Lycopene Modulates Oxidative Stress and Inflammation in Hypercholesterolemic Rats
Abstract
1. Introduction
2. Results
2.1. Effect of Lycopene on Body Weight, Organ Weight, Glycemic Features, and Kidney Function Parameters
2.2. Effect of Lycopene on Lipid Profile
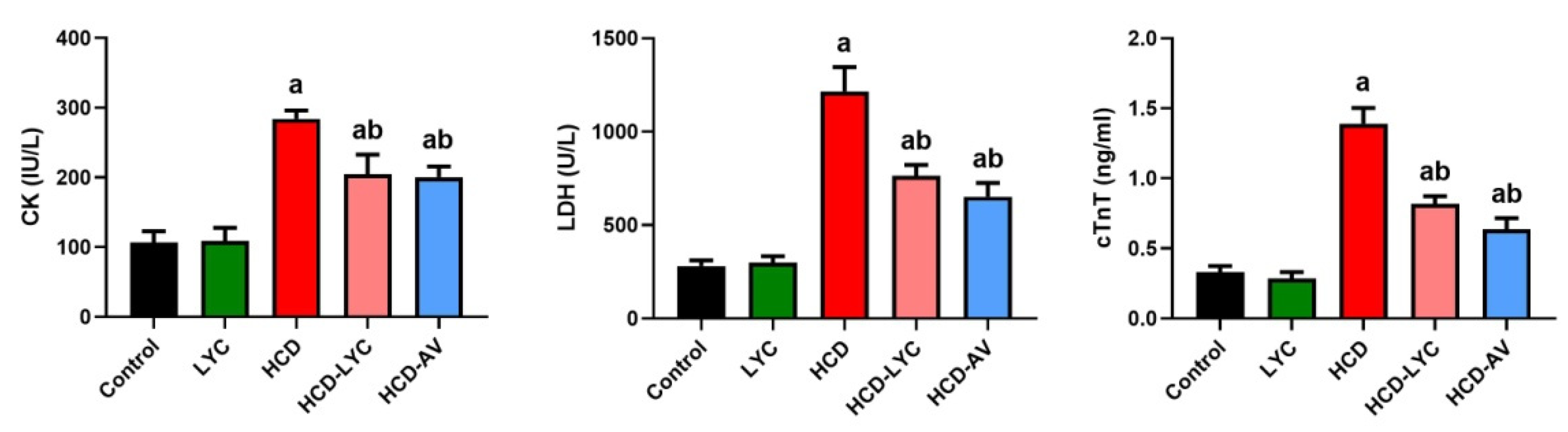
2.3. Effect of Lycopene on Cardiac Markers Following Hypercholesterolemia in Rats
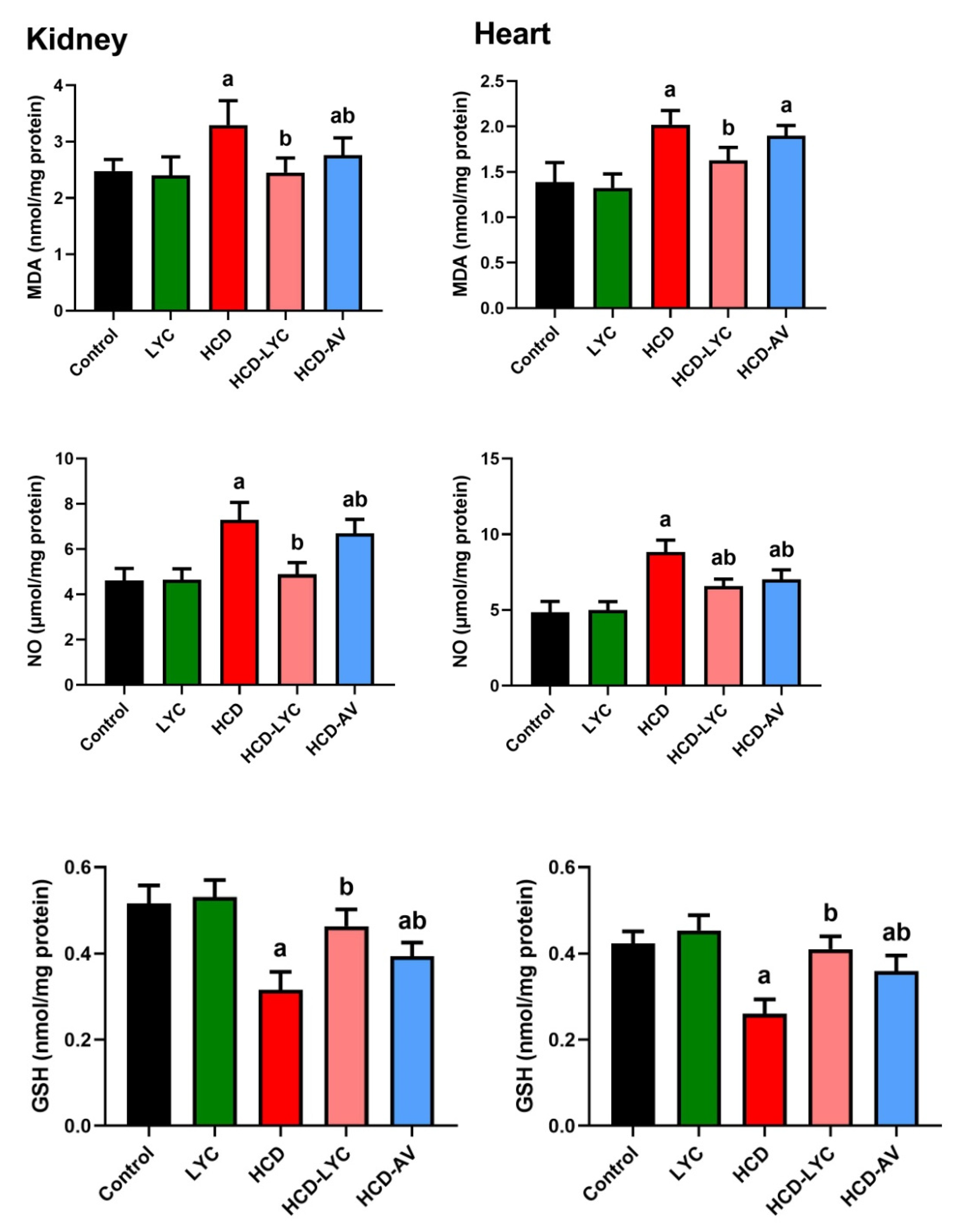
2.4. Effect of Lycopene on Redox Homeostasis Following Hypercholesterolemia in Rats
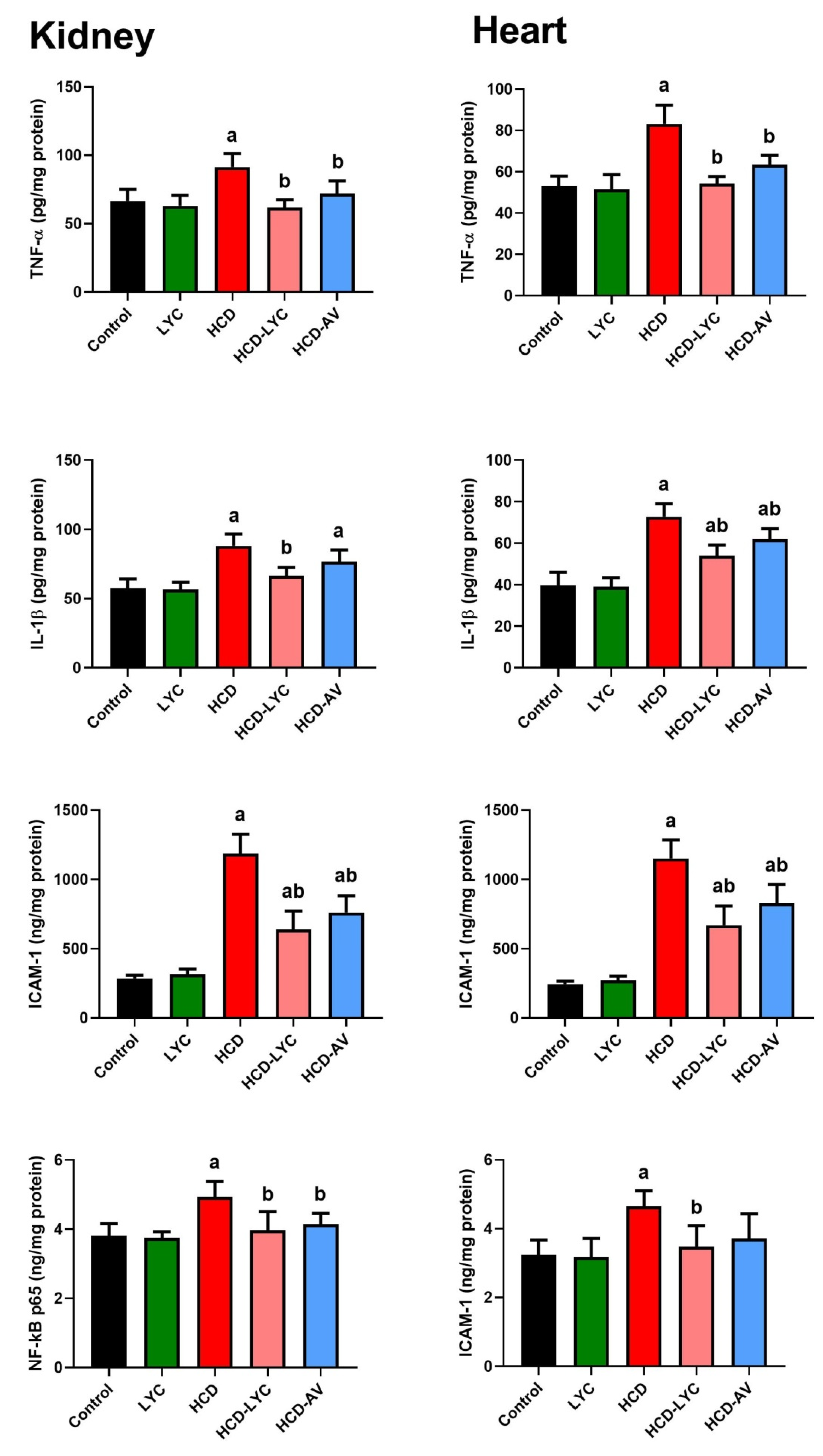
2.5. Effect of lycopene on Inflammatory Response Following Hypercholesterolemia in Rats
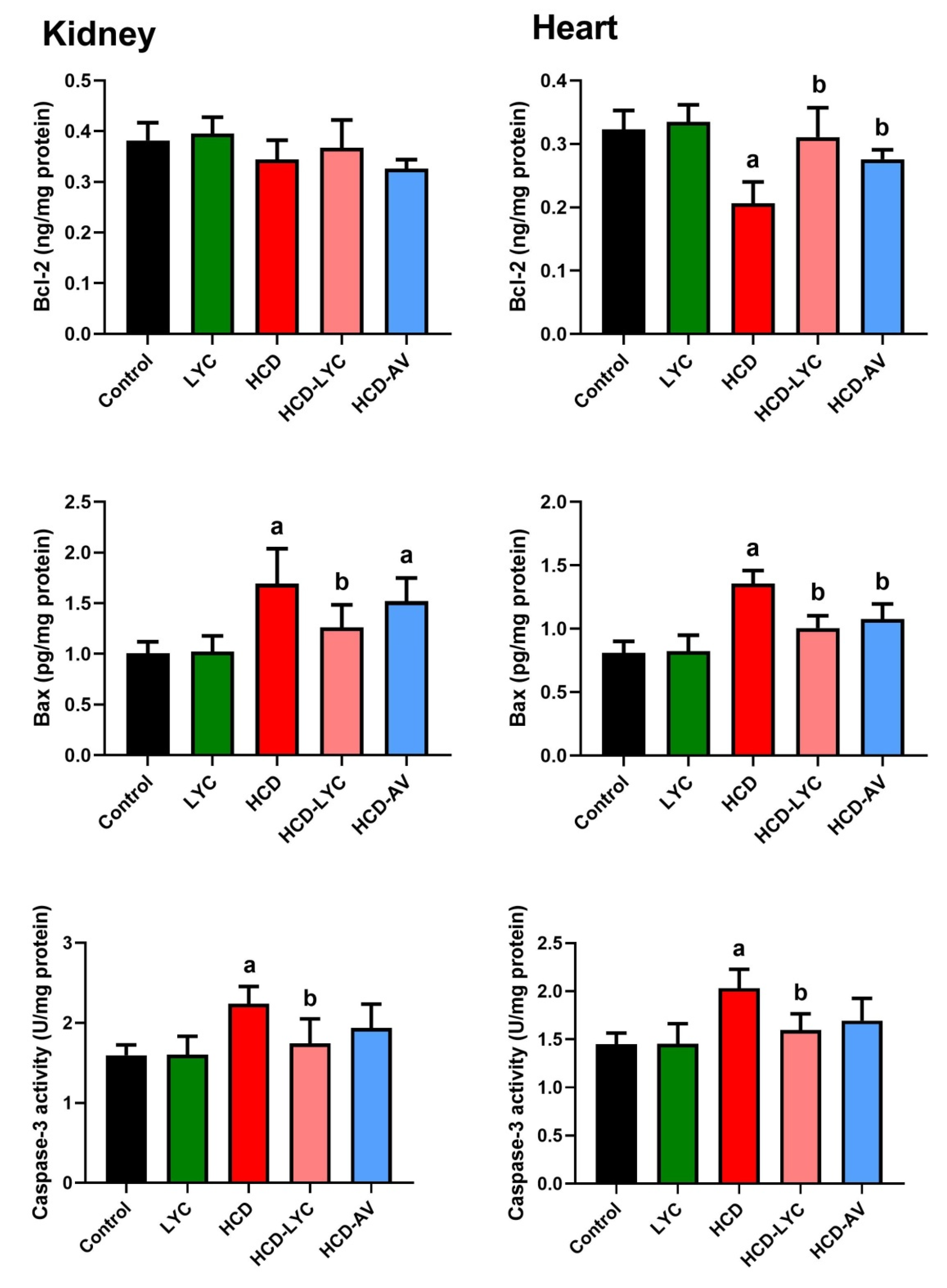
2.6. Effect of Lycopene on Apoptotic Markers Following Hypercholesterolemia in Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Sampling and Tissue Preparation
4.3. Glycemic Features and Serum Biochemical Parameters
4.4. Lipid Profile and Atherogenic Index
4.5. Investigation of Cardiac and Renal Tissue Oxidative Stress Indicators
4.6. Investigation of Antioxidant Enzyme Activity in Cardiac and Renal Tissues
4.7. Identification of Pro-Inflammatory Cytokines in Cardiac and Renal Tissues
4.8. Identifying Apoptotic Proteins in Cardiac and Renal Tissues
4.9. Analysis of Gene Expression
4.10. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Hameed, R.A.; Zahra, F.A.A.; Moneim, A.E.A. Echinops spinosus effect against diabetes and its hepatorenal complications: Total extract and flavonoids fraction. Environ. Sci. Pollut. Res. 2022, 29, 38606–38617. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Jeon, B.R.; Jeong, D.-H.; Lee, K.; Goo, Y.-K.; Kim, S.-H.; Sung, C.-K.; Roh, S.-S.; Kim, S.D.; Kim, H.-K.; et al. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng. Res. 2016, 40, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Collino, M.; Aragno, M.; Castiglia, S.; Miglio, G.; Tomasinelli, C.; Boccuzzi, G.; Thiemermann, C.; Fantozzi, R. Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation. Br. J. Clin. Pharmacol. 2010, 160, 1892–1902. [Google Scholar] [CrossRef]
- Duszka, K.; Gregor, A.; Guillou, H.; Konig, J.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity. Cells 2020, 9, 1708. [Google Scholar] [CrossRef]
- Jayakumar, V.; Ahmed, S.S.; Ebenezar, K.K. Multivariate analysis and molecular interaction of curcumin with PPARgamma in high fructose diet induced insulin resistance in rats. Springerplus 2016, 5, 1732. [Google Scholar] [CrossRef][Green Version]
- Othman, M.S.; Khaled, A.M.; Aleid, G.M.; Fareid, M.A.; Hameed, R.A.; Abdelfattah, M.S.; Aldin, D.E.; Moneim, A.E.A. Evaluation of antiobesity and hepatorenal protective activities of Salvia officinalis extracts pre-treatment in high-fat diet-induced obese rats. Environ. Sci. Pollut. Res. 2022, 29, 75043–75056. [Google Scholar] [CrossRef]
- Olorunnisola, O.S.; Bradley, G.; Afolayan, A.J. Protective effect of T. violacea rhizome extract against hypercholesterolemia-induced oxidative stress in Wistar rats. Molecules 2012, 17, 6033–6045. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Ramadan, S.S.; Almeer, R.; Albasher, G.; Abdel Moneim, A.E. Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Rev. 2021, 41, 446–456. [Google Scholar] [CrossRef]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef] [PubMed]
- Albrahim, T.; Robert, A.A. Lycopene Effects on Metabolic Syndrome and Kidney Injury in Rats Fed a High-Fat Diet: An Experimental Study. ACS Omega 2022, 7, 30930–30938. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Yi, Q.; Wang, X.; Ju, X. Protective effect of polyphenols extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules 2012, 17, 8886–8897. [Google Scholar] [CrossRef] [PubMed]
- AlAmri, O.D.; Albeltagy, R.S.; Akabawy, A.M.A.; Mahgoub, S.; Abdel-Mohsen, D.M.; Moneim, A.E.A.; Amin, H. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods 2020, 71, 103996. [Google Scholar] [CrossRef]
- Abd Al Haleem, E.N.; El-Bakly, W.M. The role of MAPK signaling pathway in selenium amelioration of high fat/high cholesterol diet-induced tauopathy in rats. Chem. Biol. Interact. 2019, 302, 108–116. [Google Scholar] [CrossRef]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; de la Jara, N.; Pena, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid. Med. Cell Longev. 2018, 2018, 7239123. [Google Scholar] [CrossRef]
- Raja, L.; Ravindran Nair, A.; Senthilpandian, S.; Ravi, V. Hypolipidemic action of Rutin on Triton WR-1339 induced hyperlipidemia in rats. J. Pre-Clin. Clin. Res. 2021, 15, 51–55. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Devakul Na Ayutthaya, W.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Lipid-Lowering and Antioxidative Activities of Aqueous Extracts of Ocimum sanctum L. Leaves in Rats Fed with a High-Cholesterol Diet. Oxid. Med. Cell Longev. 2011, 2011, 962025. [Google Scholar] [CrossRef]
- Geng, X.; Liu, H.; Yuwen, Q.; Wang, J.; Zhang, S.; Zhang, X.; Sun, J. Protective effects of zingerone on high cholesterol diet-induced atherosclerosis through lipid regulatory signaling pathway. Hum. Exp. Toxicol. 2021, 40, 1732–1745. [Google Scholar] [CrossRef]
- Cheon, S.-Y.; Chung, K.-S.; Lee, K.-J.; Choi, H.-Y.; Ham, I.-H.; Jung, D.-H.; Cha, Y.-Y.; An, H.-J. HVC1 ameliorates hyperlipidemia and inflammation in LDLR−/− mice. BMC Complement. Altern. Med. 2017, 17, 222. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; Gordon, D.M.; Hipp, J.A.; Hong, S.; Mitchell, Z.L.; Franco, N.R.; Robison, J.W.; Anderson, C.D.; Stec, D.F.; Hinds, T.D., Jr. Loss of hepatic PPARalpha promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R733–R745. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the cardiovascular system. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef]
- Tietge, U.J. Hyperlipidemia and cardiovascular disease: Inflammation, dyslipidemia, and atherosclerosis. Curr. Opin. Lipidol. 2014, 25, 94–95. [Google Scholar] [CrossRef] [PubMed]
- El-Borady, O.M.; Othman, M.S.; Atallah, H.H.; Abdel Moneim, A.E. Hypoglycemic potential of selenium nanoparticles capped with polyvinyl-pyrrolidone in streptozotocin-induced experimental diabetes in rats. Heliyon 2020, 6, e04045. [Google Scholar] [CrossRef]
- Duncan, J.G. Peroxisome proliferator activated receptor-alpha (PPARalpha) and PPAR gamma coactivator-1alpha (PGC-1alpha) regulation of cardiac metabolism in diabetes. Pediatr. Cardiol. 2011, 32, 323–328. [Google Scholar] [CrossRef]
- Wan, X.L.; Li, N.; Chen, Y.J.; Chen, X.S.; Yang, Z.; Xu, L.; Yang, H.M.; Wang, Z.Y. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult. Sci. 2021, 100, 101441. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.-X.; Luo, Y.; Cui, J.-G.; Talukder, M.; Li, J.-L. Lycopene mitigates DEHP-induced hepatic mitochondrial quality control disorder via regulating SIRT1/PINK1/mitophagy axis and mitochondrial unfolded protein response. Environ. Pollut. 2022, 292, 118390. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Aparicio, R.; Carrón, R.; Sevilla, M.Á.; Monroy-Ruiz, J.; Montero, M.J. Lycopene-supplemented diet ameliorates cardiovascular remodeling and oxidative stress in rats with hypertension induced by Angiotensin II. J. Funct. Foods 2018, 47, 279–287. [Google Scholar] [CrossRef]
- Faran, S.A.; Asghar, S.; Khalid, S.H.; Khan, I.U.; Asif, M.; Khalid, I.; Gohar, U.F.; Hussain, T. Hepatoprotective and Renoprotective Properties of Lovastatin-Loaded Ginger and Garlic Oil Nanoemulsomes: Insights into Serum Biological Parameters. Medicina 2019, 55, 579. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, S.; Zhu, T.; Sun, G.; Zhang, P.; Huang, Y.; Qu, S.; Du, X.; Mou, D. Hawthorn fruit acid consumption attenuates hyperlipidemia-associated oxidative damage in rats. Front. Nutr. 2022, 9, 936229. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Lu, X.; Jiang, Y.; Xu, L.; Zhao, X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol. 2014, 7, 5008–5015. [Google Scholar]
- Bas, H.; Pandir, D.; Kalender, S. Furan-induced hepatotoxic and hematologic changes in diabetic rats: The protective role of lycopene. Arh. Za Hig. Rada I Toksikol. 2016, 67, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef] [PubMed]
- Otocka-Kmiecik, A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Akhtar, F.; Rizvi, S.I. Protective effect of hesperidin in Poloxamer-407 induced hyperlipidemic experimental rats. Biol. Futur. 2021, 72, 201–210. [Google Scholar] [CrossRef]
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Ghany, R.A.; Habotta, O.A.; Abdel Moneim, A.E. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Jacobsson, L.T.; Turesson, C.; Gulfe, A.; Kapetanovic, M.C.; Petersson, I.F.; Saxne, T.; Geborek, P. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1213–1218. [Google Scholar]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Branen, L.; Hovgaard, L.; Nitulescu, M.; Bengtsson, E.; Nilsson, J.; Jovinge, S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arter. Thromb. Vasc. Biol. 2004, 24, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S.; Al Humayed, S.; Alkhateeb, M.A.; Shatoor, K.A.; Aldera, H.; Alassiri, M.; Shati, A.A. Crataegus Aronia protects and reverses vascular inflammation in a high fat diet rat model by an antioxidant mechanism and modulating serum levels of oxidized low-density lipoprotein. Pharm. Biol. 2019, 57, 37–47. [Google Scholar] [CrossRef]
- da Motta, N.A.V.; de Brito, F.C.F. Cilostazol exerts antiplatelet and anti-inflammatory effects through AMPK activation and NF-kB inhibition on hypercholesterolemic rats. Fundam. Clin. Pharmacol. 2016, 30, 327–337. [Google Scholar] [CrossRef]
- Liu, F.; Morris, S.; Epps, J.; Carroll, R. Demonstration of an activation regulated NF-kappaB/I-kappaBalpha complex in human platelets. Thromb. Res. 2002, 106, 199–203. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chang, M.H.; Tsai, C.C.; Chen, T.S.; Fan, C.C.; Lin, C.C.; Lai, C.H.; Tsai, F.J.; Lin, J.A.; Huang, C.Y. Garlic oil attenuates the cardiac apoptosis in hamster-fed with hypercholesterol diet. Food Chem. 2013, 136, 1296–1302. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Daghini, E.; Rodriguez-Porcel, M.; Chade, A.R.; Napoli, C.; Lerman, A.; Lerman, L.O. Redox-sensitive myocardial remodeling and dysfunction in swine diet-induced experimental hypercholesterolemia. Atherosclerosis 2007, 193, 62–69. [Google Scholar] [CrossRef]
- Osipov, R.M.; Bianchi, C.; Feng, J.; Clements, R.T.; Liu, Y.; Robich, M.P.; Glazer, H.P.; Sodha, N.R.; Sellke, F.W. Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation 2009, 120, S22–S30. [Google Scholar] [CrossRef]
- Perales, S.; Alejandre, M.J.; Palomino-Morales, R.; Torres, C.; Iglesias, J.; Linares, A. Effect of oxysterol-induced apoptosis of vascular smooth muscle cells on experimental hypercholesterolemia. J. Biomed. Biotechnol. 2009, 2009, 456208. [Google Scholar] [CrossRef]
- Zhao, X.S.; Wu, Q.; Peng, J.; Pan, L.H.; Ren, Z.; Liu, H.T.; Jiang, Z.S.; Wang, G.X.; Tang, Z.H.; Liu, L.S. Hyperlipidemia-induced apoptosis of hippocampal neurons in apoE(-/-) mice may be associated with increased PCSK9 expression. Mol. Med. Rep. 2017, 15, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tian, X.; Liu, X.; Shao, J.; Lv, Y. The p53-mediated apoptosis in hypercholesterolemia-induced renal injury of rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 408–411. [Google Scholar] [CrossRef]
- Al-Brakati, A.; Alsharif, K.F.; Alzahrani, K.J.; Kabrah, S.; Al-Amer, O.; Oyouni, A.A.; Habotta, O.A.; Lokman, M.S.; Bauomy, A.A.; Kassab, R.B.; et al. Using Green Biosynthesized Lycopene-Coated Selenium Nanoparticles to Rescue Renal Damage in Glycerol-Induced Acute Kidney Injury in Rats. Int. J. Nanomed. 2021, 16, 4335–4349. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-kappaB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. [Google Scholar] [CrossRef]
- Fan, S.; Sun, J.B.; Li, R.; Song, X.; Li, J. Lycopene protects myocardial ischemia injury through anti-apoptosis and anti-oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3096–3104. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.K.d.S.; Lima, G.d.M.; Farias, L.M.d.; Rodrigues, L.A.R.L.; Carvalho, V.B.L.d.; Pereira, C.F.d.C.; Frota, K.d.M.G.; Conde-Júnior, A.M.; Silva, A.M.O.; Rizzo, M.d.S. Lycopene-Rich Extract from Red Guava (Psidium guajava L.) Decreases Plasma Triglycerides and Improves Oxidative Stress Biomarkers on Experimentally-Induced Dyslipidemia in Hamsters. Nutrients 2019, 11, 393. [Google Scholar] [CrossRef]
- Trinder, P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Kazemi, T.; Hajihosseini, M.; Moossavi, M.; Hemmati, M.; Ziaee, M. Cardiovascular Risk Factors and Atherogenic Indices in an Iranian Population: Birjand East of Iran. Clin. Med. Insights Cardiol. 2018, 12, 1179546818759286. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Mannervik, B. Measurement of glutathione reductase activity. Curr. Protoc. Toxicol. 2001, 7, 7.2.1–7.2.4. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, N.; Jensen, P.T. Procedure for blood glutathione peroxidase determination in cattle and swine. Acta Vet. Scand. 1982, 23, 515–527. [Google Scholar] [CrossRef]


| Experimental Groups | Control | LYC | HCD | HCD-LYC | HCD-AV |
|---|---|---|---|---|---|
| Body weight at beginning (g) | 193.09 ± 9.06 | 192.67 ± 7.63 | 195.24 ± 8.43 | 194.60 ± 5.08 | 191.11 ± 5.92 |
| Body weight at end (g) | 273.27 ± 8.85 | 265.30 ± 10.07 | 384.84 ± 26.40 a | 313.52 ± 13.77 ab | 351.42 ± 15.70 ab |
| Body weight gain (g) | 80.17 ± 11.81 | 72.63 ± 16.33 | 189.60 ± 25.40 a | 118.92 ± 13.62 ab | 160.30 ± 18.96 ab |
| Absolute liver weight (g) | 8.13 ± 0.43 | 7.87 ± 0.60 | 10.56 ± 0.86 a | 9.50 ± 0.81 ab | 10.40 ± 0.65 a |
| Relative liver weight (g) | 2.97 ± 0.13 | 2.97 ± 0.17 | 2.75 ± 0.28 a | 3.03 ± 0.20 b | 2.96 ± 0.22 b |
| Absolute kidney weight (g) | 1.73 ± 0.12 | 1.74 ± 0.14 | 1.92 ± 0.18 a | 1.83 ± 0.14 | 1.93 ± 0.12 a |
| Relative kidney weight (g) | 0.63 ± 0.06 | 0.66 ± 0.07 | 0.50 ± 0.07 a | 0.58 ± 0.05 b | 0.55 ± 0.06 a |
| Absolute heart weight (g) | 1.42 ± 0.06 | 1.43 ± 0.05 | 1.86 ± 0.12 a | 1.58 ± 0.06 b | 1.61 ± 0.09 b |
| Relative heart weight (g) | 0.52 ± 0.03 | 0.54 ± 0.03 | 0.49 ± 0.05 | 0.50 ± 0.03 | 0.46 ± 0.04 a |
| Experimental Groups | Control | LYC | HCD | HCD-LYC | HCD-AV |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 86.58 ± 9.52 | 88.52 ± 11.79 | 253.69 ± 26.67 | 135.46 ± 19.15 | 115.23 ± 20.74 |
| Triglyceride (mg/dL) | 104.63 ± 15.61 | 106.01 ± 23.76 | 356.76 ± 57.36 a | 186.38 ± 39.17 ab | 162.54 ± 12.17 ab |
| LDL-c (mg/dL) | 14.71 ± 12.44 | 16.90 ± 9.00 | 154.61 ± 25.14 a | 48.98 ± 22.41 ab | 40.98 ± 25.71 ab |
| vLDL-c (mg/dL) | 20.93 ± 3.12 | 21.20 ± 4.75 | 71.35 ± 11.47 a | 37.28 ± 7.83 ab | 32.51 ± 2.45 ab |
| HDL-c (mg/dL) | 50.95 ± 8.61 | 50.41 ± 7.07 | 27.73 ± 3.74 a | 49.20 ± 6.20 b | 41.74 ± 6.59 b |
| Athrogenic index | 0.75 ± 0.44 | 0.78 ± 0.29 | 8.24 ± 1.23 a | 1.77 ± 0.39 ab | 1.86 ± 0.83 ab |
| Glucose (mg/dL) | 96.07 ± 12.54 | 100.72 ± 10.11 | 167.76 ± 25.78 a | 114.66 ± 19.18 b | 150.18 ± 15.99 a |
| Insulin (ng/mL) | 5.62 ± 0.93 | 5.72 ± 0.98 | 7.79 ± 1.78 a | 5.96 ± 1.08 b | 6.64 ± 0.91 b |
| Urea (mg/dL) | 48.52 ± 8.90 | 43.75 ± 6.88 | 132.85 ± 14.48 | 76.50 ± 10.53 ab | 106.68 ± 17.62 ab |
| Creatinine (mg/dL) | 0.54 ± 0.04 | 0.52 ± 0.07 | 1.08 ± 0.19 a | 066. ±0.07 b | 0.79 ± 0.07 ab |
| Name | Accession Number | Sense (5′→3′) | Antisense (5′→3′) |
|---|---|---|---|
| GAPDH | NM_017008.4 | CTCTCTGCTCCTCCCTGTTC | TACGGCCAAATCCGTTCACA |
| PPAR-γ | NM_001145366.1 | AGTAGCCTGGGCTGCTTTTAT | GATCACCAGCAGAGGTCCAG |
| PGC-1α | NM_031347.1 | CATGTGCAGCCAAGACTCTG | GTGAGGACCGCTAGCAAGTT |
| PON-1 | NM_032077.1 | CAGGACATGGCGAAACTGCT | TCTAAGTCTTCAGCACCCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrahim, T. Lycopene Modulates Oxidative Stress and Inflammation in Hypercholesterolemic Rats. Pharmaceuticals 2022, 15, 1420. https://doi.org/10.3390/ph15111420
Albrahim T. Lycopene Modulates Oxidative Stress and Inflammation in Hypercholesterolemic Rats. Pharmaceuticals. 2022; 15(11):1420. https://doi.org/10.3390/ph15111420
Chicago/Turabian StyleAlbrahim, Tarfa. 2022. "Lycopene Modulates Oxidative Stress and Inflammation in Hypercholesterolemic Rats" Pharmaceuticals 15, no. 11: 1420. https://doi.org/10.3390/ph15111420
APA StyleAlbrahim, T. (2022). Lycopene Modulates Oxidative Stress and Inflammation in Hypercholesterolemic Rats. Pharmaceuticals, 15(11), 1420. https://doi.org/10.3390/ph15111420






