In Vitro and In Vivo Evaluation of Hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate
Abstract
:1. Introduction
2. Results and Discussion
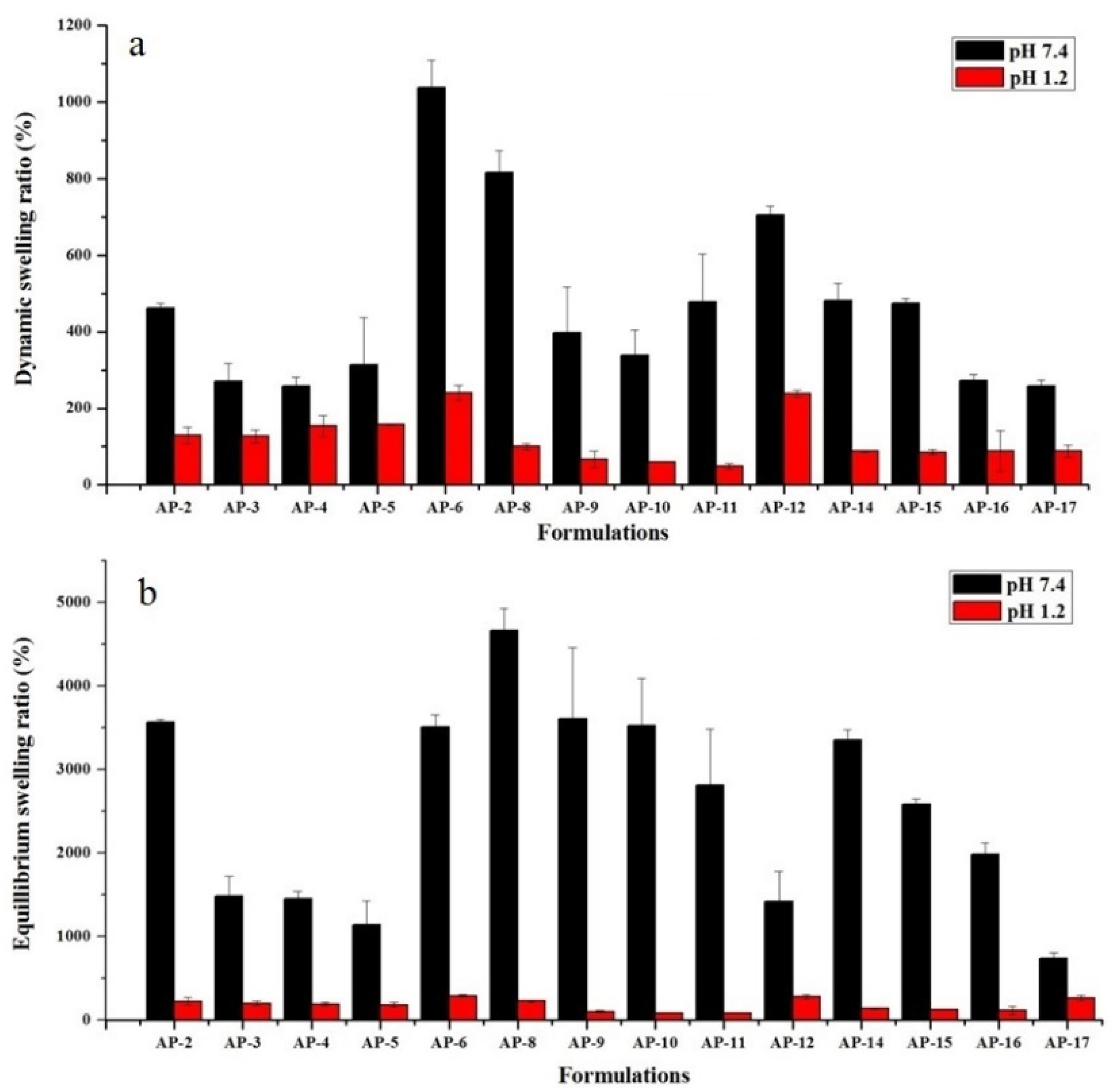
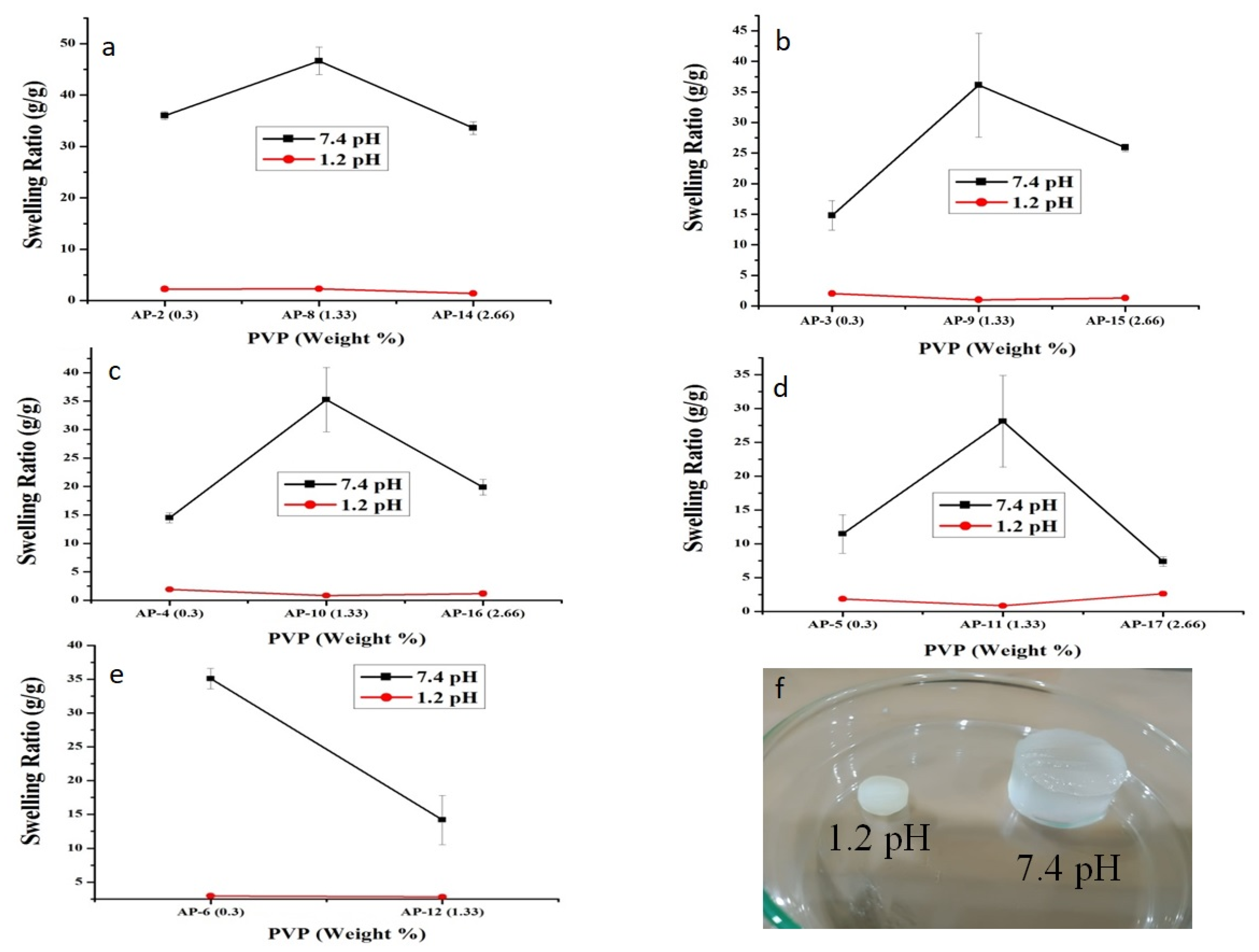
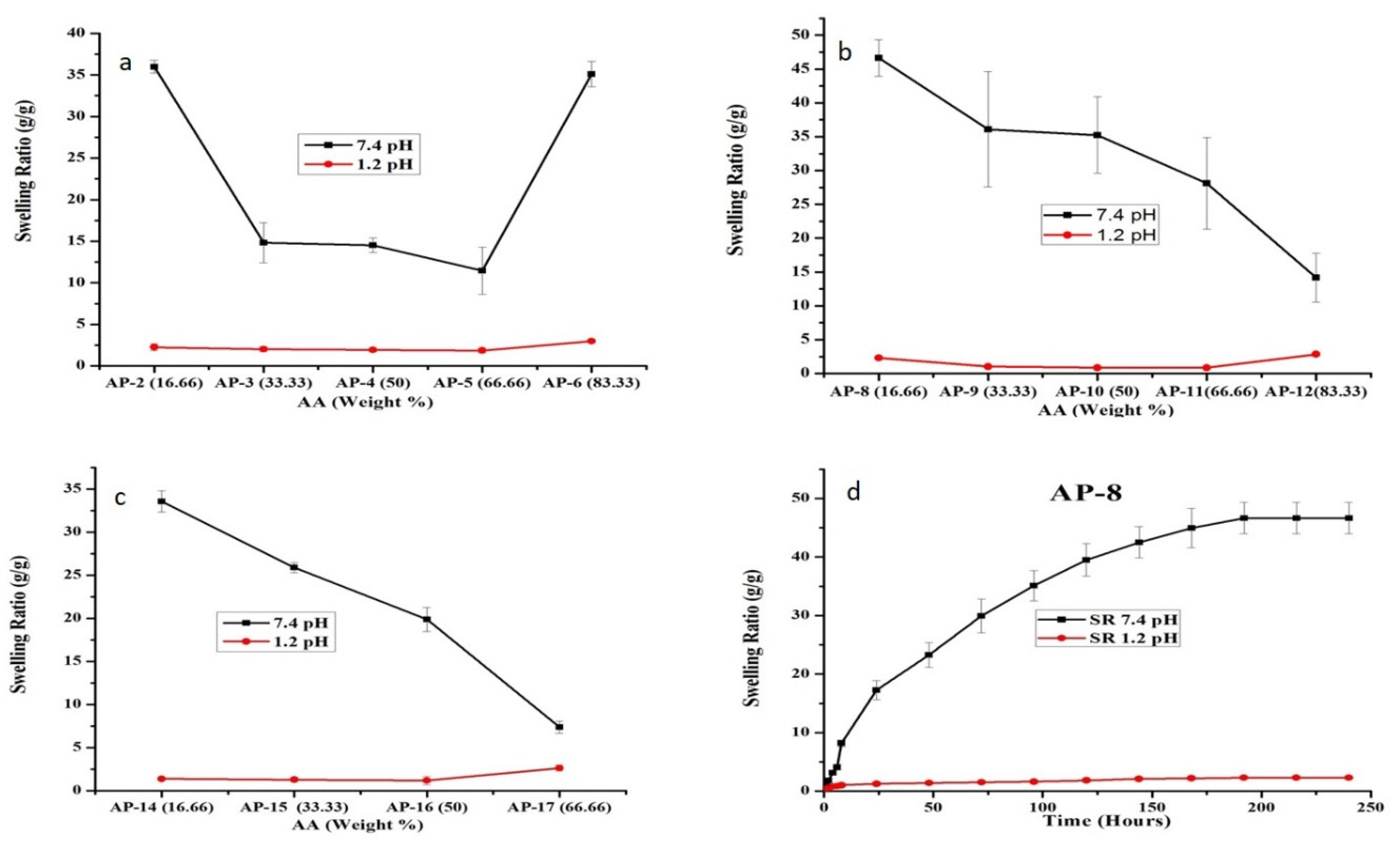
2.1. Swelling Studies
2.1.1. The pH of Medium
2.1.2. Effect of AA
2.1.3. Effect of PVP
2.2. Sol-Gel Fraction
2.3. Solid-State Characterization
2.3.1. FTIR
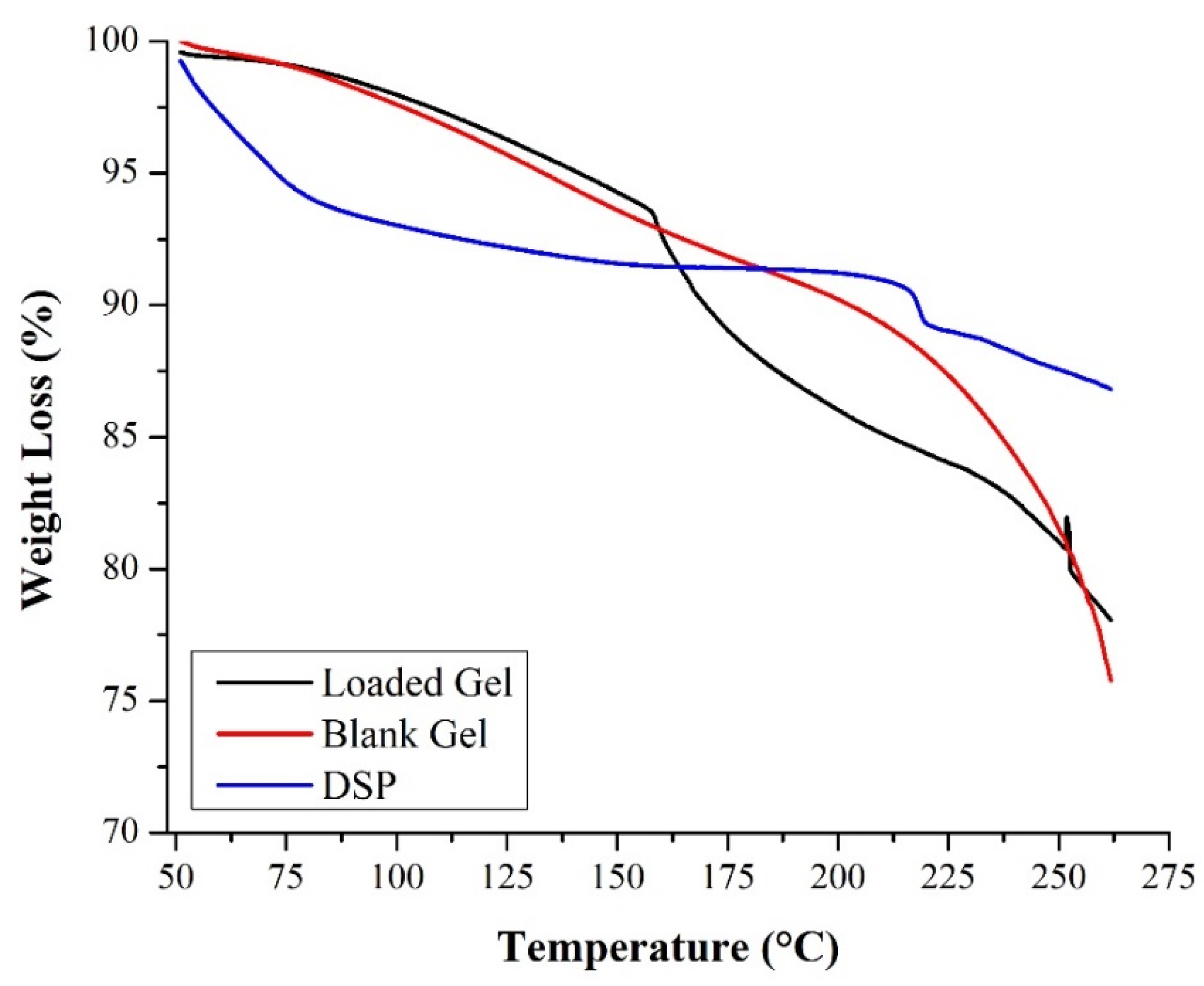
2.3.2. TGA
2.3.3. XRD
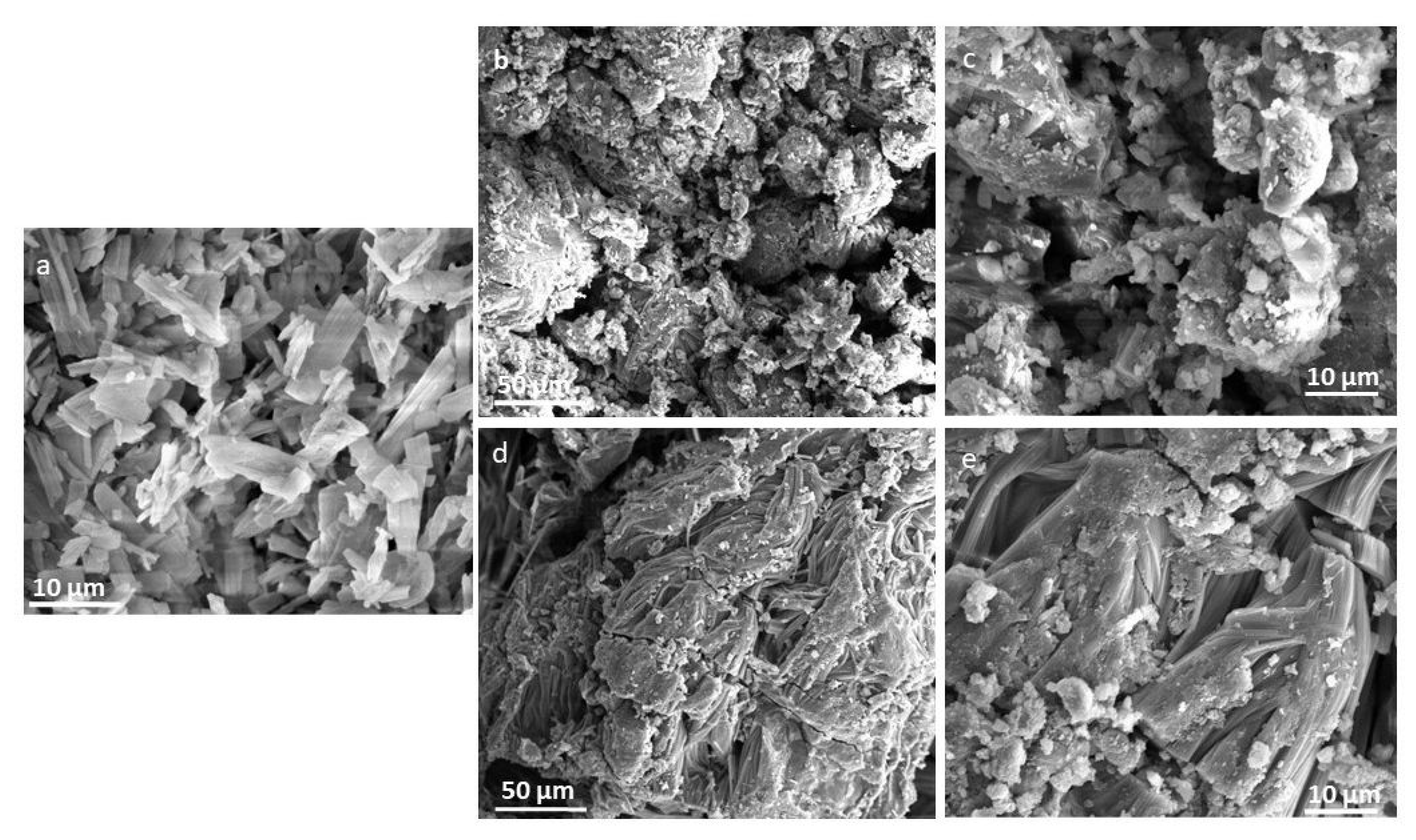
2.3.4. SEM
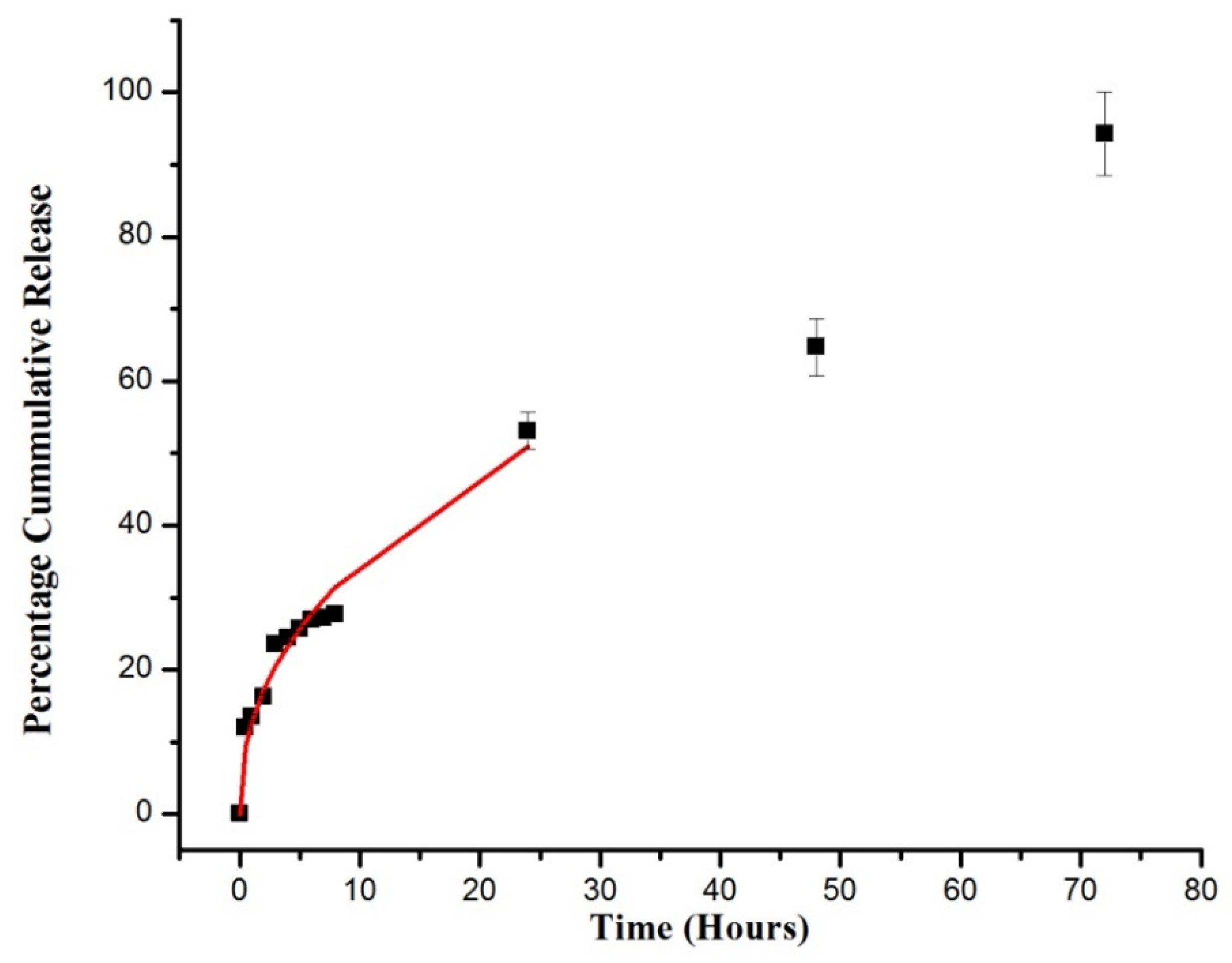
2.4. DSP Loading and In Vitro Dissolution Study
2.5. Drug Release Kinetics
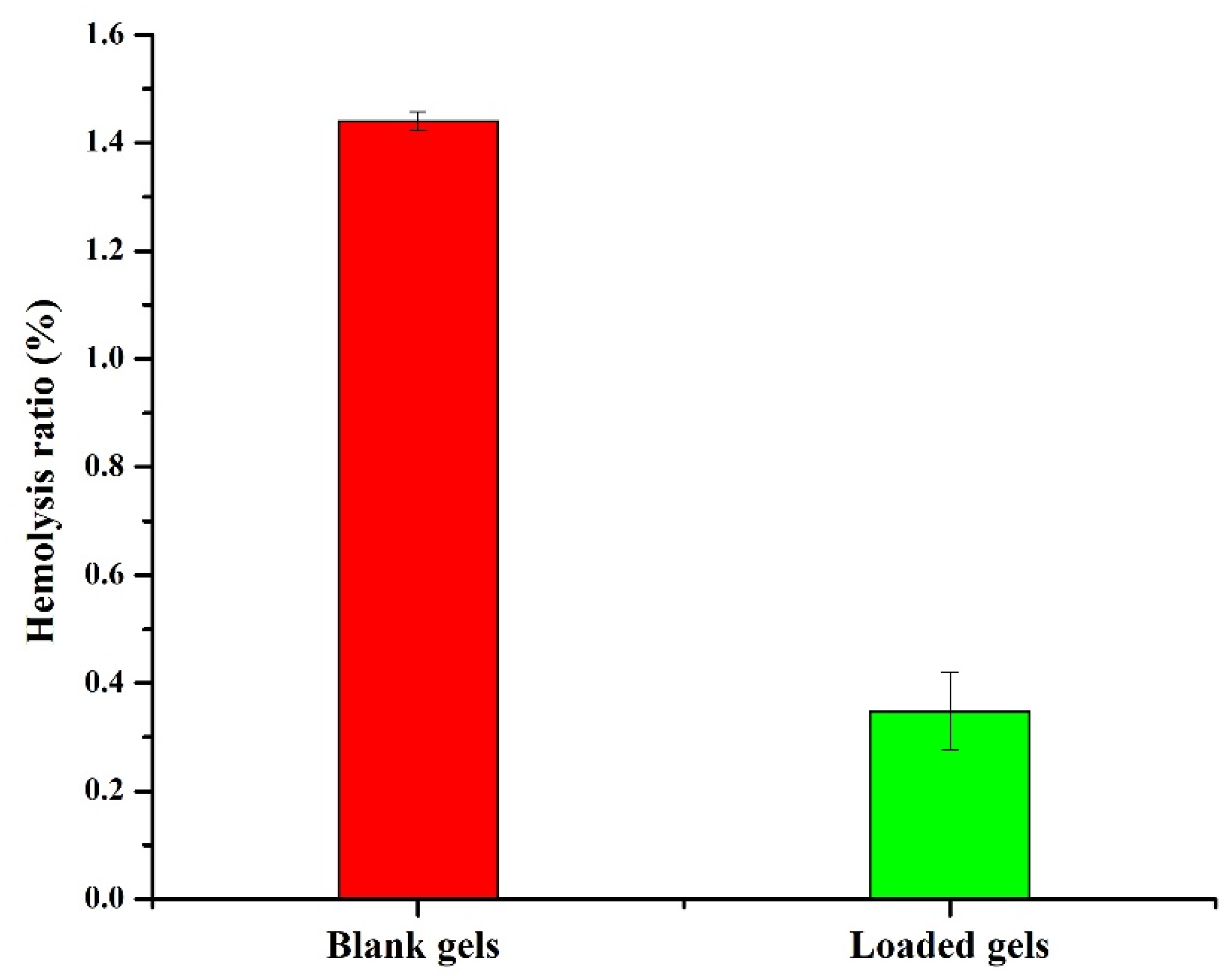
2.6. Hemocompatibility Study
2.7. Toxicity Testing
2.7.1. Monitoring the General Conditions of Rabbits
2.7.2. Hematological and Biochemical Analysis
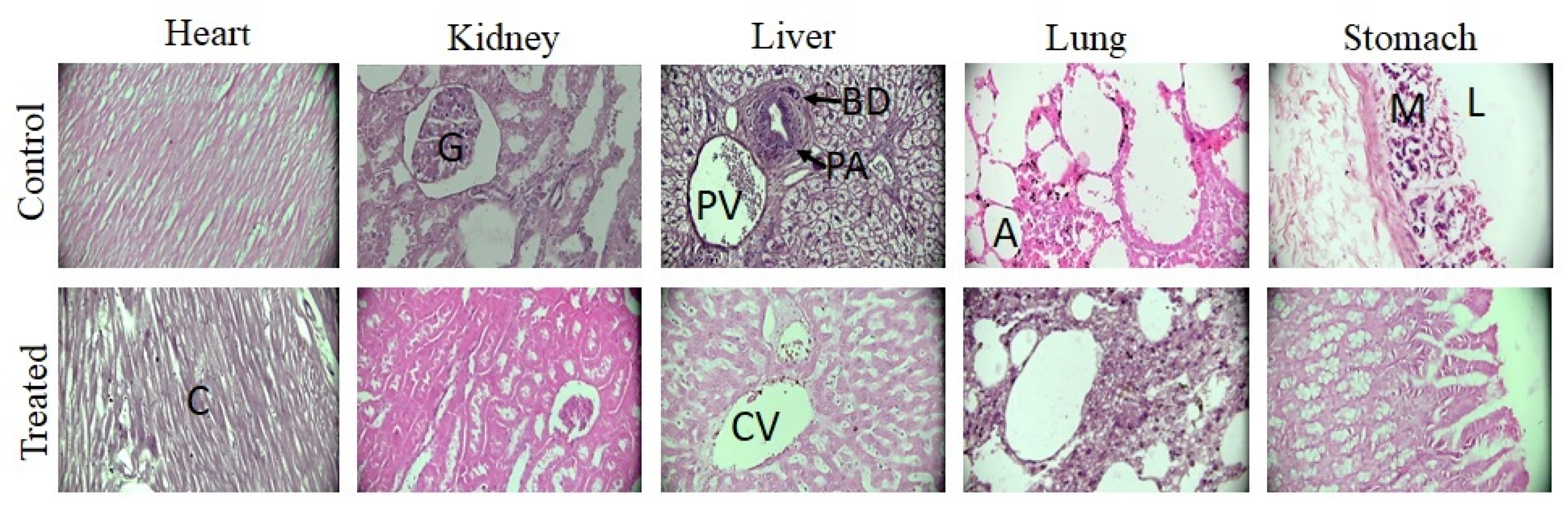
2.7.3. Histopathological Evaluation
3. Materials and Methods
3.1. Chemicals
3.2. Fabrication of HP-β-CD-g-Poly(AA)/PVP
3.3. Swelling Studies
3.4. Analysis of Sol-Gel Fraction
3.5. Drug Loading
3.6. Solid-State Characterization
3.7. In Vitro DSP Dissolution
3.8. Hemocompatibility Study
3.9. Toxicity Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jagtapa, S.D.; Mohite, A.M.; Tayadeb, S.; Supriya, S. Synthesis of Hydrogels and Their Applications in Control Fertilizer Release. J. Emerg. Technol. Innov. Res. 2019, 6, 457–468. [Google Scholar]
- Suhail, M.; Shao, Y.-F.; Vu, Q.L.; Wu, P.-C. Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation. Gels 2022, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Hussain, T.; Ansari, M.; Ranjha, N.M.; Khan, I.U.; Shahzad, Y. Chemically cross-linked poly(acrylic-co-vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Sci. World J. 2013, 2013, 340737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Khalid, S.H.; Qadir, M.I.; Massud, A.; Ali, M.; Khan, I.U.; Saleem, M.; Iqbal, M.S.; Asghar, S.; Gul, H. Water uptake and drug release behaviour of methyl methacrylateco-itaconic acid [P(MMA/IA)] hydrogels cross-linked with methylene bis-acrylamide. J. Drug Deliv. Sci. Technol. 2011, 21, 249–255. [Google Scholar] [CrossRef]
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar]
- Vaghani, S.S.; Patel, M.M. pH-sensitive hydrogels based on semi-interpenetrating network (semi-IPN) of chitosan and polyvinyl pyrrolidone for clarithromycin release. Drug Dev. Ind. Pharm. 2011, 37, 1160–1169. [Google Scholar] [CrossRef]
- Toledo, P.V.; Limeira, D.P.; Siqueira, N.C.; Petri, D.F. Carboxymethyl cellulose/poly(acrylic acid) interpenetrating polymer network hydrogels as multifunctional adsorbents. Cellulose 2019, 26, 597–615. [Google Scholar] [CrossRef]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2019, 78, 59–80. [Google Scholar]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [Green Version]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Del. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-Specific Drug Delivery of Clarithromycin Using a Semi Interpenetrating Polymeric Network Hydrogel Made of Montmorillonite and Chitosan: Synthesis, Characterization and In Vitro Drug Release Study. Adv. Pharm. Bull. 2019, 9, 159. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Wang, A. pH-responsive carboxymethylcellulose-g-poly(sodium acrylate)/polyvinylpyrrolidone semi-IPN hydrogels with enhanced responsive and swelling properties. Macromol. Res. 2011, 19, 57–65. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly(sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, X.; Wang, Y.; Xu, H.; Chen, X. Performance and characterization of irradiated poly(vinyl alcohol)/polyvinylpyrrolidone composite hydrogels used as cartilages replacement. J. Appl. Polym. Sci. 2009, 113, 736–741. [Google Scholar] [CrossRef]
- Nwosu, C.J.; Hurst, G.A.; Novakovic, K. Genipin cross-linked chitosan-polyvinylpyrrolidone hydrogels: Influence of composition and postsynthesis treatment on pH responsive behaviour. Adv. Mater. Sci. Eng. 2015, 2015, 621289. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Patel, K. Formulation and development of hydrogel for poly acrylamide-co-acrylic acid. J. Pharm. Sci. Biosci. Res. Patel. 2014, 4, 114–120. [Google Scholar]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S. Acrylic acid and methacrylic acid based hydrogels—A review. Chem. Sin. Udaipur 2015, 6, 61–72. [Google Scholar]
- Bukhari, S.M.H.; Khan, S.; Rehanullah, M.; Ranjha, N.M. Synthesis and characterization of chemically cross-linked acrylic acid/gelatin hydrogels: Effect of pH and composition on swelling and drug release. Int. J. Polym. Sci. 2015, 2015, 187961. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, N. Highly porous pH-responsive carboxymethyl chitosan-grafted-poly(acrylic acid) based smart hydrogels for 5-fluorouracil controlled delivery and colon targeting. Int. J. Polym. Sci. 2019, 2019, 6579239. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, W.; Wang, J.; Duan, Y. The swelling behavior and network parameters of guar gum/poly(acrylic acid) semi-interpenetrating polymer network hydrogels. Carbohydr. Polym. 2006, 66, 473–479. [Google Scholar] [CrossRef]
- Shah, S.; Ranjha, N.M.; Javaid, Z. Development and evaluation of pH-dependent interpenetrating network of acrylic acid/polyvinyl alcohol. Iran. Polym. J. 2013, 22, 811–820. [Google Scholar] [CrossRef]
- Fujiyoshi, T.; Carrez, O.; Imizcoz, M.; Zornoza, A.; Isasi, J.R. Interpenetrated polymer networks of Poly(β-cyclodextrin) and Polyvinylpyrrolidone with synergistic and selective sorption capacities. Carbohydr. Polym. 2019, 219, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Cesteros, L.C.; Ramírez, C.A.; Peciña, A.; Katime, I. Synthesis and Properties of Hydrophilic Networks Based on Poly(ethylene glycol) and β-Cyclodextrin. Macromol. Chem. Phys. 2007, 208, 1764–1772. [Google Scholar] [CrossRef]
- Rasheed, A. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Bal, T.; Sengupta, S.; Murthy, P.N. Formulation and evaluation of carvedilol microcapsules using Eudragit NE30D and sodium alginate. Braz. J. Pharm. Sci. 2013, 49, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sun, C.; Shi, F.; Firempong, C.K.; Yu, J.; Xu, X.; Zhang, W. Preparation, characterization, and pharmacokinetics study of capsaicin via hydroxypropyl-beta-cyclodextrin encapsulation. Pharm. Biol. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [Green Version]
- Mura, P.; Faucci, M.T.; Bettinetti, G.P. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2001, 13, 187–194. [Google Scholar] [CrossRef]
- Chowdary, K.P.R.; Srinivas, S.V. Influence of hydrophilic polymers on celecoxib complexation with hydroxypropyl β-cyclodextrin. AAPS PharmSciTech 2006, 7, E184–E189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada-Basualto, M.; Matsuhiro, B.; Mansilla, A.; Lapier, M.; Maya, J.; Olea-Azar, C. Supramolecular hydrogels of β-cyclodextrin linked to calcium homopoly-l-guluronate for release of coumarins with trypanocidal activity. Carbohydr. Polym. 2019, 204, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Madsen, F.; Larsen, K.L. Cyclodextrin modified hydrogels of PVP/PEG for sustained drug release. Drug Deliv. 2009, 16, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Muhammed, J.; Özer, A.; Ercan, M.; Hincal, A. In-vivo studies on dexamethasone sodium phosphate liposomes. J. Microencaps. 1996, 13, 293–305. [Google Scholar] [CrossRef]
- Zeng, A.; Dong, K.; Wang, M.; Sun, J.; Dong, Y.; Wang, K.; Guo, C.; Yan, Y.; Zhang, L.; Shi, X. Investigation of the colon-targeting, improvement on the side-effects and therapy on the experimental colitis in mouse of a resin microcapsule loading dexamethasone sodium phosphate. Drug Deliv. 2016, 23, 1992–2002. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Crosslinking of poly(vinylpyrrolidone)/acrylic acid with tragacanth gum for hydrogels formation for use in drug delivery applications. Carbohydr. Polym. 2017, 157, 185–195. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Controlled delivery of ibuprofen from poly(vinyl alcohol)-poly(ethylene glycol) interpenetrating polymeric network hydrogels. J. Pharm. Anal. 2019, 9, 108–116. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. pH-Responsive guar gum hydrogels for controlled delivery of dexamethasone to the intestine. Int. J. Biol. Macromol. 2015, 79, 856–863. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R. Development of interpenetrating polymeric network for controlled drug delivery and its evaluation. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 1099–1107. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Madni, A.; Bakar, A.A.; Talib, N.; Ahmad, S.; Ahmad, H. Preparation and characterization of isosorbide mononitrate hydrogels obtained by free-radical polymerization for site-specific delivery. Trop. J. Pharm. Res. 2014, 13, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Sharma, V. Design of psyllium–PVA–acrylic acid based novel hydrogels for use in antibiotic drug delivery. Int. J. Pharm. 2010, 389, 94–106. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Su, Y.; Yue, Q.; Gao, B.; Su, Y. A novel wheat straw cellulose-based semi-IPNs superabsorbent with integration of water-retaining and controlled-release fertilizers. J. Taiwan Inst. Chem. Eng. 2015, 55, 170–179. [Google Scholar] [CrossRef]
- Huang, X.; Xu, S.; Zhong, M.; Wang, J.; Feng, S.; Shi, R. Modification of Na-bentonite by polycations for fabrication of amphoteric semi-IPN nanocomposite hydrogels. Appl. Clay Sci. 2009, 42, 455–459. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Designing galacturonic acid/arabinogalactan crosslinked poly(vinyl pyrrolidone)-co-poly(2-acrylamido-2-methylpropane sulfonic acid) polymers: Synthesis, characterization and drug delivery application. Polymer 2016, 91, 50–61. [Google Scholar] [CrossRef]
- Abad, L.V.; Relleve, L.S.; Aranilla, C.T.; Rosa, A.M.D. Properties of radiation synthesized PVP-kappa carrageenan hydrogel blends. Radiat. Phys. Chem. 2003, 68, 901–908. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Zhang, F.; Chen, S.; Niu, A. Synthesis and characterization of pH-sensitivity semi-IPN hydrogel based on hydrogen bond between poly(N-vinylpyrrolidone) and poly(acrylic acid). Polymer 2006, 47, 1526–1532. [Google Scholar] [CrossRef]
- Larrañaga, A.; Petisco, S.; Villanueva, R.; Iturri, J.J.; Moya, S.; Meaurio, E.; Sarasua, J.-R. Physicochemical properties of plasma polymerized acrylic acid, ε-caprolactone and lactic acid films. In Proceedings of the European Technical Conference-Society of Plastic Engineers, Boston, MA, USA, 1–5 May 2011. [Google Scholar]
- Umemura, J.; Hayashi, S. Infrared spectra and molecular configurations of liquid and crystalline acrylic acids. Bull. Inst. Chem. Res. 1975, 52, 585–595. [Google Scholar]
- Su, J.; Chen, J.; Li, L.; Li, B.; Shi, L.; Zhang, H.; Ding, X. Preparation of natural borneol/2-hydroxypropyl-β-cyclodextrin inclusion complex and its effect on the absorption of tetramethylpyrazine phosphate in mouse. Chem. Pharm. Bull. 2012, 60, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Fares, M.M.; Assaf, S.M.; Abul-Haija, Y.M. Pectin grafted poly(N-vinylpyrrolidone): Optimization and in vitro controllable theophylline drug release. J. Appl. Polym. Sci. 2010, 117, 1945–1954. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yu, J.; Zhou, Y.; Zhang, R.; Song, Q.; Lei, L.; Li, X. Supramolecular nanofibers of dexamethasone derivatives to form hydrogel for topical ocular drug delivery. Colloids Surf. B Biointerfaces 2018, 164, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-R.; Li, A.; Mei, W.; Zhu, R.-R.; Li, K.; Sun, X.-Y.; Qian, Y.-C.; Wang, S.-L. Dexamethasone sodium phosphate intercalated layered double hydroxides and their therapeutic efficacy in a murine asthma model. RSC Adv. 2015, 5, 23826–23834. [Google Scholar] [CrossRef]
- Georgieva, D.; Kostova, B.; Ivanova, S.; Rachev, D.; Tzankova, V.; Kondeva-Burdina, M.; Christova, D. pH-Sensitive Cationic Copolymers of Different Macromolecular Architecture as Potential Dexamethasone Sodium Phosphate Delivery Systems. J. Pharm. Sci. 2014, 103, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-dissolving electrospun gelatin nanofibers encapsulating ciprofloxacin/cyclodextrin inclusion complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Soldi, M.; Pinheiro, E.; Pires, A.; Gehlen, M.H.; Soldi, V. Thermal stability of poly(N-vinyl-2-pyrrolidone-co-methacrylic acid) copolymers in inert atmosphere. Polym. Degrad. Stab. 2003, 80, 567–574. [Google Scholar] [CrossRef]
- Kamyar, A.; Khakbiz, M.; Zamanian, A.; Yasaei, M.; Yarmand, B. Synthesis of a novel dexamethasone intercalated layered double hydroxide nanohybrids and their deposition on anodized titanium nanotubes for drug delivery purposes. J. Solid State Chem. 2019, 271, 144–153. [Google Scholar] [CrossRef]
- Chu, C.-H.; Berner, B. Thermal analysis of poly(acrylic acid)/poly(oxyethylene) blends. J. Appl. Polym. Sci. 1993, 47, 1083–1087. [Google Scholar] [CrossRef]
- Qiu, N.; Cheng, X.; Wang, G.; Wang, W.; Wen, J.; Zhang, Y.; Song, H.; Ma, L.; Wei, Y.; Peng, A. Inclusion complex of barbigerone with hydroxypropyl-β-cyclodextrin: Preparation and in vitro evaluation. Carbohydr. Polym. 2014, 101, 623–630. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, I.; Usman Minhas, M.; Barkat, K.; Khan, I.U.; Syed, H.K.; Umar, A. Preparation and in vitro evaluation of Chondroitin sulfate and carbopol based mucoadhesive controlled release polymeric composites of Loxoprofen using factorial design. Eur. Polym. J. 2019, 121, 109312. [Google Scholar] [CrossRef]
- Ijaz, N.; Khan, I.U.; Khalid, I.; Khan, R.U.; Khan, H.A.; Asghar, S.; Khalid, S.H.; Shahzad, Y.; Yousaf, A.M.; Hussain, T. In vitro and toxicological assessment of dexamethasone sodium phosphate loaded pH sensitive Pectin-g-poly(AA)/PVP semi interpenetrating network. Mater. Today Commun. 2020, 25, 101325. [Google Scholar] [CrossRef]
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 79. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Temperature/pH dual sensitive Hericium erinaceus residue carboxymethyl chitin/poly(N-isopropyl acrylamide) sequential IPN hydrogels. Cellulose 2019, 27, 825–838. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Banerjee, S.; Siddiqui, L.; Bhattacharya, S.S.; Kaity, S.; Ghosh, A.; Chattopadhyay, P.; Pandey, A.; Singh, L. Interpenetrating polymer network (IPN) hydrogel microspheres for oral controlled release application. Int. J. Biol. Macromol. 2012, 50, 198–206. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Biomaterials: Design, development and biomedical applications. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–44. [Google Scholar]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Das, A.; Basu, A.; Halder, A.; Das, S.; Basu, S.; Abdullah, M.F.; Mukherjee, A.; Kundu, S. Semi-interpenetrating hydrogels from carboxymethyl guar gum and gelatin for ciprofloxacin sustained release. Int. J. Biol. Macromol. 2018, 120, 1823–1833. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Chawla, R.; Alam, M.T.; Adhikari, J.; Basu, M. Efficacy and dermal toxicity analysis of Sildenafil citrate based topical hydrogel formulation against traumatic wounds. Biomed. Pharmacother. 2019, 112, 108571. [Google Scholar] [CrossRef]
- Pereira, I.; Fraga, S.; Maltez, L.; Requicha, J.; Guardão, L.; Oliveira, J.; Prada, J.; Alves, H.; Santos, J.D.; Teixeira, J.P. In vivo systemic toxicity assessment of an oxidized dextrin-based hydrogel and its effectiveness as a carrier and stabilizer of granular synthetic bone substitutes. J. Biomed. Mater. Res. Part A 2019, 107, 1678–1689. [Google Scholar]
- Pandey, M.; Mohamad, N.; Amin, M.C.I.M. Bacterial cellulose/acrylamide pH-sensitive smart hydrogel: Development, characterization, and toxicity studies in ICR mice model. Mol. Pharm. 2014, 11, 3596–3608. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Yang, B.; Qi, X.R.; Guo, G.; Gu, Y.C.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel: Part 1—Synthesis, characterization, and acute toxicity evaluation. J. Pharm. Sci. 2009, 98, 4684–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Han, H.; Ye, T.-T.; Li, F.-X.; Wang, X.-L.; Yu, J.-Y.; Wu, D.-Q. Biodegradable and pH sensitive peptide based hydrogel as controlled release system for antibacterial wound dressing application. Molecules 2018, 23, 3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.-F.; Zeng, Q.; Wang, H.; Hao, Y. Preparation and swelling behavior of pH/temperature responsive semi-IPN hydrogel based on carboxymethyl xylan and poly(N-isopropyl acrylamide). Cellulose 2019, 26, 1909–1922. [Google Scholar] [CrossRef]
- Shabir, F.; Erum, A.; Tulain, U.R.; Hussain, M.A.; Ahmad, M.; Akhter, F. Preparation and characterization of pH sensitive crosslinked Linseed polysaccharides-co-acrylic acid/methacrylic acid hydrogels for controlled delivery of ketoprofen. Des. Monomers Polym. 2017, 20, 485–495. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Anwar, J.; Khan, S. Synthesis and characterization of biodegradable hydrogels for oral delivery of 5-fluorouracil targeted to colon: Screening with preliminary in vivo studies. Adv. Polym. Tech. 2018, 37, 221–229. [Google Scholar] [CrossRef]
- Siemoneit, U.; Schmitt, C.; Alvarez-Lorenzo, C.; Luzardo, A.; Otero-Espinar, F.; Concheiro, A.; Blanco-Méndez, J. Acrylic/cyclodextrin hydrogels with enhanced drug loading and sustained release capability. Int. J. Pharm. 2006, 312, 66–74. [Google Scholar] [CrossRef]
- Wang, N.; Kou, J.; Wang, L.; Wang, F.; Lu, K. Sensitive Electrochemical Determination of Hyperin Based on Electrochemically Activated ZrO2 Nanoparticles-Modified Carbon Paste Electrode. Nano 2019, 14, 1950052. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T. Continuous-flow encapsulation of ketoprofen in copolymer microbeads via co-axial microfluidic device: Influence of operating and material parameters on drug carrier properties. Int. J. Pharm. 2013, 441, 809–817. [Google Scholar] [CrossRef]
- Iman, M.; Barati, A.; Safari, S. Characterization, in vitro antibacterial activity, and toxicity for rat of tetracycline in a nanocomposite hydrogel based on PEG and cellulose. Cellulose 2020, 27, 347–356. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Khullar, S. Study of a cross-linked hydrogel of Karaya gum and Starch as a controlled drug delivery system. J. Biomater. Sci. Polym. Ed. 2019, 30, 1687–1708. [Google Scholar] [CrossRef]
- Muhammad, G.; Ashraf, M.U.; Naeem-ul-Hassan, M.; Bukhari, S.N.A. Appraisal of acute oral toxicity of glucuronoxylan hydrogel from Mimosa pudica seeds. Braz. J. Pharm. Sci. 2018, 54, e17579. [Google Scholar] [CrossRef]
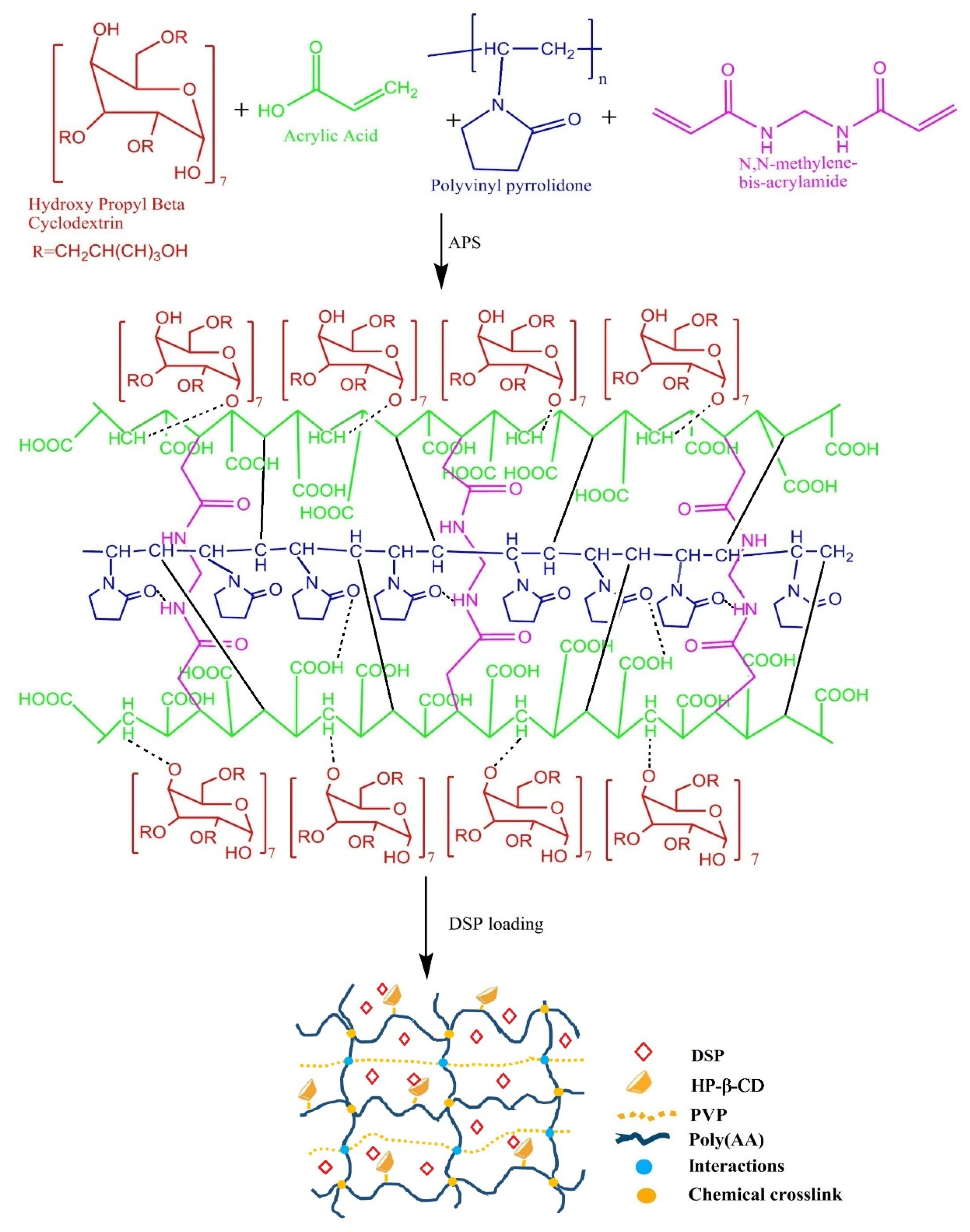


| Trial Code | AA g/100 g | PVP g/100 g | Formulation Code | AA g/100 g | PVP g/100 g | Formulation Code | AA g/100 g | PVP g/100 g |
|---|---|---|---|---|---|---|---|---|
| AP-1 * | 0 | 0.3 | AP-7 * | 0 | 1.33 | AP-13 * | 0 | 2.66 |
| AP-2 | 16.66 | AP-8 | 16.66 | AP-14 | 16.66 | |||
| AP-3 | 33.33 | AP-9 | 33.33 | AP-15 | 33.33 | |||
| AP-4 | 50 | AP-10 | 50 | AP-16 | 50 | |||
| AP-5 | 66.66 | AP-11 | 66.66 | AP-17 | 66.66 | |||
| AP-6 | 83.33 | AP-12 | 83.33 | AP-18 + | 83.33 |
| Sr No | Trial Code | Gel (%) | Sol (%) | Code | Gel (%) | Sol (%) | Code | Gel (%) | Sol (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AP-1 * | - | - | AP-7 * | - | - | AP-13 * | - | - |
| 2 | AP-2 | 95.68 | 4.31 | AP-8 | 99.59 | 0.40 | AP-14 | 96.66 | 3.33 |
| 3 | AP-3 | 92.41 | 7.58 | AP-9 | 93.48 | 6.51 | AP-15 | 92.45 | 7.54 |
| 4 | AP-4 | 92.37 | 7.62 | AP-10 | 93.41 | 6.58 | AP-16 | 90.27 | 9.72 |
| 5 | AP-5 | 94.21 | 5.78 | AP-11 | 93.33 | 6.66 | AP-17 | 89.88 | 10.11 |
| 6 | AP-6 | 97.23 | 2.76 | AP-12 | 93.28 | 6.71 | AP-18 + | - | - |
| Model | Parameter | Value |
|---|---|---|
| Korsmeyer-Peppas | Release rate constant (K) | 11.799 |
| Diffusion exponent (n) | 0.472 | |
| Correlation coefficient (R2) | 0.9800 |
| Parameters | Control | Treated |
|---|---|---|
| Signs of illness | None | None |
| Dermal toxicity | None | None |
| Ocular toxicity | None | None |
| Mortality rate | None | None |
| Body weight (kg) | ||
| Pretreatment | 1.36 ± 0.05 | 1.33 ± 0.05 |
| 1st day | 1.33 ± 0.11 | 1.30 ± 0.1 |
| 7th day | 1.23 ± 0.11 | 1.34 ± 0.23 |
| 14th day | 1.33 ± 0.15 | 1.36 ± 0.15 |
| Water Intake (mL) | ||
| Pretreatment | 183.66 ± 3.05 | 192 ± 2.64 |
| 1st day | 181.33 ± 3.21 | 186 ± 1.73 |
| 7th day | 185.66 ± 2.08 | 190 ± 1.73 |
| 14th day | 189 ± 1.73 | 193 ± 1.52 |
| Food Intake (g) | ||
| Pretreatment | 65.55 ± 1.52 | 60.33 ± 1.52 |
| 1st day | 68 ± 2.64 | 58.33 ± 1.52 |
| 7th day | 65.3 ± 3.05 | 63 ± 1 |
| 14th day | 68 ± 1.73 | 65.66 ± 1.15 |
| Hematology | Control | Treated |
|---|---|---|
| Hemoglobin (10–15 g/dL) | 12.8 ± 0.55 | 12.6 ± 0.05 |
| TLC (4.5–11 × 109 L−1) | 3.86 ± 0.56 | 3.7 ± 0.3 |
| Red Blood Cells (4.2–5.9 × 1012 L−1) | 5.55 ± 0.22 | 5.67 ± 0.01 |
| Platelets (150–400 × 109 L−1) | 159 ± 01 | 358 ± 13.11 |
| Monocytes (2–8%) | 3.66 ± 0.57 | 3.66 ± 0.57 |
| Neutrophils (40–60%) | 52 ± 1 | 20 ± 4 |
| Lymphocytes (20–40%) | 78.3 ± 0.57 | 79 ± 1 |
| Eosinophils (1–4%) | 2.33 ± 0.57 | 2 ± 1 |
| Mean Corpuscular Volume (80–96 fL) | 62.96 ± 0.55 | 61.16 ± 1.4 |
| Mean Corpuscular Hemoglobin (27–32 pg) | 22.4 ± 0.62 | 23.36 ± 0.97 |
| Mean Corpuscular Hemoglobin Concentration (32–36%) | 34.9 ± 0.26 | 38.26 ± 0.90 |
| Biochemical Analysis | Control | Treated |
|---|---|---|
| Liver profile | ||
| Alanine aminotransferase (17–77 IU/L) | 91 ± 1 | 37 ± 3 |
| Aspartate aminotransferase (54–298 IU/L) | 114.33 ± 1.52 | 53 ± 2.64 |
| Renal profile | ||
| Creatinine (0.2–0.9 mg/dL) | 0.56 ± 0.04 | 0.62 ± 0.16 |
| Urea (10–50 mg/dL) | 16 ± 2 | 19 ± 3 |
| Uric acid (3.4–7.1 mg/dL) | 3.8 ± 0.1 | 4.93 ± 0.80 |
| Lipid profile | ||
| Cholesterol (10–80 mg/dL) | 66.48 ± 1.15 | 68.3 ± 2.08 |
| Triglycerides (46–68 mg/dL) | 54.73 ± 1.48 | 54.3 ± 0.57 |
| Group | Heart (g) | Kidney (g) | Liver (g) | Lung (g) | Stomach (g) |
|---|---|---|---|---|---|
| Control | 3.68 ± 0.07 | 9.18 ± 1.52 | 35.01 ± 1.99 | 16.56 ± 2.65 | 12.25 ± 1.93 |
| Treated | 3.06 ± 0.25 | 6.90 ± 0.29 | 28.48 ± 1.01 | 7.39 ± 0.46 | 8.42 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajaz, N.; Abbas, A.; Afshan, R.; Irfan, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Rizg, W.Y.; Majrashi, K.A.; Alshehri, S.; et al. In Vitro and In Vivo Evaluation of Hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate. Pharmaceuticals 2022, 15, 1399. https://doi.org/10.3390/ph15111399
Ajaz N, Abbas A, Afshan R, Irfan M, Khalid SH, Asghar S, Munir MU, Rizg WY, Majrashi KA, Alshehri S, et al. In Vitro and In Vivo Evaluation of Hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate. Pharmaceuticals. 2022; 15(11):1399. https://doi.org/10.3390/ph15111399
Chicago/Turabian StyleAjaz, Nyla, Anum Abbas, Rabia Afshan, Muhammad Irfan, Syed Haroon Khalid, Sajid Asghar, Muhammad Usman Munir, Waleed Y. Rizg, Kamlah Ali Majrashi, Sameer Alshehri, and et al. 2022. "In Vitro and In Vivo Evaluation of Hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate" Pharmaceuticals 15, no. 11: 1399. https://doi.org/10.3390/ph15111399
APA StyleAjaz, N., Abbas, A., Afshan, R., Irfan, M., Khalid, S. H., Asghar, S., Munir, M. U., Rizg, W. Y., Majrashi, K. A., Alshehri, S., Alissa, M., Majrashi, M., Bukhary, D. M., Hussain, G., Rehman, F., & Khan, I. U. (2022). In Vitro and In Vivo Evaluation of Hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) Semi-Interpenetrating Matrices of Dexamethasone Sodium Phosphate. Pharmaceuticals, 15(11), 1399. https://doi.org/10.3390/ph15111399










