Flavonoids from Piper Species as Promising Antiprotozoal Agents against Giardia intestinalis: Structure-Activity Relationship and Drug-Likeness Studies
Abstract
:1. Introduction
2. Results and Discussion
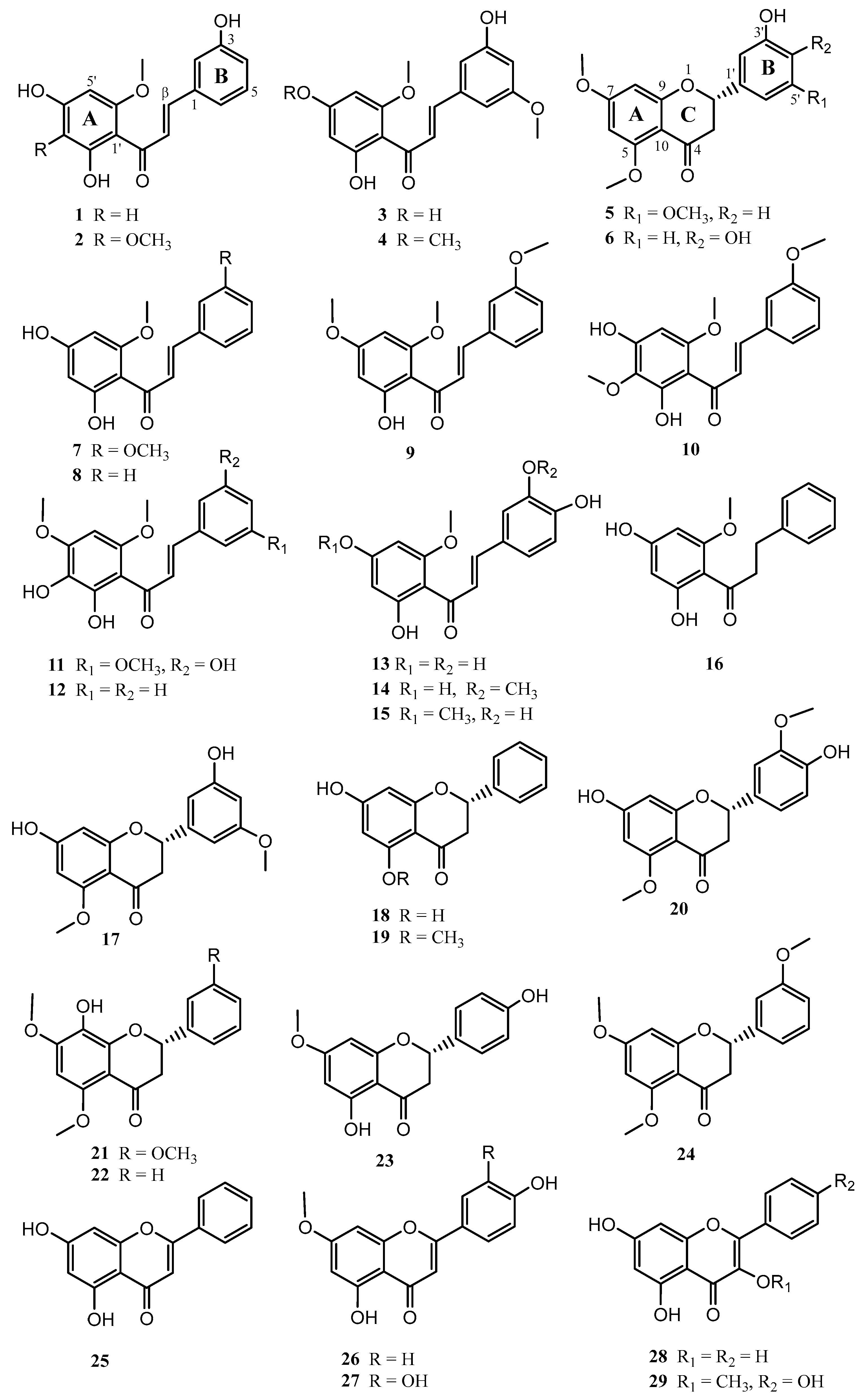
2.1. Chemistry
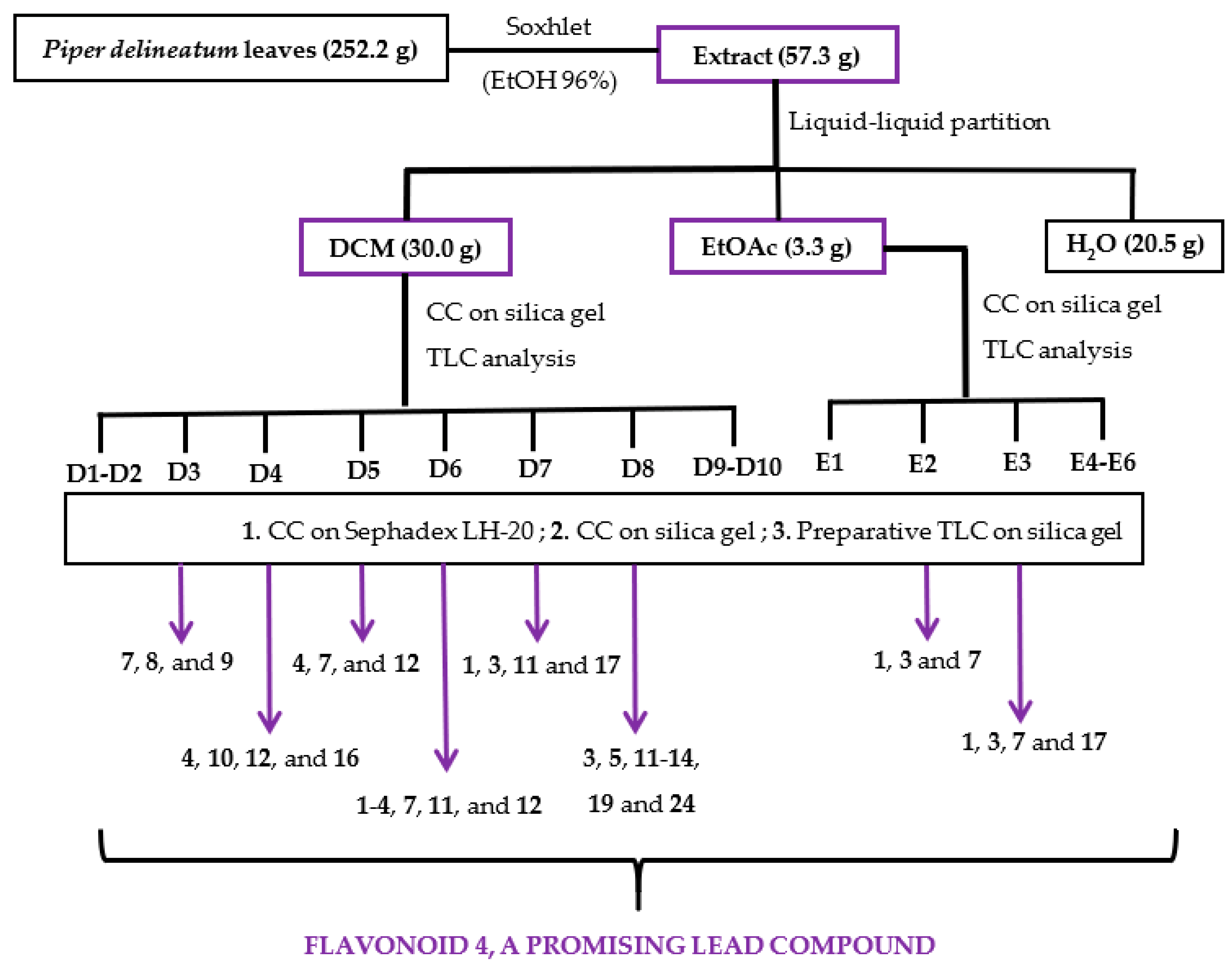
2.2. Bioassay-Guided Fractionation
2.3. Anti-Giardia Activity Assays of Pure Flavonoids
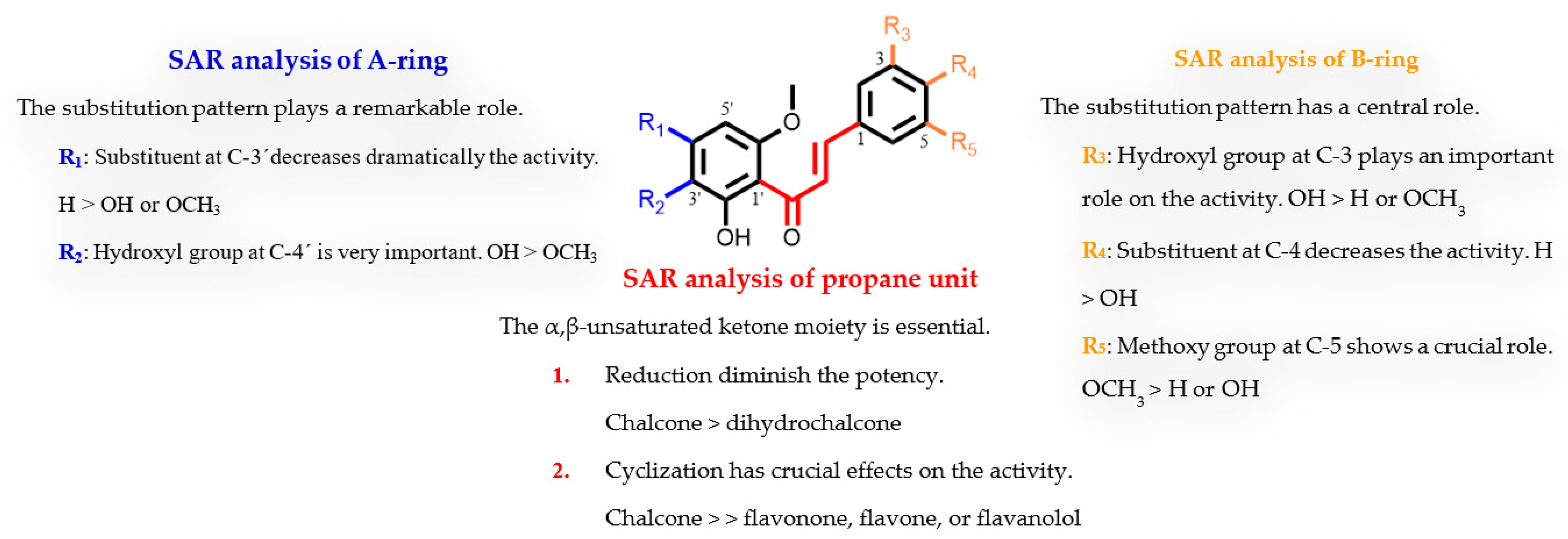
2.4. Structure-Activity Relationship Analysis
2.5. In silico Drug-Likeness Predictions
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction, Bioassay-Guided Fractionation, and Isolation
3.3.1. Compound (1). 2′,4′,3-Trihydroxy-6′-methoxychalcone
3.3.2. Compound (2). 2′,4′,3-Trihydroxy-3′,6′-dimethoxychalcone
3.3.3. Compound (3). 2′,4′,3-Trihydroxy-6′,5-dimethoxychalcone
3.3.4. Compound (4). 2′,3-Dihydroxy-4′,6′,5-trimethoxychalcone
3.3.5. Compound (5). (-)-(2S)-3′-Hydroxy-5,7,5′-trimethoxyflavanone
3.3.6. Compound (6). (-)-(2S)-3′,4′-Dihydroxy-5,7-dimethoxyflavanone
3.4. Biological Assays
3.4.1. Parasites
3.4.2. Cells
3.4.3. In Vitro Assay on Trophozoite Form of Giardia Intestinalis
3.4.4. Cytotoxicity Assay on Murine Macrophages
3.4.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capewell, P.; Krumrie, S.; Katzer, F.; Alexander, C.L.; Weir, W. Molecular Epidemiology of Giardia Infections in the Genomic Era. Trends Parasitol. 2021, 37, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Riches, A.; Christopher, J.S.; Hart, C.J.S.; Trenholme, K.R.; Skinner-Adams, T.S. Anti-Giardia Drug Discovery: Current Status and Gut Feelings. J. Med. Chem. 2020, 63, 13330–13354. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, N.A.; Nashee, H.; Elgendy, A.A.; Rabee, I.; El Amir, A. Encapsulation of Low Metronidazole Dose in Poly (D,L-lactide-co-glycolide) (PLGA) Nanoparticles Improves Giardia intestinalis Treatment. Infect. Chemother. 2020, 52, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, V.; Singh, S.; Dubey, A.; Kim, J.-J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Arbeláez, L.F.G.; Pardo, A.C.; Fantinelli, J.C.; Schinella, G.R.; Mosca, S.M.; Ríos, J.-L. Cardioprotection and natural polyphenols: An update of clinical and experimental studies. Food Funct. 2018, 9, 6129–6145. [Google Scholar] [CrossRef]
- Serafin, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef]
- Alsamhary, K.; Al-Enazi, N.; Alshehri, W.A.; Ameen, F. Gold nanoparticles synthesised by flavonoid tricetin as a potential antibacterial nanomedicine to treat respiratory infections causing opportunistic bacterial pathogens. Microb. Pathog. 2020, 139, 103928. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Diogo, G.M.; Andrade, J.S.; Junior, P.A.S.; Murta, S.M.F.; Dos Santos, V.M.R.; Taylor, J.G. Trypanocidal Activity of Flavanone Derivatives. Molecules 2020, 25, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzada, F.; Bautista, E. Plants used for the treatment of diarrhoea from Mexican flora with amoebicidal and giadicidal activity, and their phytochemical constituents. J. Ethnopharmacol. 2020, 253, 112676. [Google Scholar] [CrossRef] [PubMed]
- Bautista, E.; Calzada, F.; Yépez-Mulia, L.; Bedolla-García, B.Y.; Fragoso-Serrano, M.; Pastor-Palacios, G.; González-Juárez, D.E. Salvia connivens, a Source of Bioactive Flavones with Amoebicidal and Giardicidal Activity. Rev. Bras. Farm. 2020, 30, 729–732. [Google Scholar] [CrossRef]
- Missouri Botanical Garden. Angiosperm Phylogeny Website; University of Missouri: St. Louis, MO, USA, 2017; [Updated 24 February 2020]; Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 29 April 2020).
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [Green Version]
- Martín-Rodríguez, A.J.; Ticona, J.C.; Jiménez, I.A.; Flores, N.; Fernández, J.J.; Bazzocchi, I.L. Flavonoids from Piper delineatum modulate quorum-sensing-regulated phenotypes in Vibrio harveyi. Phytochemistry 2015, 117, 98–106. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Pino-Benitez, N.; González-Coloma, A. Volatile composition and biocidal (antifeedant and phytotoxic) activity of the essential oils of four Piperaceae species from Choco-Colombia. Ind. Crop. Prod. 2019, 138, 111463. [Google Scholar] [CrossRef]
- de Oliveira, J.S.; Ramos, N.P.; Júnior, J.L.; Xavier, L.P.; Andrade, E.H.; Mello, A.H.; Setzer, W.N.; Da Silva, J.K.R. Secondary Metabolism and Plant Growth of Piper divaricatum (Piperaceae) Inoculated with Arbuscular Mycorrhizal Fungi and Phosphorus Supplementation. Agronomy 2022, 12, 596. [Google Scholar] [CrossRef]
- Leitão, M.M.; Radai, J.A.; Ferrari, I.C.; Negrão, F.J.; Silva-Filho, S.E.; Oliveira, R.J.; Mota, J.D.S.; Kassuya, C.A. Effects of an ethanolic extract and fractions from Piper glabratum (Piperaceae) leaves on pain and inflammation. Regul. Toxicol. Pharmacol. 2020, 117, 104762. [Google Scholar] [CrossRef]
- Branquinho, L.S.; Santos, J.A.; Cardoso, C.A.L.; Mota, J.D.S.; Junior, U.L.; Kassuya, C.A.L.; Arena, A.C. Anti-inflammatory and toxicological evaluation of essential oil from Piper glabratum leaves. J. Ethnopharmacol. 2017, 198, 372–378. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Novel chalcone-based phenothiazine derivative photoinitiators for visible light induced photopolymerization with photobleaching and good biocompatibility. Prog. Org. Coat. 2022, 167, 106859. [Google Scholar] [CrossRef]
- Aponte, J.C.; Castillo, D.; Estevez, Y.; Gonzalez, G.; Arevalo, J.; Hammond, G.B.; Sauvain, M. In vitro and in vivo anti-Leishmania activity of polysubstituted synthetic chalcones. Bioorganic Med. Chem. Lett. 2010, 20, 100–103. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, X.J.; de Almeida-Neto, F.W.; Rocha, J.E.; Freitas, T.S.; Freitas, P.R.; de Araújo, A.C.; da Silva, P.T.; Nogueira, C.E.; Bandeira, P.N.; Marinho, M.M.; et al. Spectroscopic analysis by NMR, FT-Raman, ATR-FTIR, and UV-Vis, evaluation of antimicrobial activity, and in silico studies of chalcones derived from 2-hydroxyacetophenone. J. Mol. Struct. 2021, 1241, 130647. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, X.-F.; Yan, B.-Z.; Shi, H.-M.; Du, L.-B.; Zhang, Y.-Z.; Wang, L.-F.; Tang, Y.-L.; Liu, Y. A novel flavonoid from Lespedeza virgata (Thunb.) DC.: Structural elucidation and antioxidative activity. Bioorganic Med. Chem. Lett. 2007, 17, 6311–6315. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.M.; Markham, K.R. Flavonoids, Chemistry, Biochemistry, and Applications; Taylor and Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kuroyanagi, M.; Noro, T.; Fukushima, S.; Aiyama, R.; Ikuta, A.; Itokawa, H.; Morita, M. Studies on the constituents of the seeds of Alpinia katsumadai Hayata. Chem. Pharm. Bull. 1983, 31, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Boumendjel, A.; Boccard, J.; Carrupt, P.-A.; Nicolle, E.; Blanc, M.; Geze, A.; Choisnard, L.; Wouessidjewe, D.; Matera, E.-L.; Dumontet, C. Antimitotic and Antiproliferative Activities of Chalcones: Forward Structure–Activity Relationship. J. Med. Chem. 2008, 51, 2307–2310. [Google Scholar] [CrossRef]
- Chantrapromma, K.; Rat-A-Pa, Y.; Karalai, C.; Lojanapiwatana, V.; Seechammanturakit, V. A chalcone and a dihydrochalcone from Uvaria dulcis. Phytochemistry 2000, 53, 511–513. [Google Scholar] [CrossRef]
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef]
- Jun, N.; Hong, G.; Jun, K. Synthesis and evaluation of 2′,4′,6′-trihydroxychalcones as a new class of tyrosinase inhibitors. Bioorganic Med. Chem. 2007, 15, 2396–2402. [Google Scholar] [CrossRef]
- Hufford, C.D.; Oguntimein, B.O. Dihydrochalcones from Uvaria angolensis. Phytochemistry 1980, 19, 2036–2038. [Google Scholar] [CrossRef]
- Leu, Y.-L.; Hwang, T.-L.; Chung, Y.-M.; Hong, P.-Y. The Inhibition of Superoxide Anion Generation in Human Neutrophils by Viscum coloratum. Chem. Pharm. Bull. 2006, 54, 1063–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantrapromma, K.; Seechamnanturakit, V.; Pakawatchai, C.; Chinnakali, K.; Fun, H.-K.; Chantrapromma, S. 2,3-Dihydro-8-hydroxy-5,7-dimethoxy-2-phenyl-4H-1-benzopyran-4-one and 3,3,6-Tribromo-2,3-dihydro-8-hydroxy-5,7-dimethoxy-2-phenyl-4H-1-benzopyran-4-one. Acta Crystallogr. 1998, C54, IUC9800001. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Ho, D.K.; Cassady, J.M.; Cook, V.M.; Baird, W.M. Isolation of Potential Cancer Chemopreventive Agents from Eriodictyon californicum. J. Nat. Prod. 1992, 55, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sekizaki, H. Synthesis of 2-Benzylidene-3(2H)-benzofuran-3-ones (Aurones) by Oxidation of 2′-Hydroxychalcones with Mercury(II) Acetate. Bull. Chem. Soc. Jpn. 1988, 61, 1407–1409. [Google Scholar] [CrossRef] [Green Version]
- Papotti, G.; Bertelli, D.; Plessi, M.; Rossi, M.C. Use of HR-NMR to classify propolis obtained using different harvesting methods. Int. J. Food Sci. Technol. 2010, 45, 1610–1618. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.-H.; Yang, H.; Lee, Y.; Lee, E.; Lim, Y. Complete assignments of NMR data of 13 hydroxymethoxyflavones. Magn. Reson. Chem. 2007, 45, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Ali, Z.; Zahid, M.; Alam, N.; Saba, N.; Khan, T.; Qaisar, M.; Nisar, M. Phytochemical study of Salvia moorcroftiana. Fitoterapia 2000, 71, 84–85. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbono-13 NMR of Flavonoids, Studies in Organic Chemistry Series, No. 39, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 160–161. [Google Scholar] [CrossRef]
- Calzada, F.; Velázquez, C.; Cedillo-Rivera, R.; Esquivel, B. Antiprotozoal activity of the constituents of Teloxys graveolens. Phytother. Res. 2003, 17, 731–732. [Google Scholar] [CrossRef]
- Calzada, F.; Meckes, M.; Cedillo-Rivera, R. Antiamoebic and Antigiardial Activity of Plant Flavonoids. Planta Med. 1999, 65, 78–80. [Google Scholar] [CrossRef]
- Calzada, F.; Cervantes-Martínez, J.A.; Yépez-Mulia, L. In vitro antiprotozoal activity from the roots of Geranium mexicanum and its constituents on Entamoeba histolytica and Giardia lamblia. J. Ethnopharmacol. 2005, 98, 191–193. [Google Scholar] [CrossRef]
- Barbosa, E.; Calzada, F.; Campos, R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J. Ethnopharmacol. 2007, 109, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Leszczynski, J. Open access in silico tools to predict the ADMET profiling of drug candidates. Expert Opin. Drug Discov. 2020, 15, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release. 2018-2: QikProp; Schrödinger, LLC.: New York, NY, USA, 2018. [Google Scholar]
- Zhou, W.; Wang, Y.; Lu, A.; Zhang, G. Systems Pharmacology in Small Molecular Drug Discovery. Int. J. Mol. Sci. 2016, 17, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bénéré, E.; da Luz, R.A.I.; Vermeersch, M.; Cos, P.; Maes, L. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J. Microbiol. Methods 2007, 71, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Galiana-Roselló, C.; Bilbao-Ramos, P.; Dea-Ayuela, M.A.; Rolón, M.; Vega, C.; Bolás-Fernández, F.; García-España, E.; Alfonso, J.; Coronel, C.; González-Rosende, M.E. In Vitro and in Vivo Antileishmanial and Trypanocidal Studies of New N-Benzene- and N-Naphthalenesulfonamide Derivatives. J. Med. Chem. 2013, 56, 8984–8998. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 137.8 s | 137.7 s | 138.4 s | 138.3 s | ||||
| 2 | 7.19 s | 115.3 d | 7.19 s | 115.3 d | 6.81 s | 108.3 d | 6.81 s | 108.4 d |
| 3 | 158.7 s | 158.8 s | 159.8 s | 159.8 s | ||||
| 4 | 6.91 d (7.6) | 118.2 d | 6.92 d (8.0) | 118.4 d | 6.49 s | 104.3 d | 6.50 s | 104.4 d |
| 5 | 7.28 t (7.6) | 131.0 d | 7.29 t (8.0) | 130.9 d | 162.2 s | 162.3 s | ||
| 6 | 7.20 d (7.6) | 121.0 d | 7.20 d (8.0) | 120.9 d | 6.77 s | 106.2 d | 6.78 s | 106.3 d |
| C=O | 193.1 s | 193.7 s | 193.1 s | 193.4 s | ||||
| α | 7.96 d (15.6) | 128.4 d | 7.96 d (15.6) | 128.2 d | 7.93 d (15.5) | 128.6 d | 7.93 d (15.7) | 128.6 d |
| β | 7.68 d (15.6) | 142.8 d | 7.69 d (15.6) | 143.1 d | 7.62 d (15.5) | 142.8 d | 7.64 d (15.7) | 143.2 CH111 |
| 1′ | 106.3 s | 106.5 s | 106.3 s | 106.9 s | ||||
| 2′ | 169.0 s | 160.8 s | 169.0 s | 169.1 s | ||||
| 3′ | 6.00 d (1.6) | 97.0 d | 130.0 s | 6.00 s | 97.0 d | 6.10 | 94.7 d | |
| 4′ | 166.0 s | 158.1 s | 166.1 s | 167.6 s | ||||
| 5′ | 6.08 d (1.6) | 92.3 d | 6.12 s | 91.7 d | 6.08 s | 92.3 d | 6.13 | 91.9 d |
| 6′ | 164.4 s | 159.7 s | 164.3 s | 163.8 s | ||||
| OCH3-5 | 3.82 s | 55.6 q | 3.82 s | 55.6 q | ||||
| OCH3-3′ | 3.76 s | 60.6 q | ||||||
| OCH3-4′ | 3.88 s | 56.1 q | ||||||
| OCH3-6′ | 3.99 s | 56.5 t | 3.97 s | 56.5 q | 3.98 s | 56.4 q | 4.01 s | 56.6 q |
| OH-2′ | 14.17 s | 14.39 s | 14.16 s | 14.16 s | ||||
| OH-4′ | 9.04 s c | 9.20 s | 9.10 s c | |||||
| OH-3 | 9.04 s c | 8.76 s | 9.10 s c | 8.60 s |
| 5 c | 5 c | 6 d | 6 e | 6 d | |
|---|---|---|---|---|---|
| δH | δC | δH | δH | δC | |
| 2 | 5.30 dd (3.0, 13.0) | 79.1 CH | 5.32 dd (2.9, 12.6) | 5.00 dd (2.9, 12.8) | 79.9 CH |
| 3 | 2.78 dd (3.0, 16.6) 2.96 dd (13.0, 16.6) | 45.7 CH2 | 2.59 dd (2.9, 16.3) 2.94 dd (12.6, 16.3) | 2.59 dd (2.9, 16.3) 2.94 dd (12.8, 16.3) | 46.3 CH2 |
| 4 | 189.5 C | 188.4 C | |||
| 5 | 162.5 C | 163.2 C | |||
| 6 | 6.08 d (2.3) | 93.4 CH | 6.17 d (2.3) | 6.07 d (2.2) | 93.5 CH |
| 7 | 166.3 C | 166.6 C | |||
| 8 | 6.15 d (2.3) | 93.8 CH | 6.14 d (2.3) | 5.98 d (2.2) | 94.4 CH |
| 9 | 165.1 C | 165.8 C | |||
| 10 | 106.0 C | 106.7 C | |||
| 1′ | 141.4 C | 132.0 C | |||
| 2′ | 6.53 s | 105.7 CH | 7.01 s | 7.16 | 114.6 CH |
| 3′ | 157.6 C | 146.0 C | |||
| 4′ | 6.40 t (2.2) | 101.7 CH | 146.3 C | ||
| 5′ | 161.4 C | 6.85 s f | 7.01 d (8.1) | 116.0 CH | |
| 6′ | 6.56 s | 104.3 CH | 6.85 s f | 6.72 dd (1.9, 8.1) | 119.1 CH |
| OMe-5 | 3.88 s | 56.3 CH3 | 3.82 s | 3.24 s | 56.2 CH3 |
| OMe-7 | 3.82 s | 55.8 CH3 | 3.85 s | 3.40 s | 56.0 CH3 |
| OMe-5′ | 3.79 s | 55.6 CH3 | |||
| OH-3′ | 5.66 s |
| Extract or Fractions | G. intestinalis IC50 a,b (µg/mL) | Macrophages CC50 c (µg/mL) | SI d |
|---|---|---|---|
| EtOH | 1.9 ± 1.5 | 7.1 ± 0.6 | 3.7 |
| CH2Cl2 | 16.0 ± 1.2 | 6.6 ± 0.1 | 0.4 |
| D3 | 5.6 ± 1.3 | 15.5 ± 1.0 | 2.8 |
| D4 | 3.3 ± 0.7 | 9.2 ± 1.2 | 2.8 |
| D5 | 4.7 ± 0.1 | 10.9 ± 0.6 | 2.3 |
| D6 | 9.3 ± 1.5 | 17.5 ± 1.2 | 1.9 |
| D7 | 5.0 ± 1.1 | 4.5 ± 0.9 | 0.9 |
| D8 | 7.5 ± 0.5 | 10.6 ± 1.4 | 1.4 |
| EtOAc | 19.7 ± 0.7 | 4.9 ± 0.4 | 0.3 |
| E2 | 3.7 ± 1.3 | 3.2 ± 0.1 | 0.9 |
| E3 | 4.5 ± 0.9 | 2.7 ± 0.1 | 0.6 |
| Metronidazole e | 0.4 ± 0.1 | 233 | 582 |
| Compound | G. intestinalis IC50a,b (µM) | Macrophages CC50 c (µM) | SI d |
|---|---|---|---|
| 1 | 7.0 ± 0.1 | 12.9 ± 0.2 | 1.8 |
| 2 | 47.7 ± 1.2 | 184.4 ± 2.6 | 3.9 |
| 3 | 6.0 ± 0.3 | 12.0 ± 0.1 | 2.0 |
| 4 | 0.061 ± 0.001 | 14.2 ± 0.2 | 233 |
| 7 | 4.7 ± 0.1 | 15.7 ± 0.2 | 3.3 |
| 8 | 9.6 ± 0.2 | 37.8 ± 1.1 | 3.9 |
| 9 | 10.8 ± 0.1 | 22.6 ± 0.5 | 2.1 |
| 10 | 32.7 ± 1.3 | 44.2 ± 0.7 | 1.4 |
| 11 | 22.5 ± 0.8 | 48.5 ± 0.8 | 2.2 |
| 12 | 7.3 ± 0.6 | 25.3 ± 0.4 | 3.5 |
| 14 | 10.4 ± 0.2 | 16.5 ± 0.03 | 1.6 |
| 16 | 70.5 ± 0.3 | >367.2 | >5.2 |
| 23 | 52.4 ± 2.1 | 184.2 ± 2.3 | 3.5 |
| 24 | 49.3 ± 0.9 | 144.5 ± 2.3 | 2.9 |
| Metronidazole e | 2.5 ± 0.1 | >584.3 | >233 |
| Property | 1 | 3 | 4 | 7 | 8 | 9 | 12 | 14 | Range/Values |
|---|---|---|---|---|---|---|---|---|---|
| #stars | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0–5 |
| QPlogBB | −1.744 | −1.850 | −1.325 | −1.246 | −1.150 | −0.717 | −1.129 | −1.731 | −3.0 to 1.2 |
| QPPCaco | 161.650 | 162.190 | 533.788 | 523.193 | 523.287 | 1724.836 | 661.863 | 161.090 | <25 poor, >500 great |
| QPPMDCK | 69.011 | 69.259 | 250.997 | 245.616 | 245.664 | 891.757 | 316.681 | 68.752 | <25 poor, >500 great |
| QPlogKhsa | −0.022 | 0.003 | 0.179 | 0.172 | 0.158 | 0.206 | 0.175 | −0.013 | −1.5 to 1.5 |
| QPlogPo/w | 2.265 | 2.375 | 3.204 | 3.118 | 3.017 | 3.836 | 3.212 | 2.324 | −2.0 to 6.5 |
| QPlogKp | −3.299 | −3.396 | −2.441 | −2.354 | −2.255 | −1.398 | −2.111 | −3.367 | −8.0 to −1.0 |
| QPlogS | −3.555 | −3.815 | −4.242 | −4.115 | −3.869 | −4.535 | −4.072 | −3.401 | −6.5 to 0.5 |
| #metab | 4 | 5 | 5 | 4 | 3 | 4 | 4 | 5 | 1 to 8 |
| %HOA | 79.738 | 80.409 | 94.518 | 93.864 | 93.271 | 100 | 96.241 | 80.055 | >80% high, <25% poor |
| PSA | 95.0 | 103.3 | 89.0 | 80.8 | 72.5 | 66.5 | 78.0 | 102.4 | 7.0 to 200.0 |
| SASA | 555.1 | 592.0 | 616.0 | 580.9 | 543.4 | 604.5 | 578.5 | 567.6 | 300.0 to 1000.0 |
| Mol MW | 286.28 | 316.31 | 330.34 | 300.31 | 270.28 | 314.34 | 300.31 | 316.31 | 130.0 to 725.0 |
| #rotor | 8 | 9 | 9 | 8 | 7 | 8 | 8 | 9 | 0 to 15 |
| donorHB | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 2 | 0.0 to 6.0 |
| accptHB | 4 | 4.75 | 4.75 | 4 | 3.25 | 4 | 4 | 4.75 | 2.0 to 20.0 |
| volume | 929.3 | 1004.1 | 1055.4 | 981.8 | 906.5 | 1032.8 | 982.3 | 991.4 | 500.0 to 2000.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ticona, J.C.; Bilbao-Ramos, P.; Amesty, Á.; Flores, N.; Dea-Ayuela, M.A.; Bazzocchi, I.L.; Jiménez, I.A. Flavonoids from Piper Species as Promising Antiprotozoal Agents against Giardia intestinalis: Structure-Activity Relationship and Drug-Likeness Studies. Pharmaceuticals 2022, 15, 1386. https://doi.org/10.3390/ph15111386
Ticona JC, Bilbao-Ramos P, Amesty Á, Flores N, Dea-Ayuela MA, Bazzocchi IL, Jiménez IA. Flavonoids from Piper Species as Promising Antiprotozoal Agents against Giardia intestinalis: Structure-Activity Relationship and Drug-Likeness Studies. Pharmaceuticals. 2022; 15(11):1386. https://doi.org/10.3390/ph15111386
Chicago/Turabian StyleTicona, Juan C., Pablo Bilbao-Ramos, Ángel Amesty, Ninoska Flores, M. Auxiliadora Dea-Ayuela, Isabel L. Bazzocchi, and Ignacio A. Jiménez. 2022. "Flavonoids from Piper Species as Promising Antiprotozoal Agents against Giardia intestinalis: Structure-Activity Relationship and Drug-Likeness Studies" Pharmaceuticals 15, no. 11: 1386. https://doi.org/10.3390/ph15111386
APA StyleTicona, J. C., Bilbao-Ramos, P., Amesty, Á., Flores, N., Dea-Ayuela, M. A., Bazzocchi, I. L., & Jiménez, I. A. (2022). Flavonoids from Piper Species as Promising Antiprotozoal Agents against Giardia intestinalis: Structure-Activity Relationship and Drug-Likeness Studies. Pharmaceuticals, 15(11), 1386. https://doi.org/10.3390/ph15111386







