Antibody-Based In Vivo Imaging of Central Nervous System Targets—Evaluation of a Pretargeting Approach Utilizing a TCO-Conjugated Brain Shuttle Antibody and Radiolabeled Tetrazines
Abstract
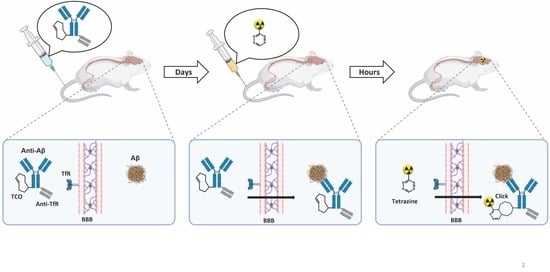
1. Introduction
2. Results
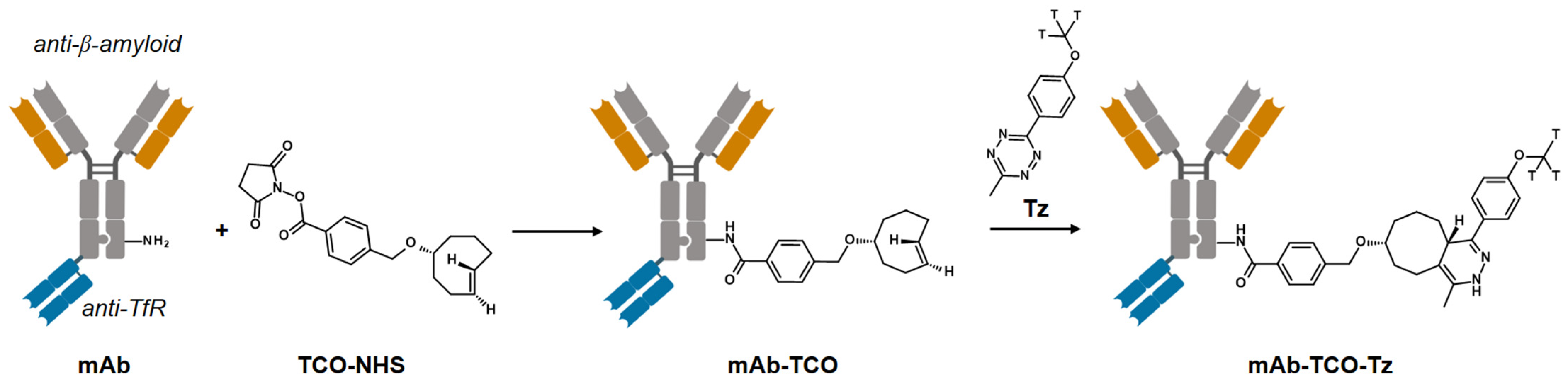
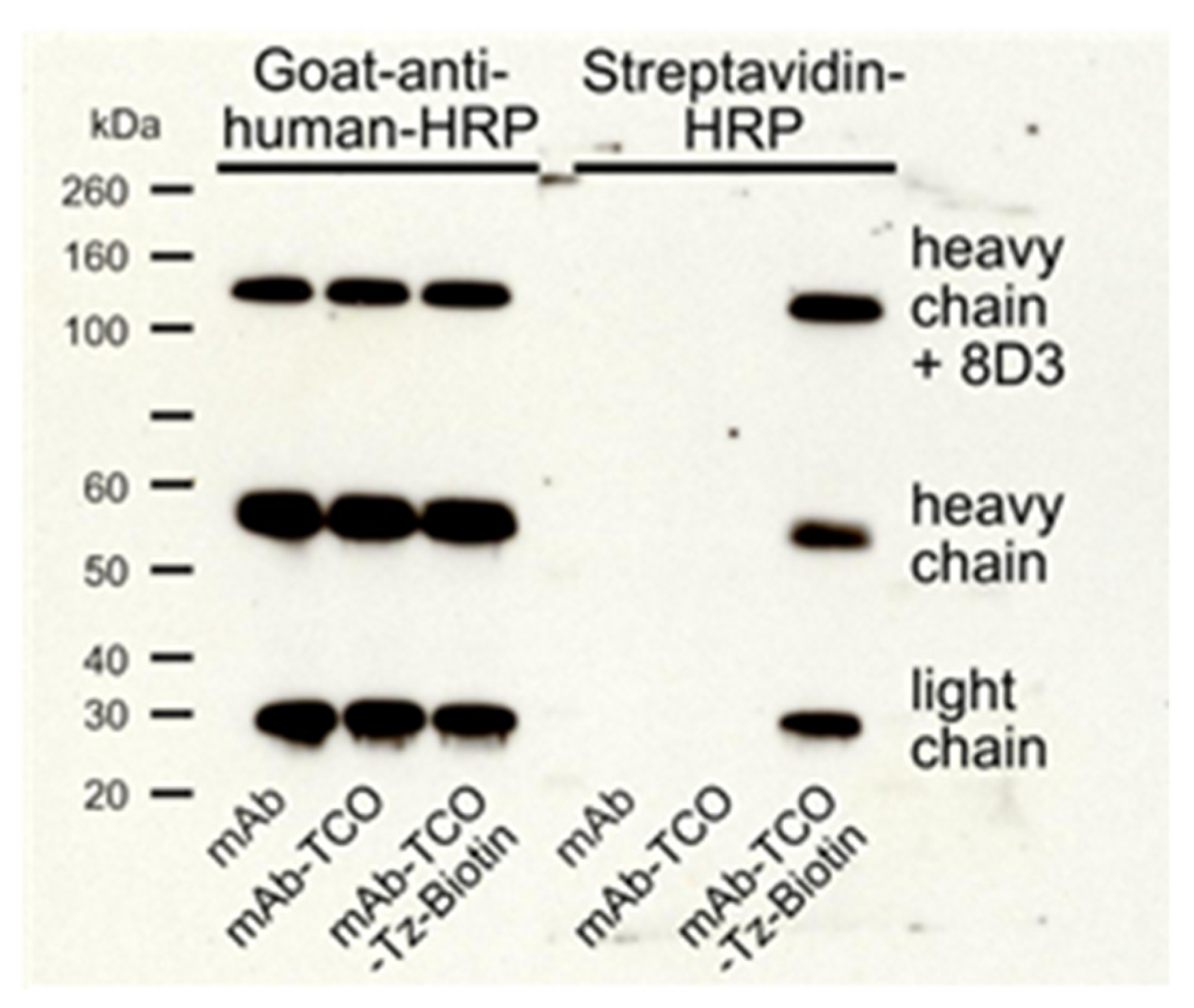
2.1. TCO Modification and Radiolabeling of the Antibody Construct
2.2. Retained Antibody Binding to Aβ Plaques after TCO modification
2.3. TCO Modified Antibody Retains Binding to Transferrin Receptor and Enters the Brain
2.4. Conjugated TCO Remains Reactive towards Tz after In Vivo Injection
2.5. In Vivo Click Reaction Did Not Reveal a Specific Radioactive Signal in the Brain
3. Discussion
4. Materials and Methods
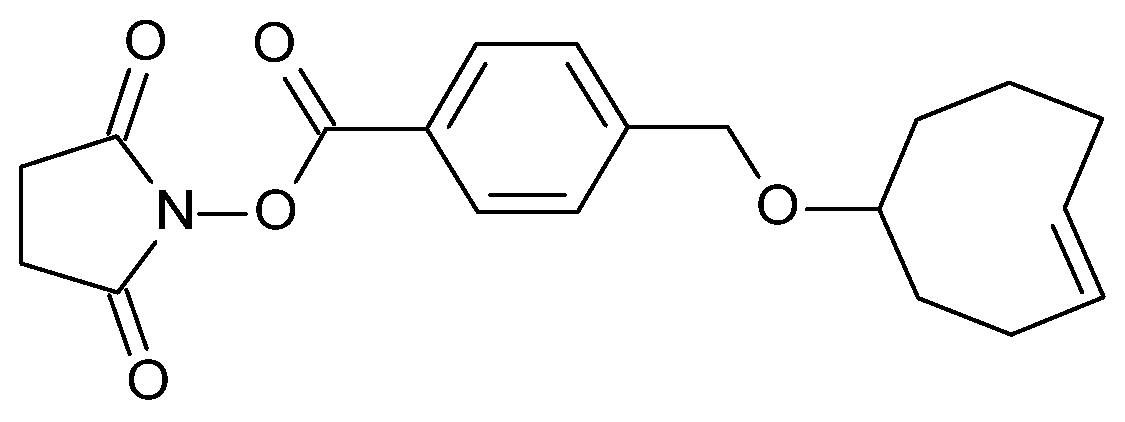
4.1. Antibody–TCO Conjugation
4.2. In Vitro Click Reaction
4.3. Intact Protein Mass Spectrometry (MS) Analysis
4.4. SDS-PAGE and Western Blot Analysis
4.5. Aβ ELISA Affinity Assay
4.6. Fluorescence-Activated Cell Sorting (FACS) Flow Cytometry Analysis
4.7. In Vivo Mouse Experiments
4.8. In Vivo Target Engagement
4.9. mAb–TCO Administration for Ex Vivo Click Reaction
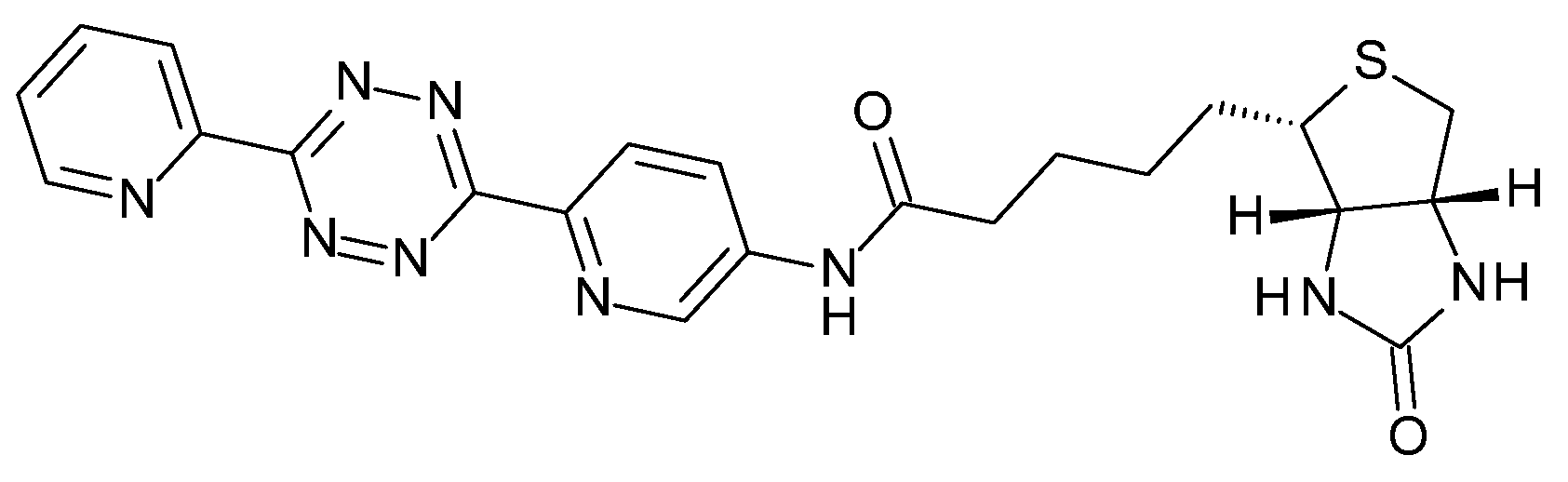
4.10. In Vivo Click Reaction
4.11. Immunohistochemistry, Radio-Immunohistochemistry
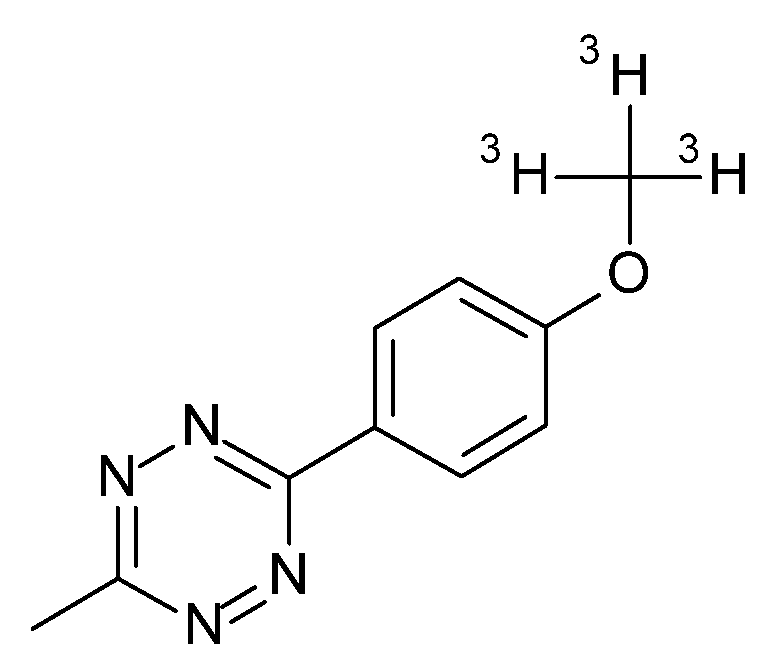
4.12. Radioactive Assays, Autoradiography, and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Houghton, J.L.; Evans, M.J.; Viola-Villegas, N.; Lewis, J.S. Underscoring the influence of inorganic chemistry on nuclear imaging with radiometals. Inorg. Chem. 2014, 53, 1880–1899. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Goldenberg, D.M. Cancer radioimmunotherapy. Immunotherapy 2011, 3, 349–370. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.D.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A pretargeted PET imaging strategy based on bioorthogonal Diels–Alder click chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Brand, C.; Abdel-Atti, D.; Carnazza, K.E.; Cook, B.E.; Carlin, S.; Reiner, T.; Lewis, J.S. Optimization of a pretargeted strategy for the PET imaging of colorectal carcinoma via the modulation of radioligand pharmacokinetics. Mol. Pharm. 2015, 12, 3575–3587. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- Moro, M.; Pelagi, M.; Fulci, G.; Paganelli, G.; Dellabona, P.; Casorati, G.; Siccardi, A.G.; Corti, A. Tumor cell targeting with antibody-avidin complexes and biotinylated tumor necrosis factor α. Cancer Res. 1997, 57, 1922–1928. [Google Scholar] [PubMed]
- Yao, Z.; Zhang, M.; Kobayashi, H.; Sakahara, H.; Nakada, H.; Yamashina, I.; Konishi, J. Improved targeting of radiolabeled streptavidin in tumors pretargeted with biotinylated monoclonal antibodies through an avidin chase. J. Nucl. Med. 1995, 36, 837–841. [Google Scholar]
- Liu, G.; Dou, S.; Liu, Y.; Wang, Y.; Rusckowski, M.; Hnatowich, D.J. 90Y labeled phosphorodiamidate morpholino oligomer for pretargeting radiotherapy. Bioconjug. Chem. 2011, 22, 2539–2545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossin, R.; Renart Verkerk, P.; van den Bosch, S.M.; Vulders, R.C.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted immuno-PET of pancreatic cancer: Overcoming circulating antigen and internalized antibody to reduce radiation doses. J. Nucl. Med. 2016, 57, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; van Duijnhoven, S.M.; Läppchen, T.; van den Bosch, S.M.; Robillard, M.S. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels–Alder reaction. Mol. Pharm. 2014, 11, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.E.; Adumeau, P.; Membreno, R.; Carnazza, K.E.; Brand, C.; Reiner, T.; Agnew, B.J.; Lewis, J.S.; Zeglis, B.M. Pretargeted PET imaging using a site-specifically labeled immunoconjugate. Bioconjug. Chem. 2016, 27, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Mandikian, D.; Rafidi, H.; Adhikari, P.; Venkatraman, P.; Nazarova, L.; Fung, G.; Figueroa, I.; Ferl, G.Z.; Ulufatu, S.; Ho, J. Site-specific conjugation allows modulation of click reaction stoichiometry for pretargeted SPECT imaging. Mabs-Austin 2018, 10, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Sarrett, S.M.; Keinänen, O.; Dayts, E.J.; Roi, D.-L.; Rodriguez, C.; Carnazza, K.E.; Zeglis, B.M. Inverse electron demand Diels–Alder click chemistry for pretargeted PET imaging and radioimmunotherapy. Nat. Protoc. 2021, 16, 3348–3381. [Google Scholar] [CrossRef]
- Rossin, R.; Versteegen, R.M.; Wu, J.; Khasanov, A.; Wessels, H.J.; Steenbergen, E.J.; Ten Hoeve, W.; Janssen, H.M.; van Onzen, A.H.; Hudson, P.J. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Li, H.; Conde, J.; Guerreiro, A.; Bernardes, G.J. Tetrazine Carbon Nanotubes for Pretargeted In Vivo “Click-to-Release” Bioorthogonal Tumour Imaging. Angew. Chem. Int. Ed. 2020, 59, 16023–16032. [Google Scholar] [CrossRef]
- Handula, M.; Chen, K.-T.; Seimbille, Y. IEDDA: An Attractive Bioorthogonal Reaction for Biomedical Applications. Molecules 2021, 26, 4640. [Google Scholar] [CrossRef]
- Darko, A.; Wallace, S.; Dmitrenko, O.; Machovina, M.M.; Mehl, R.A.; Chin, J.W.; Fox, J.M. Conformationally strained trans-cyclooctene with improved stability and excellent reactivity in tetrazine ligation. Chem. Sci. 2014, 5, 3770–3776. [Google Scholar] [CrossRef]
- Debets, M.F.; Van Berkel, S.S.; Dommerholt, J.; Dirks, A.J.; Rutjes, F.P.; Van Delft, F.L. Bioconjugation with strained alkenes and alkynes. Acc. Chem. Res. 2011, 44, 805–815. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels− Alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Devaraj, N.K. Inverse Electron-Demand Diels–Alder Bioorthogonal Reactions. In Cycloadditions in Bioorthogonal Chemistry Topics in Current Chemistry Collections; Vrabel, M., Carell, T., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 109–130. [Google Scholar]
- Han, H.-S.; Devaraj, N.K.; Lee, J.; Hilderbrand, S.A.; Weissleder, R.; Bawendi, M.G. Development of a bioorthogonal and highly efficient conjugation method for quantum dots using tetrazine− norbornene cycloaddition. J. Am. Chem. Soc. 2010, 132, 7838–7839. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Hilderbrand, S.; Upadhyay, R.; Mazitschek, R.; Weissleder, R. Bioorthogonal turn-on probes for imaging small molecules inside living cells. Angew. Chem. 2010, 122, 2931–2934. [Google Scholar] [CrossRef]
- Haun, J.B.; Devaraj, N.K.; Hilderbrand, S.A.; Lee, H.; Weissleder, R. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat. Nanotechnol. 2010, 5, 660–665. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand diels–alder click chemistry. Bioconjug. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Schoch, J.; Staudt, M.; Samanta, A.; Wiessler, M.; Jäschke, A. Site-specific one-pot dual labeling of DNA by orthogonal cycloaddition chemistry. Bioconjug. Chem. 2012, 23, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Asare-Okai, P.; Agustin, E.; Fabris, D.; Royzen, M. Site-specific fluorescence labelling of RNA using bio-orthogonal reaction of trans-cyclooctene and tetrazine. Chem. Commun. 2014, 50, 7844–7847. [Google Scholar] [CrossRef] [PubMed]
- Reiner, T.; Lacy, J.; Keliher, E.J.; Yang, K.S.; Ullal, A.; Kohler, R.H.; Vinegoni, C.; Weissleder, R. Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia 2012, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, H.; Hassink, M.; Blackman, M.L.; Brown, R.C.; Conti, P.S.; Fox, J.M. Tetrazine–trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 2010, 46, 8043–8045. [Google Scholar] [CrossRef]
- Syvanen, S.; Fang, X.T.; Faresjo, R.; Rokka, J.; Lannfelt, L.; Olberg, D.E.; Eriksson, J.; Sehlin, D. Fluorine-18-labeled antibody ligands for PET imaging of amyloid-β in brain. ACS Chem. Neurosci. 2020, 11, 4460–4468. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L.; Berg, C.T. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc. Natl. Acad. Sci. USA 1994, 91, 5705–5709. [Google Scholar] [CrossRef] [PubMed]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.-L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Yu, Y.J.; Atwal, J.K.; Zhang, Y.; Tong, R.K.; Wildsmith, K.R.; Tan, C.; Bien-Ly, N.; Hersom, M.; Maloney, J.A.; Meilandt, W.J. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014, 6, 261ra154. [Google Scholar] [CrossRef]
- Syvänen, S.; Fang, X.T.; Hultqvist, G.; Meier, S.R.; Lannfelt, L.; Sehlin, D. A bispecific Tribody PET radioligand for visualization of amyloid-beta protofibrils–a new concept for neuroimaging. Neuroimage 2017, 148, 55–63. [Google Scholar] [CrossRef]
- Gustavsson, T.; Syvänen, S.; O’Callaghan, P.; Sehlin, D. SPECT imaging of distribution and retention of a brain-penetrating bispecific amyloid-β antibody in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Hersom, M.; Helms, H.C.; Pretzer, N.; Goldeman, C.; Jensen, A.I.; Severin, G.; Nielsen, M.S.; Holm, R.; Brodin, B. Transferrin receptor expression and role in transendothelial transport of transferrin in cultured brain endothelial monolayers. Mol. Cell. Neurosci. 2016, 76, 59–67. [Google Scholar] [CrossRef]
- Moos, T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J. Comp. Neurol. 1996, 375, 675–692. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Bohrmann, B.; Niewoehner, J.; Fischer, J.A.; Rueger, P.; Tiefenthaler, G.; Moelleken, J.; Bujotzek, A.; Brady, K.; Singer, T. Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 2018, 22, 149–162. [Google Scholar] [CrossRef]
- Richards, J.G.; Higgins, G.A.; Ouagazzal, A.-M.; Ozmen, L.; Kew, J.N.; Bohrmann, B.; Malherbe, P.; Brockhaus, M.; Loetscher, H.; Czech, C. PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J. Neurosci. 2003, 23, 8989–9003. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, A.; Petersen, I.N.; Herth, M.M.; Kristensen, J.L. Novel Tetrazine Compounds for in vivo Imaging. WO2020/108720. A1 Patent WO2020/108720, 04 June 2020. [Google Scholar]
- Rossin, R.; Läppchen, T.; Van Den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels–Alder reaction for tumor pretargeting: In vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Billaud, E.M.; Belderbos, S.; Cleeren, F.; Maes, W.; Van de Wouwer, M.; Koole, M.; Verbruggen, A.; Himmelreich, U.; Geukens, N.; Bormans, G. Pretargeted PET imaging using a bioorthogonal 18F-labeled trans-cyclooctene in an ovarian carcinoma model. Bioconjug. Chem. 2017, 28, 2915–2920. [Google Scholar] [CrossRef]
- Rossin, R.; Van Den Bosch, S.M.; Ten Hoeve, W.; Carvelli, M.; Versteegen, R.M.; Lub, J.; Robillard, M.S. Highly reactive trans-cyclooctene tags with improved stability for Diels–Alder chemistry in living systems. Bioconjug. Chem. 2013, 24, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.R.; Hauri, S. Functional in vitro assessment of modified antibodies: Impact of label on protein properties. PLoS ONE 2021, 16, e0257342. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.; Ty, N.; Bequignat, J.-B.; Quintana, M.; Briat, A.; Witkowski, T.; Bouchon, B.; Boucheix, C.; Miot-Noirault, E.; Pouget, J.-P. Antibody PEGylation in bioorthogonal pretargeting with trans-cyclooctene/tetrazine cycloaddition: In vitro and in vivo evaluation in colorectal cancer models. Sci. Rep. 2017, 7, 14918. [Google Scholar] [CrossRef]
- Cook, B.E.; Membreno, R.; Zeglis, B.M. Dendrimer scaffold for the amplification of in vivo pretargeting ligations. Bioconjug. Chem. 2018, 29, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Membreno, R.; Keinänen, O.M.; Cook, B.E.; Tully, K.M.; Fung, K.C.; Lewis, J.S.; Zeglis, B.M. Toward the optimization of click-mediated pretargeted radioimmunotherapy. Mol. Pharm. 2019, 16, 2259–2263. [Google Scholar] [CrossRef]
- Maggi, A.; Ruivo, E.; Fissers, J.; Vangestel, C.; Chatterjee, S.; Joossens, J.; Sobott, F.; Staelens, S.; Stroobants, S.; Van Der Veken, P. Development of a novel antibody–tetrazine conjugate for bioorthogonal pretargeting. Org. Biomol. Chem. 2016, 14, 7544–7551. [Google Scholar] [CrossRef] [PubMed]
- Billaud, E.M.; Shahbazali, E.; Ahamed, M.; Cleeren, F.; Noël, T.; Koole, M.; Verbruggen, A.; Hessel, V.; Bormans, G. Micro-flow photosynthesis of new dienophiles for inverse-electron-demand Diels–Alder reactions. Potential applications for pretargeted in vivo PET imaging. Chem. Sci. 2017, 8, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Jørgensen, J.T.; Denk, C.; Battisti, U.M.; Nørregaard, K.; Edem, P.E.; Bratteby, K.; Shalgunov, V.; Wilkovitsch, M.; Svatunek, D. Lipophilicity and click reactivity determine the performance of bioorthogonal tetrazine tools in pretargeted in vivo chemistry. ACS Pharmacol. Trans. Sci. 2021, 4, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Myrhammar, A.; Vorobyeva, A.; Westerlund, K.; Yoneoka, S.; Orlova, A.; Tsukahara, T.; Tolmachev, V.; Karlström, A.E.; Altai, M. Evaluation of an antibody-PNA conjugate as a clearing agent for antibody-based PNA-mediated radionuclide pretargeting. Sci. Rep. 2020, 10, 20777. [Google Scholar] [CrossRef]
- Freskgård, P.-O.; Urich, E. Antibody therapies in CNS diseases. Neuropharmacology 2017, 120, 38–55. [Google Scholar] [CrossRef]
- van den Broek, S.L.; Shalgunov, V.; Sehlin, D.; Syvanen, S.; Herth, M. Development of trans-cyclooctene modified antibodies for pretargeted imaging within the central nervous system. J. Nucl. Med. 2020, 61, 196. [Google Scholar]
- Meyer, J.-P.; Tully, K.M.; Jackson, J.; Dilling, T.R.; Reiner, T.; Lewis, J.S. Bioorthogonal masking of circulating antibody–TCO groups using tetrazine-functionalized dextran polymers. Bioconjug. Chem. 2018, 29, 538–545. [Google Scholar] [CrossRef]
- Bohrmann, B.; Baumann, K.; Benz, J.; Gerber, F.; Huber, W.; Knoflach, F.; Messer, J.; Oroszlan, K.; Rauchenberger, R.; Richter, W.F. Gantenerumab: A novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimers Dis. 2012, 28, 49–69. [Google Scholar] [CrossRef]
- Muri, D.; Edelmann, M.R. Tools for work-up and prepurification of tritium-labeled small molecules. J. Label. Compds. Radiopharm. 2018, 61, 912–915. [Google Scholar] [CrossRef]


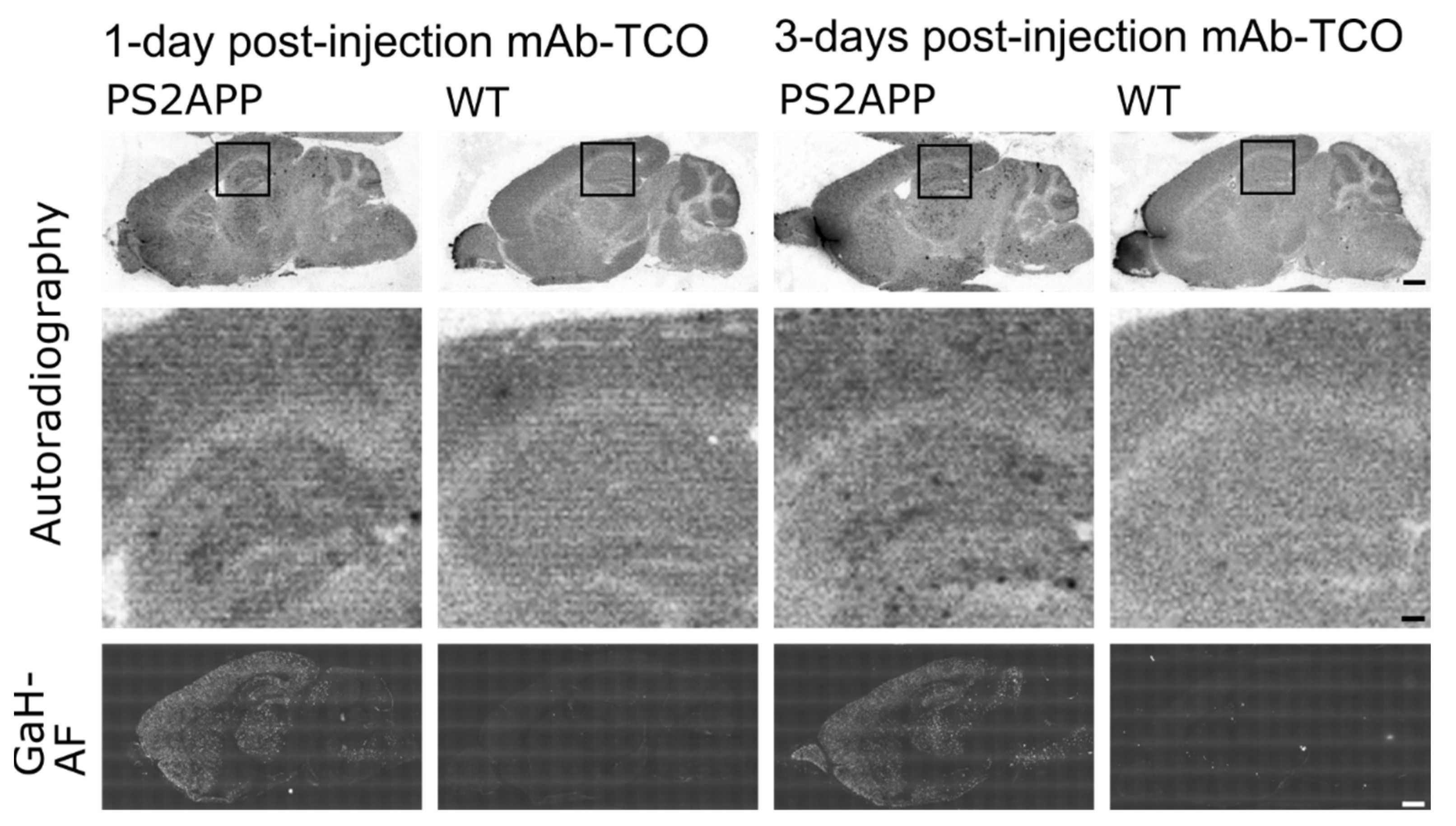
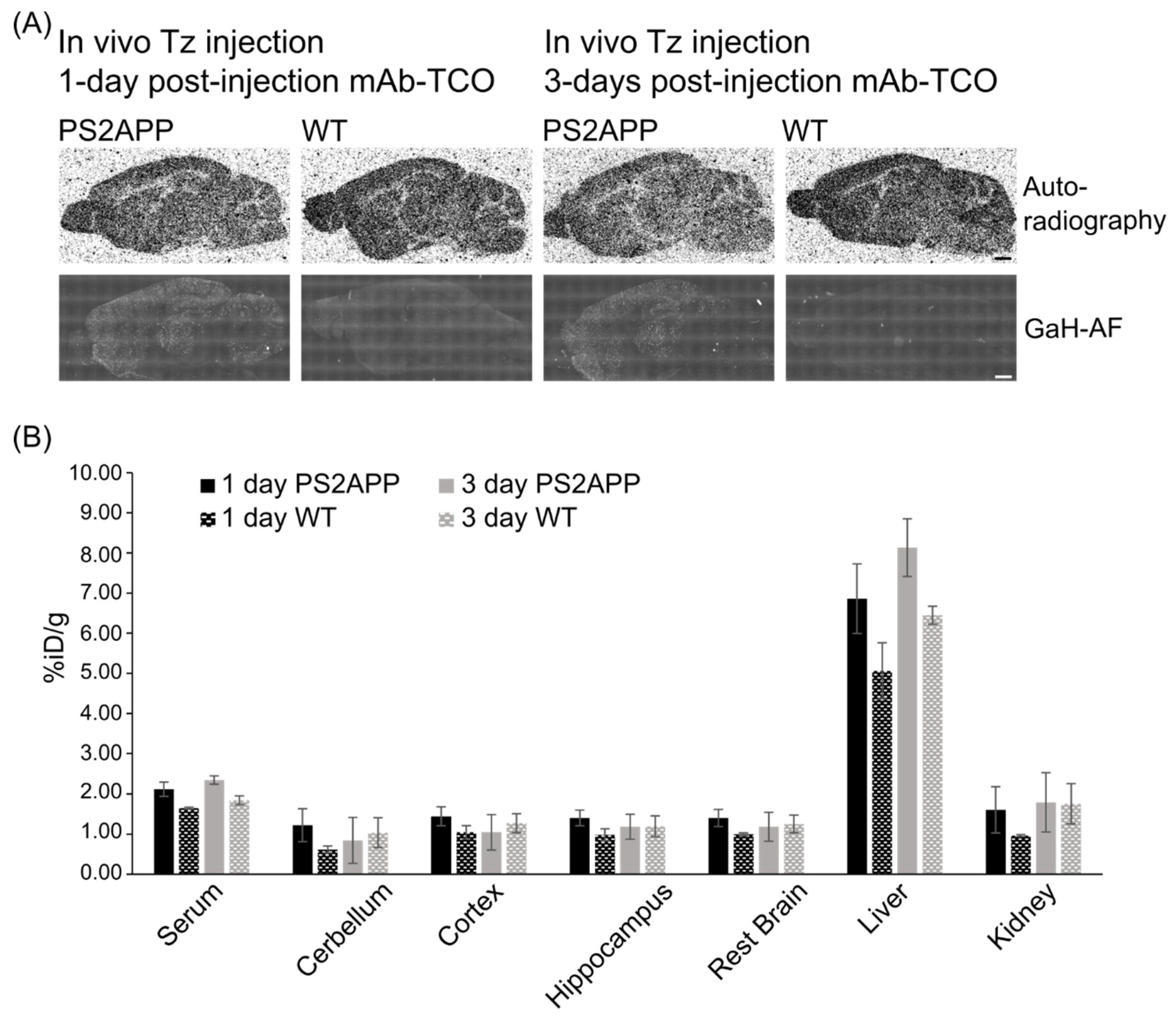
| Plaque Density | 1-Day Post-Injection | 3-Days Post-Injection | ||
|---|---|---|---|---|
| PS2APP | WT | PS2APP | WT | |
| Cortex | 12.40 ± 2.13 | 3.63 ± 0.95 | 14.55 ± 3.27 | 3.30 ± 0.49 |
| Hippocampus | 12.12 ± 1.42 | 1.85 ± 0.47 | 11.84 ± 3.00 | 1.77 ± 0.44 |
| Thalamus | 9.45 ± 0.96 | 1.26 ± 0.40 | 9.15 ± 1.34 | 0.66 ± 0.18 |
| Experiment | Animals Used | Antibody Injected In Vivo | Time (d) * | Tz Administered | |
|---|---|---|---|---|---|
| WT | PS2APP | ||||
| Brain Uptake | 1× | mAb–TCO–Tz | 3 | in vitro, in vial | |
| 2× | mAb–TCO–Tz | 12 | in vitro, in vial | ||
| In Vivo Stability of mAb–TCO | 1× | 1× | mAb–TCO | 1 | ex vivo, on slide |
| 1× | 1× | mAb–TCO | 3 | ex vivo, on slide | |
| 1× | 1× | mAb–TCO | 6 | ex vivo, on slide | |
| 1× | 1× | mAb–TCO | 12 | ex vivo, on slide | |
| In Vivo Click Reaction | 2× | 3× | mAb–TCO | 1 | in vivo, intravenous |
| 3× | 3× | mAb–TCO | 3 | in vivo, intravenous | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredack, C.; Edelmann, M.R.; Borroni, E.; Gobbi, L.C.; Honer, M. Antibody-Based In Vivo Imaging of Central Nervous System Targets—Evaluation of a Pretargeting Approach Utilizing a TCO-Conjugated Brain Shuttle Antibody and Radiolabeled Tetrazines. Pharmaceuticals 2022, 15, 1445. https://doi.org/10.3390/ph15121445
Bredack C, Edelmann MR, Borroni E, Gobbi LC, Honer M. Antibody-Based In Vivo Imaging of Central Nervous System Targets—Evaluation of a Pretargeting Approach Utilizing a TCO-Conjugated Brain Shuttle Antibody and Radiolabeled Tetrazines. Pharmaceuticals. 2022; 15(12):1445. https://doi.org/10.3390/ph15121445
Chicago/Turabian StyleBredack, Christoph, Martin R. Edelmann, Edilio Borroni, Luca C. Gobbi, and Michael Honer. 2022. "Antibody-Based In Vivo Imaging of Central Nervous System Targets—Evaluation of a Pretargeting Approach Utilizing a TCO-Conjugated Brain Shuttle Antibody and Radiolabeled Tetrazines" Pharmaceuticals 15, no. 12: 1445. https://doi.org/10.3390/ph15121445
APA StyleBredack, C., Edelmann, M. R., Borroni, E., Gobbi, L. C., & Honer, M. (2022). Antibody-Based In Vivo Imaging of Central Nervous System Targets—Evaluation of a Pretargeting Approach Utilizing a TCO-Conjugated Brain Shuttle Antibody and Radiolabeled Tetrazines. Pharmaceuticals, 15(12), 1445. https://doi.org/10.3390/ph15121445