006, 23, 11–22. [Google Scholar] [CrossRef]
- King, R.H. The role of glycation in the pathogenesis of diabetic polyneuropathy. Mol. Pathol. 2001, 54, 400–408. [Google Scholar]
- Vincent, A.M.; McLean, L.L.; Backus, C.; Feldman, E.L. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005, 19, 638–640. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, T.; Yang, X.W.; Huang, R.; Yang, B.; Tang, L.; Liu, Y. Chemical and biological aspects of marine sponges of the genus Xestospongia. Chem. Biodivers. 2010, 7, 2201–2227. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Al-Massarani, S.M.; Shaala, L.A.; Alahdald, A.M.; Al-Said, M.S.; Ashour, A.E.; Kumar, A.; Abdel-Kader, M.S.; Abdel-Mageed, W.M.; Youssef, D.T. Cytotoxic Compounds from the Saudi Red Sea Sponge Xestospongia testudinaria. Mar. Drugs 2016, 14, 82. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Shaala, L.A.; Abbas, A.T.; Abdel-Dayem, U.A.; Azhar, E.I.; Ali, S.S.; van Soest, R.W.; Youssef, D.T. Evaluation of the Anti-Inflammatory, Antioxidant and Immunomodulatory Effects of the Organic Extract of the Red Sea Marine Sponge Xestospongia testudinaria against Carrageenan Induced Rat Paw Inflammation. PLoS ONE 2015, 10, e0138917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liolios, C.C.; Sotiroudis, G.T.; Chinou, I. Fatty acids, sterols, phenols and antioxidant activity of Phoenix theophrasti fruits growing in Crete, Greece. Plant Foods Hum. Nutr. 2009, 64, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Swantara, M.D.; Rita, W.S.; Suartha, N.; Agustina, K.K. Anticancer activities of toxic isolate of Xestospongia testudinaria sponge. Vet. World 2019, 12, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Magadmi, R.M.; Alsulaimani, M.A.; Al-Rafiah, A.R.; Esmat, A. The Neuroprotective Effect of Carvedilol on Diabetic Neuropathy: An in vitro Study. J. Diabetes Res. 2021, 2021, 6927025. [Google Scholar] [CrossRef] [PubMed]
- Magadmi, R.M.; Alsulaimani, M.A.; Al-Rafiah, A.R.; Ahmad, M.S.; Esmat, A. Carvedilol Exerts Neuroprotective Effect on Rat Model of Diabetic Neuropathy. Front. Pharmacol. 2021, 12, 613634. [Google Scholar] [CrossRef]
- Vincent, A.M.; Stevens, M.J.; Backus, C.; McLean, L.L.; Feldman, E.L. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid. Redox Signal. 2005, 7, 1494–1506. [Google Scholar] [CrossRef]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- Longair, M.H.; Baker, D.A.; Armstrong, J.D. Simple Neurite Tracer: Open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 2011, 27, 2453–2454. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.N.; Negi, G.; Kharatmal, S.B.; Mule, N.K.; Sharma, D.; Sharma, S.S. Short-term extracellular glucose exposure alters neurite outgrowth and intracellular reactive oxygen species without altering viability in neuronal cells. Indian J. Exp. Biol. 2017, 55, 648–654. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology, Mosby: St. Louis, MO, USA, 1980.
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.M.; Brownlee, M.; Russell, J.W. Oxidative stress and programmed cell death in diabetic neuropathy. Ann. N. Y. Acad. Sci. 2002, 959, 368–383. [Google Scholar] [CrossRef]
- Cízková, D.; Lukácová, N.; Marsala, M.; Marsala, J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res. Bull. 2002, 58, 161–171. [Google Scholar] [CrossRef]
- Hodgson, E. A Textbook of Modern Toxicology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Shir, Y.; Seltzer, Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci. Lett. 1990, 115, 62–67. [Google Scholar] [CrossRef]
- Abbas, A.T.; El-Shitany, N.A.; Shaala, L.A.; Ali, S.S.; Azhar, E.I.; Abdel-Dayem, U.A.; Youssef, D.T.A. Red Sea Suberea mollis Sponge Extract Protects against CCl4-Induced Acute Liver Injury in Rats via an Antioxidant Mechanism. Evid.-Based Complement. Altern. Med. eCAM 2014, 2014, 745606. [Google Scholar] [CrossRef] [Green Version]
- Cederberg, J.; Basu, S.; Eriksson, U.J. Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia 2001, 44, 766–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Kawazoe, K.; Kitagawa, I. Araguspongines B, C, D, E, F, G, H, and J, new vasodilative bis-1-oxaquinolizidine alkaloids from an okinawan marine sponge, Xestospongia sp. Chem. Pharm. Bull. 1989, 37, 1676–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Mittal, R. Nrf2: A potential therapeutic target for diabetic neuropathy. Inflammopharmacology 2017, 25, 393–402. [Google Scholar] [CrossRef]
- Endo, M.; Nakagawa, M.; Hamamoto, Y.; Ishihama, M. Pharmacologically active substances from southern Pacific marine invertebrates. Pure Appl. Chem. 1986, 58, 387–394. [Google Scholar] [CrossRef]
- Papanas, N.; Ziegler, D. Efficacy of alpha-lipoic acid in diabetic neuropathy. Expert Opin. Pharmacother. 2014, 15, 2721–2731. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Abreu, L.F.M.; Gomes, P.S.; Gonzaga, R.M.; Veloso, C.A.; Nogueira-Machado, J.A. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid. Med. Cell. Longev. 2014, 2014, 479587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, E.; Ghosh, S. Inhibition of NF-κB by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S. Bioactive Compounds and Their Neuroprotective Effects in Diabetic Complications. Nutrients 2016, 8, 472. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magadmi, R.; Borouk, K.; Youssef, D.T.A.; Shaala, L.A.; Alrafiah, A.R.; Shaik, R.A.; Alharthi, S.E. Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models. Pharmaceuticals 2022, 15, 1309. https://doi.org/10.3390/ph15111309
Magadmi R, Borouk K, Youssef DTA, Shaala LA, Alrafiah AR, Shaik RA, Alharthi SE. Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models. Pharmaceuticals. 2022; 15(11):1309. https://doi.org/10.3390/ph15111309
Chicago/Turabian StyleMagadmi, Rania, Kariman Borouk, Diaa T. A. Youssef, Lamiaa A. Shaala, Aziza R. Alrafiah, Rasheed A. Shaik, and Sameer E. Alharthi. 2022. "Neuroprotective Effect of Red Sea Marine Sponge Xestospongia testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models" Pharmaceuticals 15, no. 11: 1309. https://doi.org/10.3390/ph15111309





