Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance
Abstract
1. Introduction
2. Chemistry and Biology of Synthetic Hemorphin Analogs Containing Non-Natural Amino Acids
- -
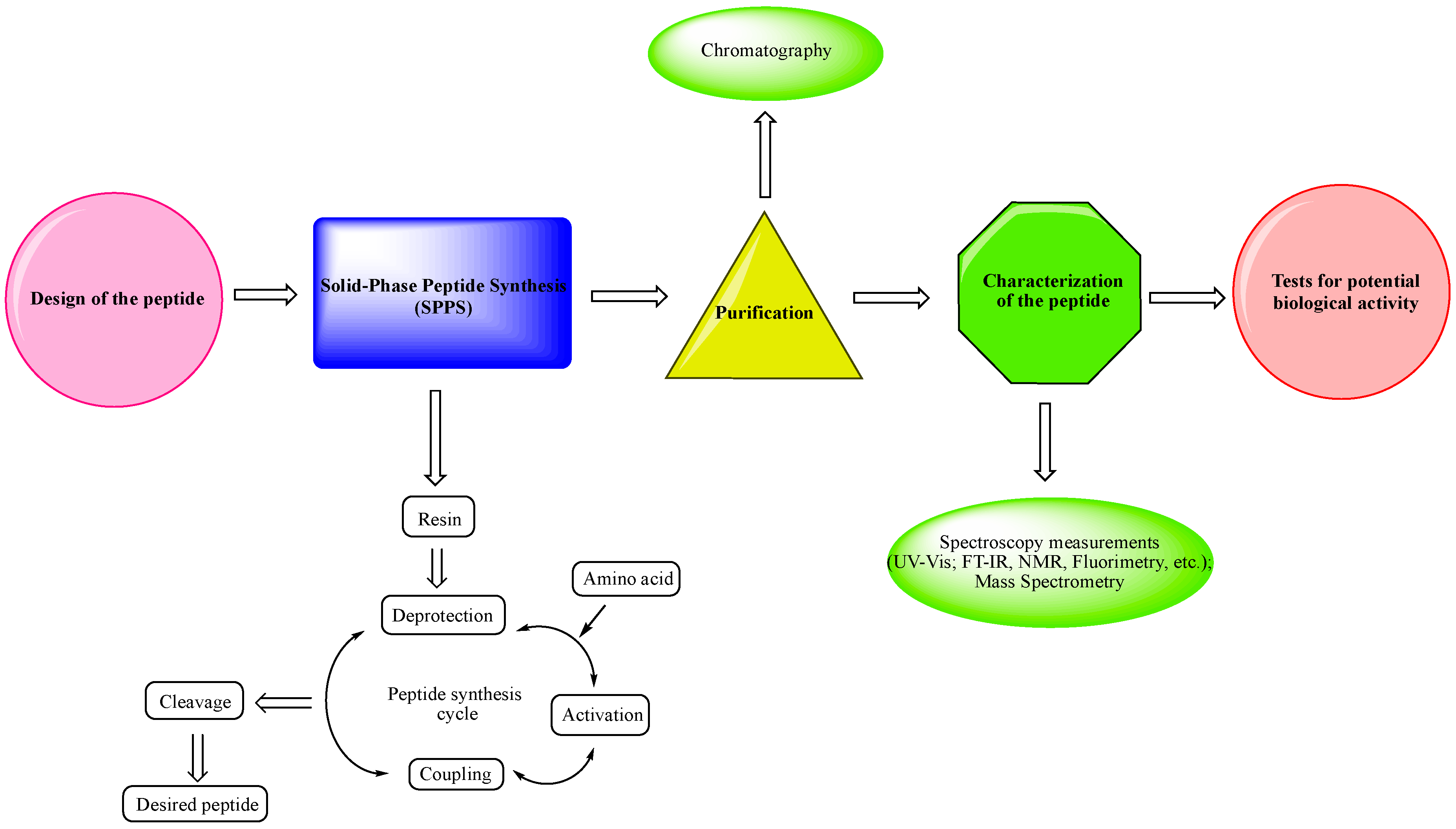
- Design of the peptide—planning of the desired peptide compound with expected biological activity, what modifications to be made, in which part of the molecule to be made, what properties we expect to obtain, etc.
- -
- Choice of a reliable method used to obtain the desired peptide—peptide synthesis in solution or solid-phase peptide synthesis (SPPS). The solid-phase peptide synthesis by the Fmoc-strategy is the most widespread and acceptable method due to the number of its advantages, including reduced reaction time for creating a peptide bond; quantitative progression of condensation reactions; the easy removal of excess reagents and solvents by washing the peptidyl-resin; minimal losses when receiving the final product.
- -
- The synthesized peptide must be purified using chromatography (the most used is reversed-phase high-performance liquid chromatography (RP-HPLC)).
- -
- Followed by the complete characterization of the peptide using modern instrumental methods and techniques: spectroscopy measurements (UV-Vis; FT-IR, NMR, fluorimetry, etc.); mass spectrometry.
- -
- Screening tests for potential biological activity.
2.1. Analytical Characteristics of Hemorphin Analogs
2.2. Electrochemical Behavior of Hemorphin Analogs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mielczarek, P.; Hartman, K.; Drabik, A.; Hung, H.-Y.; Huang, E.Y.-K.; Gibula-Tarlowska, E.; Kotlinska, J.H.; Silberring, J. Hemorphins—From Discovery to Functions and Pharmacology. Molecules 2021, 26, 3879. [Google Scholar] [CrossRef]
- Fukui, K.; Shiomi, H.; Takagi, H.; Hayashi, K.; Kiso, Y.; Kitagawa, K. Isolation from bovine brain of a novel analgesic pentapeptide, neo-kyotorphin, containing the Tyr-Arg (kyotorphin)unit. Neuropharmacology 1983, 22, 191–196. [Google Scholar] [CrossRef]
- Schechter, A.N. Hemoglobin research and the origins of molecular medicine. Blood J. Am. Soc. Hematol. 2008, 112, 3927–3938. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, M.; Stamler, J.S. Hemoglobin induction in mouse macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 6643–6647. [Google Scholar] [CrossRef]
- Newton, D.A.; Rao, K.M.K.; Dluhy, R.A.; Baatz, J.E. Hemoglobin is expressed by alveolar epithelial cells. J. Biol. Chem. 2006, 281, 5668–5676. [Google Scholar] [CrossRef]
- Wride, M.A.; Mansergh, F.C.; Adams, S.; Everitt, R.; Minnema, S.E.; Rancourt, D.E.; Evans, M.J. Expression profiling and gene discovery in the mouse lens. Mol. Vis. 2003, 9, 360–396. [Google Scholar]
- Setton-Avruj, C.P.; Musolino, P.L.; Salis, C.; Allo, M.; Bizzozero, O.; Villar, M.J.; Pasquini, J.M. Presence of α-globin mRNA and migration of bone marrow cells after sciatic nerve injury suggests their participation in the degeneration/regeneration process. Exp. Neurol. 2007, 203, 568–578. [Google Scholar] [CrossRef]
- Ohyagi, Y.; Yamada, T.; Goto, I. Hemoglobin as a novel protein developmentally regulated in neurons. Brain Res. 1994, 635, 323–327. [Google Scholar] [CrossRef]
- Schelshorn, D.W.; Schneider, A.; Kuschinsky, W.; Weber, D.; Krüger, C.; Dittgen, T.; Maurer, M.H. Expression of hemoglobin in rodent neurons. J. Cereb. Blood Flow Metab. 2009, 29, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P.; Chesselet, M.F. Neurons express hemoglobin α-and β-chains in rat and human brains. J. Comp. Neurol. 2009, 515, 538–547. [Google Scholar] [CrossRef]
- Ivanov, V.T.; Karelin, A.A.; Philippova, M.M.; Nazimov, I.V.; Pletnev, V.Z. Hemoglobin as a source of endogenous bioactive peptides: The concept of tissue-specific peptide pool. Pept. Sci. 1997, 43, 171–188. [Google Scholar] [CrossRef]
- Karelin, A.A.; Blishchenko, E.Y.; Ivanov, V.T. Fragments of Functional Proteins: Role in Endocrine Regulation. Neurochem. Res. 1999, 24, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Giardina, B. Hemoglobin: Multiple molecular interactions and multiple functions. An example of energy optimization and global molecular organization. Mol. Asp. Med. 2022, 84, 101040. [Google Scholar] [CrossRef]
- Amanat, A.; Alzeyoudi, C.A.; Almutawa, A.C.; Alnajjar, A.H.; Vijayan, R. Molecular basis of the therapeutic properties of hemorphins, Pharmacol. Res. 2020, 158, 104855. [Google Scholar]
- Yoshikawa, M. Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides 2015, 72, 208–225. [Google Scholar] [CrossRef] [PubMed]
- Brantl, V.; Gramsch, C.; Lottspeich, F.; Mertz, R.; Jaeger, K.H.; Herz, A. Novel opioid peptides derived from hemoglobin: Hemorphins. Eur. J. Pharmacol. 1986, 125, 309–310. [Google Scholar] [CrossRef]
- Yang, Y.R.; Chiu, T.H.; Chen, C.L. Structure–activity relationships of naturally occurring and synthetic opioid tetrapeptides acting on locus coeruleus neurons. Eur. J. Pharmacol. 1999, 372, 229–236. [Google Scholar] [CrossRef]
- Mollica, A.; Pinnen, F.; Stefanucci, A.; Mannina, L.; Sobolev, A.P.; Lucente, G.; Davis, P.; Lai, J.; Ma, S.-W.; Porreca, F.; et al. cis-4-Amino-l-proline Residue as a Scaffold for the Synthesis of Cyclic and Linear Endomorphin-2 Analogues: Part 2. J. Med. Chem. 2012, 55, 19–8477. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Costante, R.; Novellino, E. Pyroglutamic Acid Derivatives: Building Blocks for Drug Discovery. HETEROCYCLES 2014, 89, 1801. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Costante, R.; Hruby, V.J. Chapter 2—Rational Approach to the Design of Bioactive Peptidomimetics: Recent Developments in Opioid Agonist Peptides. Stud. Nat. Prod. Chem. 2015, 46, 27–68. [Google Scholar] [CrossRef]
- Schiller, P.W. Development of opioid peptide analogs as pharmacologic tools and as potential drugs: Current status and future directions. NIDA Res. Monogr. 1991, 112, 180–197. [Google Scholar] [PubMed]
- Blishchenko, E.Y.; Sazonova, O.V.; Kalinina, O.A.; Yatskin, O.N.; Philippova, M.M.; Surovoy, A.Y.; Karelin, A.A.; Ivanov, V.T. Family of hemorphins: Co-relations between amino acid sequences and effects in cell cultures. Peptides 2002, 23, 903–910. [Google Scholar] [CrossRef]
- Maurer, R.; Römer, D.; Büscher, H.H.; Gähwiler, B.H.; Thies, P.W.; David, S. Valorphin: A novel chemical structure with opioid activity. Neuropeptides 1985, 5, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Erchegyi, J.; Kastin, A.J.; Zadina, J.E.; Qiu, X.D. Isolation of a heptapeptide Val-Val-Tyr-Pro- Trp-Thr-Gln (valorphin) with some opiate activity. Int. J. Pept. Protein Res. 1992, 39, 477–484. [Google Scholar] [CrossRef]
- Blishchenko, E.; Sazonova, O.; Surovoy, A.; Khaidukov, S.; Sheikine, Y.; Sokolov, D.; Ivanov, V. Antiproliferative action of valorphin in cell cultures. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2002, 8, 438–452. [Google Scholar] [CrossRef]
- Blishchenko, E.Y.; Mernenko, O.A.; Mirkina, I.I.; Satpaev, D.K.; Ivanov, V.S.; Tchikin, L.D.; Ivanov, V.T. Tumor cell cytolysis mediated by valorphin, an opioid-like fragment of hemoglobin β-chain. Peptides 1997, 18, 79–85. [Google Scholar] [CrossRef]
- Song, C.; Rahim, R.T.; Davey, P.C.; Bednar, F.; Bardi, G.; Zhang, L.; Zhang, N.; Oppenheim, J.J.; Rogers, T.J. PKC mediates-opioid receptor-induced cross-desensitization of chemokine receptor CCR5. J. Biol. Chem. 2011, 286, 20354–20365. [Google Scholar] [CrossRef]
- Blishchenko, E.Y.; Sazonova, O.V.; Kalinina, O.A.; Moiseeva, E.V.; Vass, A.A.; Karelin, A.A.; Ivanov, V.T. Antitumor effect of valorphin in vitro and in vivo: Combined action with cytostatic drugs. Cancer Biol. Ther. 2005, 4, 125–131. [Google Scholar] [CrossRef]
- Karelin, A.A.; Philippova, M.M.; Karelina, E.V.; Ivanov, V.T. Isolation of endogenous hemorphin-related hemoglobin fragments from bovine brain. Biochem. Biophys. Res. Commun. 1994, 202, 410–415. [Google Scholar] [CrossRef]
- Moeller, I.; Lew, R.A.; Mendelsohn, F.A.; Smith, A.I.; Brennan, M.E.; Tetaz, T.J.; Chai, S.Y. The globin fragment LVV-hemorphin-7 is an endogenous ligand for the AT4 receptor in the brain. J. Neurochem. 1997, 68, 2530–2537. [Google Scholar] [CrossRef]
- Murillo, L.; Piot, J.M.; Coitoux, C.; Fruitier-Arnaudin, I. Brain processing of hemorphin-7 peptides in various subcellular fractions from rats. Peptides 2006, 27, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Moisan, S.; Harvey, N.; Beaudry, G.; Forzani, P.; Burhop, K.E.; Drapeau, G.; Rioux, F. Structural requirements and mechanism of the pressor activity of Leu-Val-Val-hemorphin-7, a fragment of hemoglobin β-chain in rats. Peptides 1998, 19, 119–131. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, L.; Jing, Y. Hemoglobin-derived peptides and mood regulation. Peptides 2020, 127, 170268. [Google Scholar] [CrossRef]
- Hung, H.Y.; Chow, L.H.; Kotlinska, J.H.; Drabik, A.; Silberring, J.; Chen, Y.H.; Huang, E.Y. LVV-hemorphin-7 (LVV-H7) plays a role in antinociception in a rat model of alcohol-induced pain disorders. Peptides 2021, 136, 170455. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Baby, B.; Soman, S.S.; Vijayan, R. Molecular insights into the interaction of hemorphin and its targets. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Alzeyoudi, S.A.R.; Almutawa, S.A.; Alnajjar, A.N.; Al Dhaheri, Y.; Vijayan, R. Camel hemorphins exhibit a more potent angiotensin-I converting enzyme inhibitory activity than other mammalian hemorphins: An in silico and in vitro study. Biomolecules 2020, 10, 486. [Google Scholar] [CrossRef]
- Caballero, J. Considerations for docking of selective angiotensin-converting enzyme inhibitors. Molecules 2020, 25, 295. [Google Scholar] [CrossRef]
- Amanat, A.; Soman, S.S.; Vijayan, R. Dynamics of camel and human hemoglobin revealed by molecular simulations. Sci. Rep. 2022, 12, 122. [Google Scholar] [CrossRef]
- Ojima, I.; Lin, S.; Wang, T. Recent advances in the medicinal chemistry of taxoids with novel beta-amino acid side chains. Curr. Med. Chem. 1999, 6, 927–954. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, U.H.; Raaschou-Nielsen, M.; Breddam, K. Recognition of C-terminal amide groups by (serine) carboxypeptidase Y investigated by site-directed mutagenesis. J. Biol. Chem. 1994, 269, 15528–15532. [Google Scholar] [CrossRef]
- Pogozheva, I.D.; Przydzial, M.J.; Mosberg, H.I. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J. 2005, 7, E434–E448. [Google Scholar] [CrossRef] [PubMed]
- Gademann, K.; Hintermann, T.; Schreiber, J.V. Beta-peptides: Twisting and turning. Curr. Med. Chem. 1999, 6, 905–925. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, F. The chemistry of 2-aminocycloalkanecarboxylic acids. Chem. Rev. 2001, 101, 2181–2204. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S.; Rangelov, M.; Todorova, N. Structure–activity relationship study on new Hemorphin-4 analogues containing steric restricted amino acids moiety for evaluation of their anticonvulsant activity. Amino Acids 2020, 52, 375–1390. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Georgieva, S.; Tchekalarova, J.; Rangelov, M.; Todorova, N. Synthesis and characterization of new 5,5’-dimethyl- and 5,5’-diphenylhydantoin-conjugated hemorphin derivatives designed as potential anticonvulsant agents. New J. Chem. 2022, 46, 2198–2217. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Peneva, P.; Tchekalarova, J. Spectral and electrochemical solvatochromic investigations of newly synthesized peptide-based chemosensor bearing azobenzene side chain bio photoswitch. Dye. Pigment. 2021, 91, 109348. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Synthesis of new modified with Rhodamine B peptides for antiviral protection of textile materials. Molecules 2021, 26, 6608. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Pechlivanova, D.; Georgieva, S.; Dzhambazova, E. Synthesis, characterization and nociceptive screening of new VV-hemorphin-5 analogues. Bioorganic Med. Chem. Lett. 2018, 28, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Rangelov, M.; Peneva, P.; Todorova, N.; Tchekalarova, J. Anticonvulsant evaluation and docking analysis of VV-Hemorphin-5 analogues. Drug Dev. Res. 2019, 80, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Rangelov, M.; Georgieva, S.; Todorova, N. Synthesis, characterization and anticonvulsant activity of new series of N-modified analogues of VV-Hemorphin-5 with aminophosphonate moiety. Amino Acids 2019, 51, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Assenov, B.; Pechlivanova, D.; Dzhambazova, E.; Peneva, P.; Todorov, P. Antinociceptive Effects of VV-Hemorphin-5 Peptide Analogues Containing Aminophosphonate Moiety in Mouse Formalin Model of Pain. Protein Pept. Lett. 2021, 28, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Vasileva-Tonkova, E.; Grabchev, I. Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric. Molecules 2022, 27, 4770. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Peneva, P.; Tchekalarova, J. Investigation of the structure–activity relationship in a series of new LVV-and VV-hemorphin-7 analogues designed as potential anticonvulsant agents. Amino Acids 2022, 54, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.; Todorov, P.; Nikolov, S.; Dzhambazova, E.; Peneva, P.; Assenov, B.; Pechlivanova, D. New N-and C-modified RGD-hemorphins as potential biomedical application on Ti-surface materials: Synthesis, characterization and antinociceptive activity. Mol. Divers. 2022, 1–18. [Google Scholar] [CrossRef]
- De Rosa, T.F. Maximal Electroshock Seizure (MESs) are a model of generalized tonic-clonic seizures. From: Models of Seizures and Epilepsy, 2006. In Significant Pharmaceuticals Reported in US Patents, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-08-045344-6. [Google Scholar] [CrossRef]
- Potschka, H. Animal models of drug-resistant epilepsy. Epileptic Disord. 2012, 14, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Gredičak, M.; Supek, F.; Kralj, M.; Majer, Z.; Hollosi, M.; Smuc, T.; Mlinaric-Majerski, K.; Horvat, Š. Computational structure–activity study directs synthesis of novel antitumor enkephalin analogs. Amino Acids 2010, 38, 1185–1191. [Google Scholar] [CrossRef]
- Löscher, W.; Klotz, U.; Zimprich, F.; Schmidt, D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia 2009, 50, 1–23. [Google Scholar] [CrossRef]
- Alachkar, A.; Ojha, S.K.; Sadeq, A.; Adem, A.; Frank, A.; Stark, H.; Sadek, B. Experimental Models for the Discovery of Novel Anticonvulsant Drugs: Focus on Pentylenetetrazole-Induced Seizures and Associated Memory Deficits. Current Pharm. Design 2020, 26, 1693–1711. [Google Scholar] [CrossRef]
- Prasad, S.; Rao, R.B.; Balaram, P. Contrasting solution conformations of peptides containing α, α-dialkylated residues with linear and cyclic side chains. Biopolym. Orig. Res. Biomol. 1995, 35, 11–20. [Google Scholar] [CrossRef]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed]
- Field, M.J.; Li, Z.; Schwarz, J.B. Ca2+ channel α2-δ ligands for the treatment of neuropathic pain. J. Med. Chem. 2007, 50, 2569–2575. [Google Scholar] [CrossRef]
- Bryans, J.S.; Davies, N.; Gee, N.S.; Dissanayake, V.U.; Ratcliffe, G.S.; Horwell, D.C.; O’Neill, J.A. Identification of novel ligands for the gabapentin binding site on the α2δ subunit of a calcium channel and their evaluation as anticonvulsant agents. J. Med. Chem. 1998, 41, 1838–1845. [Google Scholar] [CrossRef]
- Mart, R.J.; Allemann, R.K. Azobenzene photocontrol of peptides and proteins. Chem. Commun. 2016, 52, 12262–12277. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, W.; Beierle, J.M.; Kistemaker, H.A.; Velema, W.A.; Feringa, B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef] [PubMed]
- Piotto, S.; Trapani, A.; Bianchino, E.; Ibarguren, M.; López, D.J.; Busquets, X.; Concilio, S. The effect of hydroxylated fatty acid-containing phospholipids in the remodeling of lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1509–1517. [Google Scholar] [CrossRef]
- Mendive-Tapia, L.; Preciado, S.; García, J.; Ramón, R.; Kielland, N.; Albericio, F.; Lavilla, R. New peptide architectures through C–H activation stapling between tryptophan–phenylalanine/tyrosine residues. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Chiba, T.; Li, Y.H.; Yamane, T.; Ogikubo, O.; Fukuoka, M.; Arai, R.; Matsui, N. Inhibition of recombinant dipeptidyl peptidase III by synthetic hemorphin-like peptides. Peptides 2003, 24, 773–778. [Google Scholar] [CrossRef]
- Jung, K.Y.; Moon, H.D.; Lee, G.E.; Lim, H.H.; Park, C.S.; Kim, Y.C. Structure- activity relationship studies of spinorphin as a potent and selective human P2X3 receptor antagonist. J. Med. Chem. 2007, 50, 4543–4547. [Google Scholar] [CrossRef]
- Desiderio, C.; D’Angelo, L.; Rossetti, D.V.; Iavarone, F.; Giardina, B.; Castagnola, M.; Di Rocco, C. Cerebrospinal fluid top-down proteomics evidenced the potential biomarker role of LVV-and VV-hemorphin-7 in posterior cranial fossa pediatric brain tumors. Proteomics 2012, 12, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Dakubo, G.D. Brain Cancer Biomarkers in Proximal Fluids. In Cancer Biomarkers in Body Fluids; Springer: Cham, Switzerland, 2019; pp. 211–218. [Google Scholar] [CrossRef]
- Sanderson, K.; Nyberg, F.; Khalil, Z. Modulation of peripheral inflammation by locally administered hemorphin-7. Inflamm. Res. 1998, 47, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, K.R.; Ianzer, D.; Turones, L.C.; Reis, L.L.; Camargo-Silva, G.; Mendonça, M.M.; Xavier, C.H. Behavioral effects evoked by the beta globin-derived nonapeptide LVV-H6. Peptides 2019, 115, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.C.; Tao, P.L.; Cheng, Y.Y.; Huang, E.Y.K. LVV-hemorphin 7 and angiotensin IV in correlation with antinociception and anti-thermal hyperalgesia in rats. Peptides 2012, 36, 9–16. [Google Scholar] [CrossRef]
- Lee, J.; Albiston, A.L.; Allen, A.M.; Mendelsohn, F.A.O.; Ping, S.E.; Barrett, G.L.; Chai, S.Y. Effect of ICV injection of AT4 receptor ligands, NLE1-angiotensin IV and LVV-hemorphin 7, on spatial learning in rats. Neuroscience 2004, 124, 341–349. [Google Scholar] [CrossRef]
- Prabhulkar, S.; Tian, H.; Wang, X.; Zhu, J.J.; Li, C.Z. Engineered proteins: Redox properties and their applications. Antioxid. Redox Signal. 2012, 17, 1796–1822. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Berglund, G.I.; Carlsson, G.H.; Smith, A.T.; Szöke, H.; Henriksen, A.; Hajdu, J. The catalytic pathway of horseradish peroxidase at high resolution. Nature 2002, 417, 463–468. [Google Scholar] [CrossRef]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef]
- Berry, S.M.; Ralle, M.; Low, D.W.; Blackburn, N.J.; Lu, Y. Probing the role of axial methionine in the blue copper center of azurin with unnatural amino acids. J. Am. Chem. Soc. 2003, 125, 8760–8768. [Google Scholar] [CrossRef]
- Bi, Y.H.; Huang, Z.L.; Zhao, Y.D. The interface behavior and biocatalytic activity of superoxide dismutase at carbon nanotube. Biosens. Bioelectron. 2006, 21, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Bixon, M.; Jortner, J. Electron transfer—From isolated molecules to biomolecules. Adv. Chem. Phys. Electron Transf. Isol. Mol. Biomol. Part 1 1999, 106, 35–202. [Google Scholar] [CrossRef]
- Boireau, W.; Bombard, S.; Sari, M.A.; Pompon, D. Bioengineering and characterization of DNA–protein assemblies floating on supported membranes. Biotechnol. Bioeng. 2002, 77, 225–231. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Roscoe, S.G. Electrochemical oxidation reactions of tyrosine, tryptophan and related dipeptides. Electrochim. Acta 1997, 42, 1189–1200. [Google Scholar] [CrossRef]
- Chiku, M.; Nakamura, J.; Fujishima, A.; Einaga, Y. Conformational Change Detection in Nonmetal Proteins by Direct Electrochemical Oxidation Using Diamond Electrodes. Anal. Chem. 2008, 80, 5783–5787. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Stradolini, F.; Abate, G.; Uberti, D.; Serpelloni, M.; Carrara, S.; Sardini, E. Electrochemical detection of different p53 conformations by using nanostructured surfaces. Sci. Rep. 2019, 9, 17347. [Google Scholar] [CrossRef] [PubMed]





| № | Abbreviations Given in Articles | Peptide | Molecular Formula | Biological Activity, Reference |
|---|---|---|---|---|
| Hemorphin-4 analogs | ||||
| 1 | P4-1 | Tyr-Ac5c-Trp-Thr-NH2 | C30H38N6O6 | anticonvulsant activity, [44] |
| 2 | P4-2 | Tyr-Ac6c-Trp-Thr-NH2 | C31H40N6O6 | anticonvulsant activity, [44] |
| 3 | P4-3 | Aaa-Tyr-Pro-Trp-Thr-NH2 | C40H50N6O7 | anticonvulsant activity, [44] |
| 4 | P4-4 | Aaa-Tyr-Ac5c-Trp-Thr-NH2 | C41H52N6O7 | anticonvulsant activity, [44] |
| 5 | P4-5 | Aaa-Tyr-Ac6c-Trp-Thr-NH2 | C42H54N6O7 | anticonvulsant activity, [44] |
| 6 | Dm-4 |  | C36H44N8O9 | anticonvulsant activity, [45] |
| 7 | Ph-4 |  | C46H48N8O9 | anticonvulsant activity, [45] |
| 8 | Az-H4 |  | C43H47N9O7 | anticonvulsant activity, [46] |
| 9 | Rh-1 | rhodamineB-Gly-Tyr-Pro-Trp-Thr-NH2 | C59H69N9O9 | antiviral activity, [47] |
| 10 | Rh-2 | rhodamineB-β-Ala-Tyr-Pro-Trp- Thr-NH2 | C60H71N9O9 | antiviral activity, [47] |
| 11 | Rh-3 | rhodamineB-γ-Abu-Tyr-Pro-Trp-Thr-NH2 | C61H73N9O9 | antiviral activity, [47] |
| Hemorphin-5 analogs | ||||
| 12 | V2/H2 | Val-Val-Tyr-Pro-Trp-Thr-Dap-NH2 | C42H60N10O9 | antinociceptive and anticonvulsant activity, [48,49] |
| 13 | V3/H3 | Val-Val-Tyr-Pro-Trp-Thr-Dab-NH2 | C43H62N10O9 | antinociceptive and anticonvulsant activity, [48,49] |
| 14 | V4/H4 | Val-Val-Tyr-Pro-Trp-Thr-Orn-NH2 | C44H64N10O9 | antinociceptive and anticonvulsant activity, [48,49] |
| 15 | V5/H5 | Val-Val-Tyr-Pro-Trp-Thr-Lys-NH2 | C45H66N10O9 | antinociceptive and anticonvulsant activity, [48,49] |
| 16 | V6/H6 | Ile-Val-Val-Tyr-Pro-Trp-Thr-Gln-NH2 | C50H73N11O11 | antinociceptive and anticonvulsant activity, [48,49] |
| 17 | V7/H7 | Aib-Val-Val-Tyr-Pro-Trp-Thr-Gln-NH2 | C48H69N11O11 | antinociceptive and anticonvulsant activity, [48,49] |
| 18 | V2p |  | C42H60N9O12P | antinociceptive and anticonvulsant activity, [50,51] |
| 19 | V3p |  | C43H62N9O12P | antinociceptive and anticonvulsant activity, [50,51] |
| 20 | V4p |  | C47H69N10O13P | antinociceptive and anticonvulsant activity, [50,51] |
| 21 | V5p |  | C48H71N10O13P | antinociceptive and anticonvulsant activity, [50,51] |
| 22 | V6p |  | C53H80N11O14P | antinociceptive and anticonvulsant activity, [50,51] |
| 23 | Dm-5 |  | C51H70N12O13 | anticonvulsant activity, [45] |
| 24 | Ph-5 |  | C61H74N12O13 | anticonvulsant activity, [45] |
| 25 | C-V | Cys-Val-Val-Tyr-Pro-Trp-Thr-Glu-NH2 | C47H66N10O12S | antiviral and antibacterial activity, [52] |
| 26 | H-V | His-Val-Val-Tyr-Pro-Trp-Thr-Glu-NH2 | C50H68N12O12 | antiviral and antibacterial activity, [52] |
| 27 | AC-V | Aaa-Cys-Val-Val-Tyr-Pro-Trp-Thr-Glu-NH2 | C58H80N10O13S | antiviral and antibacterial activity, [52] |
| 28 | AH-V | Aaa-His-Val-Val-Tyr-Pro-Trp-Thr-Glu-NH2 | C61H82N12O13 | antiviral and antibacterial activity, [52] |
| Hemorphin-7 analogs | ||||
| 29 | 2 | Val-Val-Tyr-Ac5c-Trp-Thr-Gln-Arg-Phe-NH2 | C60H85N15O12 | anticonvulsant activity, [53] |
| 30 | 3 | Val-Val-Tyr-Ac6c-Trp-Thr-Gln-Arg-Phe-NH2 | C61H87N15O12 | anticonvulsant activity, [53] |
| 31 | 4 | Val-Val-Tyr-Pro-Trp-Thr-Dap-Arg-Phe-NH2 | C57H81N15O11 | anticonvulsant activity, [53] |
| 32 | 5 | Val-Val-Tyr-Pro-Trp-Thr-Dab-Arg-Phe-NH2 | C58H83N15O11 | anticonvulsant activity, [53] |
| 33 | 6 | Val-Val-Tyr-Ac5c-Trp-Thr-Dap-Arg-Phe-NH2 | C58H83N15O11 | anticonvulsant activity, [53] |
| 34 | 7 | Val-Val-Tyr-Ac5c-Trp-Thr-Dab-Arg-Phe-NH2 | C59H85N15O11 | anticonvulsant activity, [53] |
| 35 | 8 | Val-Val-Tyr-Ac6c-Trp-Thr-Dap-Arg-Phe-NH2 | C59H85N15O11 | anticonvulsant activity, [53] |
| 36 | 9 | Val-Val-Tyr-Ac6c-Trp-Thr-Dab-Arg-Phe-NH2 | C60H87N15O11 | anticonvulsant activity, [53] |
| 37 | H7-1 | Ile-Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-D-Phe-NH2 | C65H94N16O13 | anticonvulsant activity, [54] |
| 38 | H7-2 | Ile-Val-Tyr-Pro-Trp-Thr-Gln-Arg-D-Phe-NH2 | C60H85N15O12 | anticonvulsant activity, [54] |
| 39 | H7-3 | D-Leu-Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-D-Phe-NH2 | C65H94N16O13 | anticonvulsant activity, [54] |
| 40 | H7-4 | D-Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-D-Phe-NH2 | C59H83N15O12 | anticonvulsant activity, [54] |
| 41 | H7-5 |  | C68H101N16O16P | anticonvulsant activity, [54] |
| 42 | H7-6 |  | C68H101N16O16P | anticonvulsant activity, [54] |
| 43 | H7-7 |  | C62H90N15O15P | anticonvulsant activity, [54] |
| 44 | H7-8 |  | C62H90N15O15P | anticonvulsant activity, [54] |
| 45 | Dm-7 |  | C72H102N18O16 | anticonvulsant activity, [45] |
| 46 | Ph-7 |  | C82H106N18O16 | anticonvulsant activity, [45] |
| 47 | RGD1 | Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-Phe-Arg-Gly-Asp-NH2 | C71H103N21O17 | antinociceptive activity, [55] |
| 48 | RGD2 | Asp-Gly-Arg-Val-Val-Tyr-Pro-Trp-Thr-Gln-Arg-Phe-Arg-Gly-Asp-NH2 | C83H123N27O22 | antinociceptive activity, [55] |
| 49 | NH7C | Nic-Leu-Val-Val-Tyr-Pro-Trp-Thr-Glu-Arg-Phe-Cys-NH2 | C74H101N17O16S | antiviral and antibacterial activity, [52] |
| 50 | NCH7 | Nic-Cys-Leu-Val-Val-Tyr-Pro-Trp-Thr-Glu-Arg-Phe-NH2 | C74H101N17O16S | antiviral and antibacterial activity, [52] |
| Drug | TPE a | ED50 b µg | 95% Confidence Interval | TD50 c | PI d |
|---|---|---|---|---|---|
| (min) | |||||
| Phenytoin | 60 | 4.92 mg.kg−1 | (2.57–9.39) | >100 mg.kg−1 | >20.35 |
| Hemorphin-4 analogs | 10 | ||||
| P4-1 | - | - | - | - | |
| P4-2 | 2.33 | (1.13–4.83) | >10 | >4.29 | |
| P4-3 | 1.66 | (1.24–2.24) | >10 | >6.02 | |
| P4-4 | 2.33 | (1.13–4.83) | >10 | >4.29 | |
| P4-5 | 0.41 | (0.19–0.90) | >10 | >24.39 | |
| Peptide-based chemosensor bearing azobenzene side chain bio photoswitch | 10 | ||||
| Cis Az-H4 | 1.71 | (1.16–2.51) | >10 | >5.85 | |
| Trans A-H4 | 1.51 | (1.04–2.02) | >10 | >6.62 | |
| VV-Hemorphin-5 analogs | 10 | ||||
| V2 | - | - | - | - | |
| V3 | - | - | - | - | |
| V4 | 3.63 | (2.45–5.38) | >20 | >5.51 | |
| V5 | 3.19 | (2.62–3.87) | >20 | >6.27 | |
| V6 | 16.77 | (11.08–25.36) | >20 | >1.19 | |
| V7 | 16.55 | (12.78–21.41) | >20 | >1.21 | |
| 5,5-dimethyl- and 5,5-diphenylhydantoin-conjugated hemorphin derivatives | 10 | ||||
| Dm-4 | 0.36 | (0.13–1.0) | >3 | >8.33 | |
| Dm-5 | 0.74 | (0.06–8.8) | >5 | >6.76 | |
| Dm-7 | 0.7 | (0.05–9.58) | >10 | >14.29 | |
| Ph-4 | 0.56 | (0.06–5.34) | >8 | >14.29 | |
| Ph-5 | 0.25 | (0.10–0.60) | >5 | >20 | |
| LVV- and VV-hemorphin-7 analogs | 10 | ||||
| H7-1 | - | - | - | - | |
| H7-2 | 0.94 | (0.36–2.47) | >8 | >8.51 | |
| H7-3 | 0.68 | (0.19–2.51) | >8 | >11.76 | |
| H7-4 | 2.54 | (1.38–4.64) | >15 | >5.91 | |
| H7-5 | 1.53 | (0.60–3.88) | >3 | >1.96 | |
| H7-6 | 0.38 | (0.13–1.15) | >3 | >7.89 | |
| H7-7 | 1.58 | (0.68–3.70) | >5 | >3.16 | |
| H7-8 | 1.67 | (1.11–2.51) | >7 | >4.19 |
| Drug | TPE a | ED50 b | 95% Confidence Interval | TD50 c | PI d |
|---|---|---|---|---|---|
| (min) | µg | ||||
| Hemorphin-4 analogs | 10 | ||||
| P4-1 | 0.52 | (0.33–0.82) | >5 | >9.62 | |
| P4-2 | 2.16 | (1.87–2.49) | >5 | >2.31 | |
| P4-3 | 0.83 | (0.57–1.19) | >5 | >6.02 | |
| P4-4 | 0.44 | (0.25–0.78) | >5 | >11.36 | |
| P4-5 | 0.64 | (0.40–1.02) | >5 | >7.81 | |
| VV-Hemorphin-5 analogs | 10 | ||||
| V2 | 9.97 | (9.07−10.90) | >20 | 2 | |
| V4 | 5.09 | (4.31–6.02) | >20 | 3.93 | |
| V5 | 9.89 | (8.64–11.34) | >20 | 2.02 | |
| V6 | 5.55 | (5.51–5.58) | >20 | 7.84 | |
| V7 | 6.61 | (6.59–6.62) | >20 | 3.03 | |
| N-modified analogs of VV-hemorphin-5 with aminophosphonate moiety | 10 | ||||
| V2p | |||||
| V3p | 6.47 | (3.96–10.57) | >20 | 3.09 | |
| V4p | 4.31 | (2.76–10.47) | >20 | 4.64 | |
| V5p | 12.55 | (9.26–16.99) | >30 | 2.39 | |
| V6p | 14.11 | (9.17 –21.47) | >40 | 2.83 | |
| 5,5-dimethyl- and 5,5-diphenylhydantoin-conjugated hemorphin derivatives | 10 | ||||
| Dm-4 | 0.53 | (0.38–0.73) | >5 | 9.43 | |
| Dm-5 | 0.64 | (0.40–1.01) | >5 | 7.81 | |
| Dm-7 | 0.54 | (0.26–1.11) | >5 | 9.26 | |
| Ph-4 | 0.22 | (0.13–0.37) | >5 | 22.72 | |
| Ph-5 | 0.27 | (0.11–0.69) | >5 | 18.52 | |
| Ph-7 | 0.23 | (0.10–0.52) | >5 | 21.74 | |
| VV Hemorphin-7 analogs containing unnatural amino acids | 10 | ||||
| VV-H-2 | 5.69 | (3.67–8.81) | >30 | >5.27 | |
| VV-H-3 | 5.69 | (3.67–8.81) | >30 | >5.27 | |
| VV-H-4 | 3.83 | (1.50–9.76) | >20 | >5.22 | |
| VV-H-5 | 0.89 | (0.54–1.46) | >20 | >22.47 | |
| VV-H-6 | 2.67 | (4.67–8.19) | >20 | >7.49 | |
| VV-H-7 | 0.89 | (0.66–2.00) | >20 | >22.47 | |
| VV-H-8 | 1.01 | (0.29–3.55) | >20 | >19.80 | |
| VV-H-9 | 1.09 | (0.40–3.00) | >30 | >27.52 | |
| LVV- and VV-hemorphin-7 analogs | 10 | ||||
| H7-1 | 0.33 | (0.32–0.33) | >5 | >15.15 | |
| H7-2 | 2.6 | (1.58–4.55) | >5 | >1.87 | |
| H7-3 | - | - | - | - | |
| H7-4 | - | - | - | - | |
| H7-5 | 2.16 | (1.70–2.74) | >5 | >2.31 | |
| H7-6 | 2.44 | (1.41–4.21) | >5 | >2.05 | |
| H7-7 | 2.16 | (1.70–2.74) | >5 | >2.31 | |
| H7-8 | 3.03 | (2.44–3.74) | >5 | >1.65 |
| Drug | TPE a | Comparison of Activity Related to the Threshold Dose (µg/10 µL) for Clonic Seizures |
|---|---|---|
| (min) | ||
| Hemorphin-4 analogs | 10 | |
| P4 | ||
| P4-2 | P4-4 = P4-5 > P4-2 = P4-3 > P4 | |
| P4-3 | ||
| P4-4 | ||
| P4-5 | ||
| VV-Hemorphin-5 analogs | 10 | |
| V1 | ||
| V2 | V1 = V4 > V2 | |
| V4 | ||
| V5 | ||
| V6 | ||
| V7 | ||
| N-modified analogs of VV-hemorphin-5 with aminophosphonate moiety | 10 | V1 = V3p |
| V1 | ||
| V2p | ||
| V3p | ||
| V4p | ||
| V5p | ||
| V6p | ||
| Hemorphin-7 analogs containing unnatural amino acids | 10 | VV–H7 = V–H4 |
| VV-H7 | ||
| VV-H-2 | ||
| VV-H-3 | ||
| VV-H-4 | ||
| VV-H-5 | ||
| VV-H-6 | ||
| VV-H-7 | ||
| VV-H-8 | ||
| VV-H-9 | ||
| LVV- and VV-hemorphin-7 analogs | 10 | |
| H7 | H7-5> H7 = H7-3 = H7-6 = H7-7 = H7-8 > H7-1 | |
| H7-1 | ||
| H7-2 | ||
| H7-3 | ||
| H7-4 | ||
| H7-5 | ||
| H7-6 | ||
| H7-7 | ||
| H7-8 |
| Drug | Test | Concentration/Dose | Effect | Reference |
|---|---|---|---|---|
| Endogenous tetrapeptides endomorphin-1 (Tyr-Pro-Trp-Phe-NH2), endomorphin-2 (Tyr-Pro-Phe-Phe-NH2), morphiceptin (Tyr-Pro-Phe-Pro-NH2), hemorphin-4 (Tyr-Pro-Trp-Thr), Tyr-MIF-1 (Tyr-Pro-Leu-Gly-NH2), Tyr-W-MIF-1 (Tyr-Pro-Trp-Gly-NH2), TAPS (Tyr-D-Arg-Phe-Sar), and DALDA (Tyr-D-Arg-Phe-Lys-NH2) | in vitro LC neurons | TAPS IC50 = 1.9 nM > endomorphin-1 (IC50 = 8.8 nM) and endomorphin-2 (IC50 = 5.3 nM) > DALDA IC50 = 20 nM) > morphiceptin (IC50 = 65 nM) > Tyr-W-MIF-I IC50 = 3.8 µM > hemorphin-4 IC50 = 6.7 µM > Tyr-MIF-1 IC50 = 37.5 µM | inhibition of the spontaneous firing | [17] |
| VV-Hemorphin-5 Valorphin (endogenous Hb β-chain (33–39) fragment) Valorphin Valorphin Valorphin | hot plate, tail flick test in mice; Randall–Selitto test in rat in vitro cerebellar Purkinje cells the guinea pig ileum muscle preparation L929 and K562 tumor cells tumor (L929 and A549) cell cultures, primary culture of murine bone marrow cells and in murine model of breast carcinoma in vivo | IC50 of 14 nM IC50 of 10 µM 10−7–10−13 M concentration range +1 µM | opioid analgesic activity binds to rat mu-opioid receptor inhibition of the spontaneous firinginhibition of the electrically induced contractions of mu-opioid receptor Tumor cell cytolysis additive effects 0.5 µM epirubicin added 24 h prior to 1 µM valorphin; 1 µM valorphin added 48 h prior to 0.1 µM epirubicin - 100% cell death | [23] [24] [26] [27] |
| Hemorphin-6 and Hemorphin-7 LVV-hemorphin-7 LVV-hemorphin-7 LVV-hemorphin-7 and alanine-containing derivates of Lev-Val-Val-Hemorphin-7 LVV-hemorphin-6 LVV-hemorphin-7 LVV-hemorphin-7 | (in vitro) distal end of the sciatic nerve radiotelemetry (blood pressure) in WK rats and SHRs Tail-flick and hot plate tests in chronic alcohol-exposed rat model, ELISA Rat blood pressure assay OF, EPM, FST tests in rat carrageenan-induced hyperalgesia at the spinal level Barnes circular maze in rat | 20 and 200 µM 100 µg/kg - ED25 128.0 (nmoles/kg) 153 nmol/kg i.p. 27.2 nmol i.t. 100 pmol | electrical stimulation Reduce blood pressure Decreased plasma level of LVV-hemorphin-7 pressor and tachycardic activities mediated by the SNS anxiolytic and antidepressant effect decrease enhanced spatial learning | [74] [29] [34] [32] [75] [76] [77] |
| Peptide | pKa1; pKa2 Constants | |
|---|---|---|
| Hemorphin-4 Analogs | Method Determination | Constants, Reference |
| P4 | by potentiometric titration | 3.80; 6.44, [44] |
| P4-1 | 3.89; 6.52, [44] | |
| P4-2 | 3.93; 6.71, [44] | |
| P4-3 | 3.88; 6.93, [44] | |
| P4-4 | 6.16; 8.90, [44] | |
| P4-5 | 6.20; 9.06, [44] | |
| Dm-4 | by potentiometric titration | 2.86; [45] |
| Ph-4 | by potentiometric titration | 2.98; [45] |
| Rh-1 | by potentiometric titration | 2.81; 6.60, [47] |
| Rh-2 | 2.78;6.38, [47] | |
| Rh-3 | 2.86;6.39, [47] | |
| Hemorphin-5 analogs | ||
| V2/H2 | by potentiometric titration | 9.23, [48,49] |
| V3/H3 | 8.12, [48,49] | |
| V4/H4 | 7.83, [48,49] | |
| V5/H5 | 8.24, [48,49] | |
| V6/H6 | 8.01, [48,49] | |
| V7/H7 | 8.17, [48,49] | |
| V2p | by potentiometric titration and voltamperometry | 8.93, [50,51] |
| V3p | 8.83, [50,51] | |
| V4p | 7.92, [50,51] | |
| V5p | 8.97, [50,51] | |
| V6p | 9.05, [50,51] | |
| Dm-5 | by potentiometric titration | 3.06; 7.14, [45] |
| Ph-5 | 3.09; 6.98, [45] | |
| C-V | by fluorimetry | 5.18, [52] |
| H-V | 4.75, [52] | |
| AC-V | 5.43, [52] | |
| AH-V | 4.84, [52] | |
| Hemorphin-7 analogs | ||
| 2 | by potentiometric titration | 8.04(Val); 5.34(Tyr), [53] |
| 3 | 7.49(Val); 4.83(Tyr), [53] | |
| 4 | 7.10(Val);5.46(Dap, Dab); 3.14(Tyr), [53] | |
| 5 | 8.08(Val);7.21(Dap, Dab); 5.98(Tyr), [53] | |
| 6 | 8.15(Val);6.87(Dap, Dab); 4.72(Tyr), [53] | |
| 7 | 8.21(Val);7.26(Dap, Dab); 4.70(Tyr), [53] | |
| 8 | 9.20(Val);8.03(Dap, Dab); 5.27(Tyr), [53] | |
| 9 | 9.08(Val);8.80(Dap, Dab); 4.66(Tyr), [53] | |
| H7-1 | by potentiometric titration | 2.98; 6.12, [54] |
| H7-2 | 3.09; 6.62, [54] | |
| H7-3 | 3.05; 6.78, [54] | |
| H7-4 | 3.22; 6.52, [54] | |
| H7-5 | 3.17; 6.23, [54] | |
| H7-6 | 2.98; 5.85, [54] | |
| H7-7 | 3.15; 6.09, [54] | |
| H7-8 | 2.78; 5.52, [54] | |
| Dm-7 | by potentiometric titration | 3.19; 5.11, [45] |
| Ph-7 | 3.23; 6.45, [45] | |
| RGD1 | by potentiometric titration | 3.53; 6.42, [55] |
| RGD2 | 3.48; 6.34, [55] | |
| NH7C | by fluorimetry | 5.07, [52] |
| NCH7 | 4.78, [52] | |
| Peptide | Working Electrode/Electrolyte | Ep,a [V] | Ep,c [V] | Nature of the Process, Reference | |
|---|---|---|---|---|---|
| Hemorphin-4 Analogs | Electrod | Electrolyte | |||
| Dm-4 | Hg(HMDE) | Methanol/ tetrabutylamonium persulfat (0.043 mol L−1) | 0.050 | - | IR [45] |
| Ph-4 | Hg(HMDE) | Ep1 = 0.116 Ep2 = −0.181 Ep3 = −0.657 | - Ep2 = −0.171 Ep3 = −0.518 | R [45] | |
| Az-H4 | Hg(HMDE) | (1) pH 6.86 (phosphate-buffered solution, 0.1 mol L−1)(2) AcCN | (1) −0.547 (2) −0.603 | (1) 0.458 (2) 0.355 | (1) R * [46] (2) R [46] |
| Hemorphin-5 analogs | |||||
| V2/H2 | Pt-working electrode SW | phosphates buffer at pH 6.86, | 0.385 0.217 | - −0.106 | QR [48,49] |
| V3/H3 | 0.355 0.156 | - −0.175 | QR [48,49] | ||
| V4/H4 | 0.400 0.171 | - −0.045 | QR [48,49] | ||
| V5/H5 | 0.370 0.187 | - −0.060 | QR [48,49] | ||
| V6/H6 | 0.370 0.170 | - −0.045 | QR [48,49] | ||
| V7/H7 | 0.370 0.171 | - −0.075 | QR [48,49] | ||
| V2p | glass carbonic (GC) electrode SW | Phosphate-buffered solution (pH 6.86) | −0.708 −0.0476 0.315 0.661 | 0.00488 −0.465 −0.751 | [50,51] |
| V3p | −0.708 −0.0476 0.315 −0.661 | 0.00488 −0.465 −0.751 | [50,51] | ||
| V4p | −0.661 0.143 0.411 | −0.013 0.727 - | [50,51] | ||
| V5p | −0.576 0.638 | −0.0534 −0.708 | [50,51] | ||
| V6p | −0.436 0.254 0.526 | 0.144 −0.732 - | [50,51] | ||
| Dm-5 | Hg(HMDE) | Methanol/ tetrabutylamonium persulfat (0.043 mol L−1) | Ep1 = 0.105 Ep1 = −0.252 ** | Ep1 = 0.270 | QR[45] |
| Ph-5 | Hg(HMDE) | Ep1 = 0.186 Ep1 = −0.439 ** | Ep1 = 374 | QR[45] | |
| C-V | glass-carbon (GC) | phosphate-buffered solution at (1) pH 6.87 and (2) 7.34 DPP | (1) −0.625(Trp) −1.26(Tyr) (2) −0.625(Trp) −1.26(Tyr) 1.82(Cys) | - | IR [52] |
| H-V | (1) −0.661(Trp) −1.297(Tyr) (2) −0.661(Trp) −1.297(Tyr) 1.82(His) | - | IR [52] | ||
| AC-V | (1) −0.619(Trp) −1.19(Tyr) (2) −0.619(Trp) −1.19(Tyr) 1.75 (Cys) | - | IR [52] | ||
| AH-V | (1) −0.631(Trp) −1.270(tyr) (2) −0.631(Trp) −1.270(tyr) 1.78(His) | - | IR [52] | ||
| Hemorphin-7 analogs | |||||
| 2 | (1) HMDE electrode (SW) (2) Au electrode (SW) | LiOH/LiCl, pH = 10.65 | (1) −1.70 (2) 0.179 1.27 | (1) −1.74 (2) −0.0563 0.501 | (1) R [53] |
| 3 | (1) −1.74 (2) 0.179 1.27 | (1) −1.80 (2) −0.0563 0.501 | (1) R [53] | ||
| 4 | (1) −1.72 (2) 0.179 | (1) −1.80 (2) −0.0592 | (1) R [53] | ||
| 5 | (1) −1.73 (2) 0.179 | (1) −1.78 (2) −0.0592 | (1) R [53] | ||
| 6 | (1) −1.71 (2) 0.160 | (1) −1.76 (2) −0.0560 −0.219 0.341 | (1) R [53] | ||
| 7 | (1) −1.66 (2) −0.0465 0.569 0.733 1.27 | (1) −1.70 (2) 0.453 0.304 −0.220 −0.0560 | (1) R [53] | ||
| 8 | (1) −1.75 (2) 0.179 | (1) −1.76 (2) −0.0392 0.441 | (1) R [53] | ||
| 9 | (1) −1.73 (2) 0.179 | (1) −1.78 (2) −0.0592 0.461 | (1) R [53] | ||
| H7-1 | glass carbonic (GC) electrode DPP | phosphate-buffered solution at pH 7.04 | 0.762 | - | IR [54] |
| H7-2 | 0.762 | - | IR [54] | ||
| H7-3 | 0.756 | - | IR [54] | ||
| H7-4 | 0.696 | - | IR [54] | ||
| H7-5 | 0.756 | - | IR [54] | ||
| H7-6 | 0.756 | - | IR [54] | ||
| H7-7 | 0.756 | - | IR [54] | ||
| H7-8 | 0.756 | - | IR [54] | ||
| Dm-7 | Hg(HMDE) | Methanol/tetrabutylamonium persulfat (0.043 mol L−1) | Ep1 = 0.093 Ep2 = −0.608 | - Ep2 = −0.470 | IR [45] |
| Ph-7 | Hg(HMDE) | Ep1 = 0.106 Ep2 = −0.618 | - Ep1 = −607 | R [45] | |
| RGD1 | Hg(HMDE) (CV) | Methanol/tetrabutylamonium persulfat (0.043 mol L-1) | −0.44 | −0.54 | R[55] |
| RGD2 | Hg(HMDE) (CV) | −0.460 | −0.560 | R[55] | |
| NH7C | glass-carbon (GC) DPP | phosphate-buffered solution at (1) pH 6.87 and (2) 7.34 | (1) −0.518(Trp) −1.196(Tyr) (2) −0.518(Trp) −1.196(Tyr) | - | (1) QR [52] |
| NCH7 | (1) −0.613(Trp) −1.17(Tyr) (2) −0.613(Trp) −1.17(Tyr) | - | (1) QR [52] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, P.; Georgieva, S.; Tchekalarova, J. Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance. Pharmaceuticals 2022, 15, 1425. https://doi.org/10.3390/ph15111425
Todorov P, Georgieva S, Tchekalarova J. Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance. Pharmaceuticals. 2022; 15(11):1425. https://doi.org/10.3390/ph15111425
Chicago/Turabian StyleTodorov, Petar, Stela Georgieva, and Jana Tchekalarova. 2022. "Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance" Pharmaceuticals 15, no. 11: 1425. https://doi.org/10.3390/ph15111425
APA StyleTodorov, P., Georgieva, S., & Tchekalarova, J. (2022). Recent Synthesis, Characterization, and Pharmacological Evaluation of Multifunctional Hemorphins Containing Non-Natural Amino Acids with Potential Biological Importance. Pharmaceuticals, 15(11), 1425. https://doi.org/10.3390/ph15111425








