A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
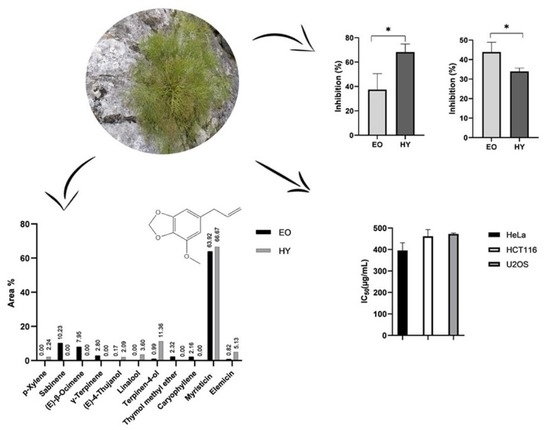
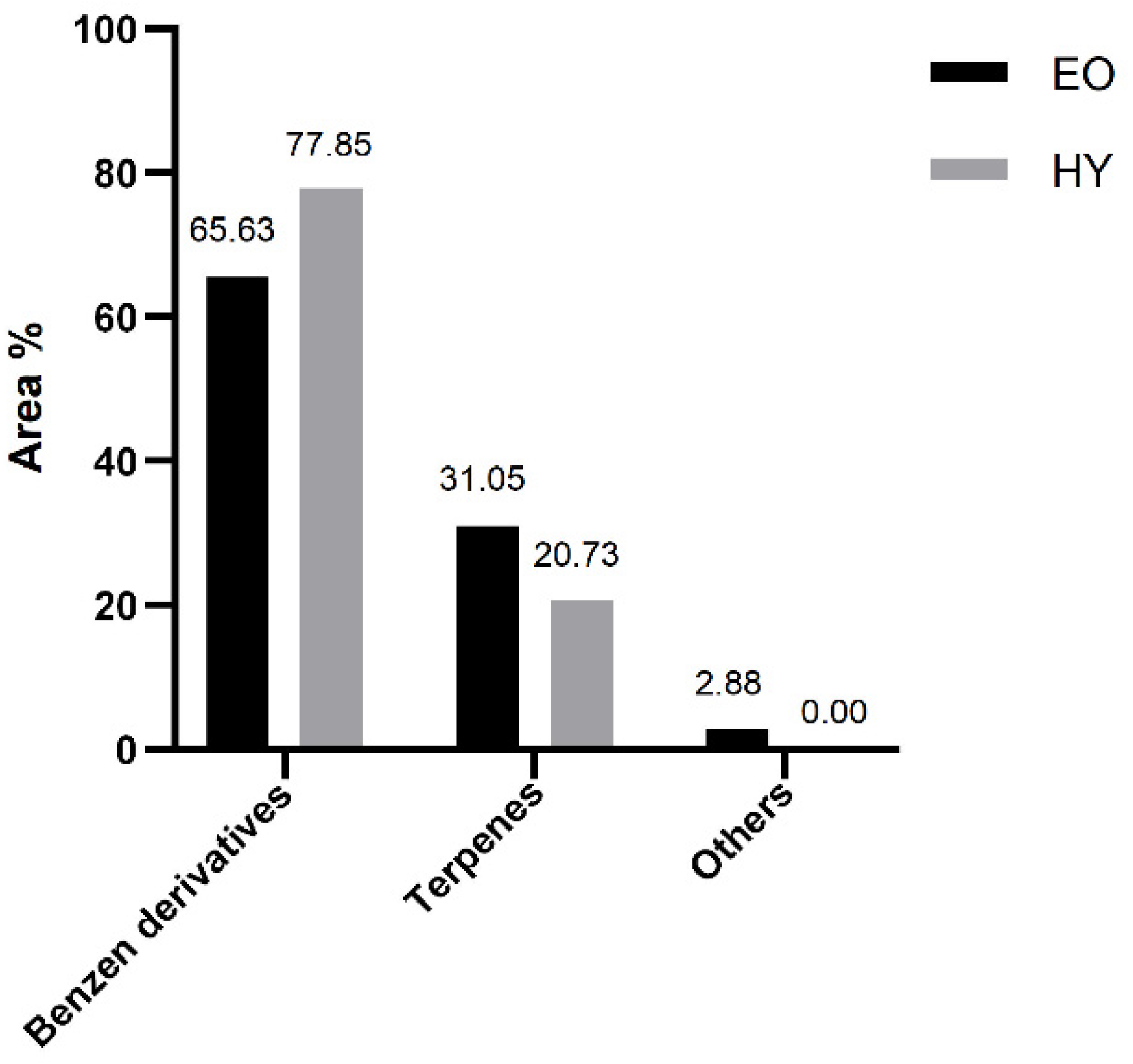
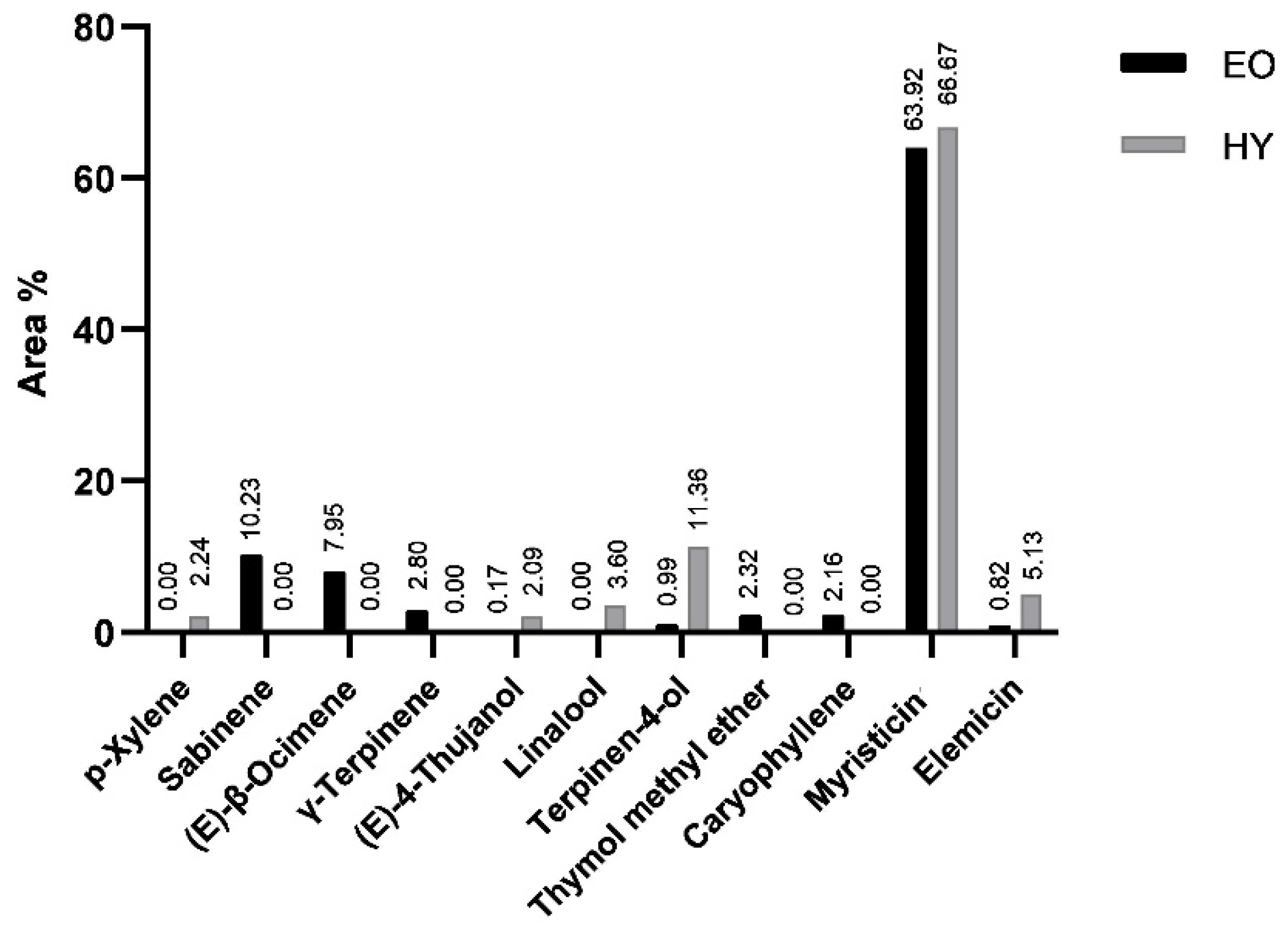
2.1. Composition of Volatile Organic Compounds Obtained by Hydrodistillation—Essential Oil and Hydrosol
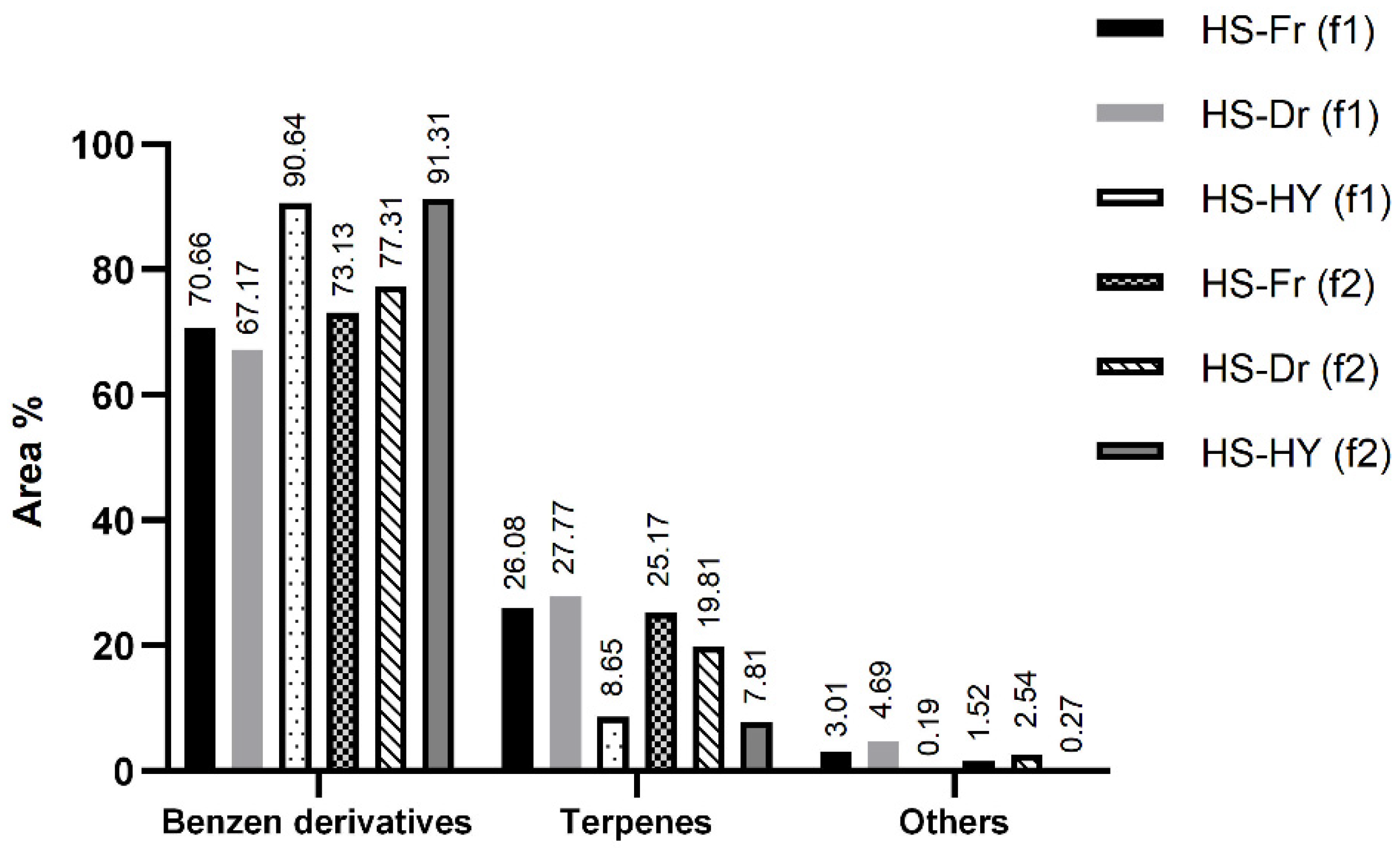
2.2. Headspace Composition of the Volatile Organic Compounds Isolated by HS-SPME—Plant and Hydrosol
2.3. Non-Target Screening of Non-Volatile Compounds in Methanol Extract
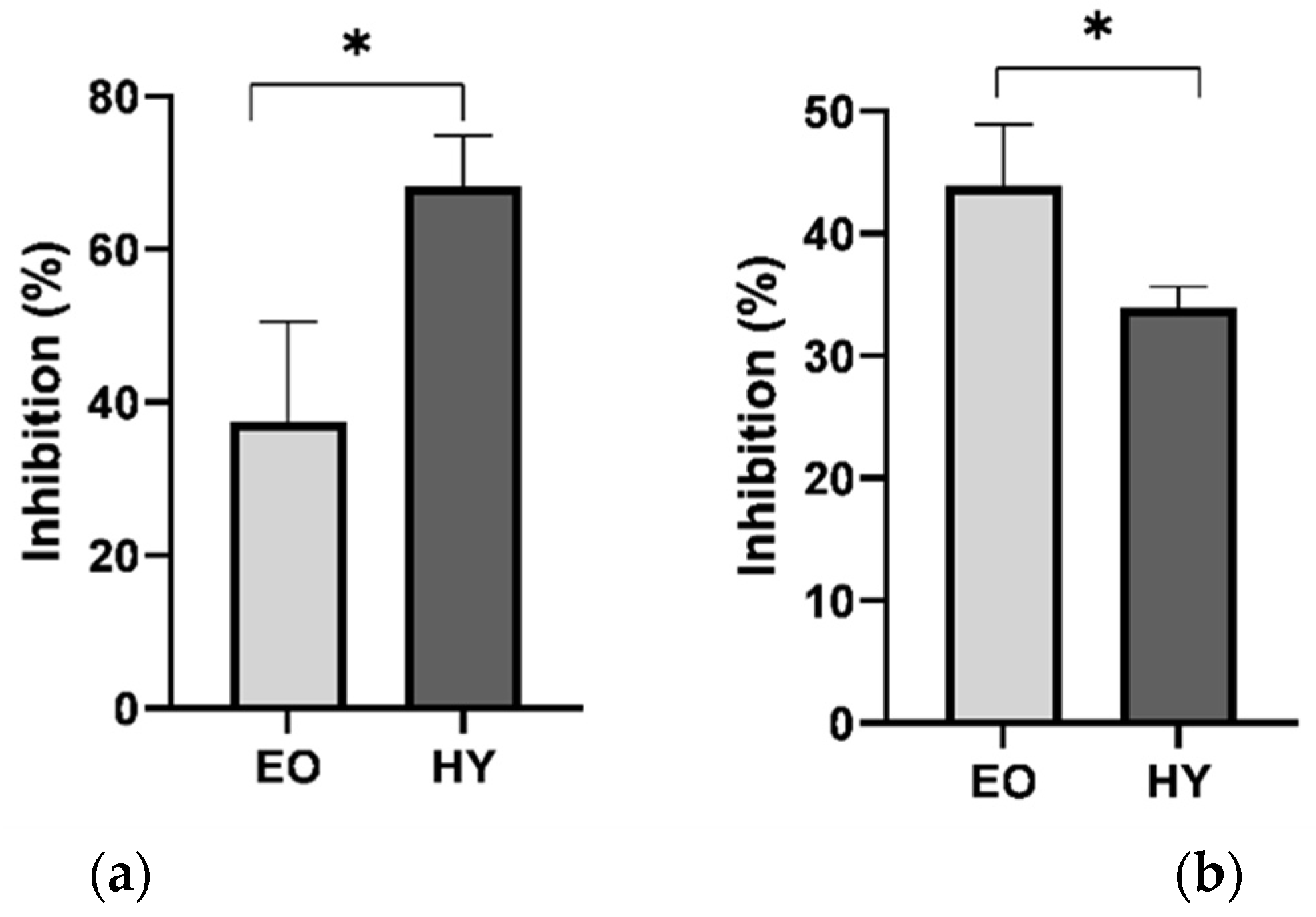
2.4. Antiproliferative Activity of Hydrosol and Methanol Extract of P. ramosissima
2.5. Antiphytoviral Activity
3. Materials and Methods
3.1. Herbal Material
3.2. Hydrodistillation
3.3. Methanol Extract
3.4. Headspace Solid-Phase Microextraction (HS-SPME)
3.5. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
3.6. Ultra High-Performance Liquid Chromatography—High-Resolution Mass Spectrometry (UHPLC-ESI-HRMS) of Methanol Extract
3.7. Antiproliferative Analysis
3.8. Antiphytoviral Activity Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolić, T. Sistematska Botanika: Raznolikost i Evolucija Biljnog Svijeta; Alfa d.d.: Zagreb, Croatia, 2013; pp. 1–882. [Google Scholar]
- Nikolić, T. Flora Croatica: Vaskularna Flora Republike Hrvatske; Ključevi za determinaciju s pratećim Podatcima: Magnoliidae-porodice FAG-ZYG; Alfa d.d.: Zagreb, Croatia, 2020; Volume 3, pp. 1–704. [Google Scholar]
- Maleš, Ž.; Plazibat, M.; Bučar, F. Essential Oil of Portenschlagiella ramosissima from Croatia, a Rich Source of Myristicin. Croat. Chem. Acta 2009, 82, 725–728. [Google Scholar]
- Soković, M.D.; Glamočlija, J.; Ćirić, A.; Grubišić, D.; Stojković, D.; Ristić, M. Antimicrobial Activity of Essential Oils Isolated from Different Parts of Endemic Plant Portenschlagiella ramosissima Tutin. J. Essent. Oil Res. 2011, 20, 369–372. [Google Scholar] [CrossRef]
- Beeby, E.; Magalhães, M.; Poças, J.; Collins, T.; Lemos, M.F.L.; Barros, L.; Ferreira, I.C.F.R.; Cabral, C.; Pires, I.M. Secondary metabolites (essential oils) from sand-dune plants induce cytotoxic effects in cancer cells. J. Ethnopharmacol. 2020, 258, 112803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Isman, M.B.; Tak, J.H. Insecticidal Activity of 28 Essential Oils and a Commercial Product Containing Cinnamomum cassia Bark Essential Oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamočlija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuko, E.; Rusak, G.; Dunkić, V.; Kremer, D.; Kosalec, I.; Rađa, B.; Bezić, N. Inhibition of Satellite RNA Associated Cucumber Mosaic Virus Infection by Essential Oil of Micromeria croatica (Pers.) Schott. Molecules 2019, 24, 1342. [Google Scholar] [CrossRef] [Green Version]
- Bishop, C.D. Antiviral activity of the essential oil of Melaleuca alternifolia (Maiden amp; Betche) cheel (tea tree) against tobacco mosaic virus. J. Essent. Oil Res. 1995, 7, 641–644. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Ušjak, L.J.; Petrović, S.D.; Drobac, M.M.; Soković, M.D.; Stanojković, T.P.; Ćirić, A.D.; Grozdanić, N.; Niketić, M.S. Chemical Composition, Antimicrobial and Cytotoxic Activity of Heracleum verticillatum Pančić and H. ternatum Velen. (Apiaceae) Essential Oils. Chem. Biodivers. 2016, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic Essential Oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) Growing in Central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, S.; Znati, M.; Jabrane, A.; Casanova, J.; Ben Jannet, H. Chemical Composition, Antimicrobial, Anti-acetylcholinesterase and Cytotoxic Activities of the Root Essential oil from the Tunisian Ferula lutea (Poir.) Maire (Apiaceae). J. Essent. Oil-Bearing Plants 2016, 19, 897–906. [Google Scholar] [CrossRef]
- Karakaya, S.; Özdemir, Ö.; Koca, M.; Demirci, B.; Aksakal, Ö.; Turkez, H.; Baser, K.H.C. Cytotoxic effect and molecular docking studies of essential oils of Cymbocarpum erythraeum (DC.) Boiss. (Apiaceae) as potential inhibitors of cholinesterase. J. Essent. Oil Res. 2020, 32, 436–448. [Google Scholar] [CrossRef]
- Önder, A.; Çinar, A.S.; Bakar-Ateş, F.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Chemical composition and cytotoxic potency of essential oil from Seseli petraeum M. Bieb. (Apiaceae). J. Res. Pharm. 2021, 25, 249–257. [Google Scholar] [CrossRef]
- Jamalova, D.N.; Gad, H.A.; Akramov, D.K.; Tojibaev, K.S.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. Discrimination of the Essential Oils Obtained from Four Apiaceae Species Using Multivariate Analysis Based on the Chemical Compositions and Their Biological Activity. Plants 2021, 10, 1529. [Google Scholar] [CrossRef]
- Gomaa, S.E.; Friedersdorf, M.; El Enshasy, H.A.; Abou-Donia, M.B. In vitro Comparative Study for Anti-proliferative Activity of Some Plant Extracts, Fam. Apiaceae, on HeLa Cell Line. Indones. J. Pharm. 2020, 31, 108. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamočilja, J.; Soković, M.; Jekő, J.; Cziáky, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Baradaran, B.; Orangi, M.; Zamani, F. In vitro Cytotoxic Activity of Four Plants Used in Persian Traditional Medicine. Adv. Pharm. Bull. 2013, 3, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Prakash, E.; Gupta, D.K. Cytotoxic Activity of Ethanolic Extract of Cuminum cyminum Linn against Seven Human Cancer Cell Line. Univers. J. Agric. Res. 2014, 2, 27–30. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae Vegetables Carrot, Celery, Fennel, Parsley, and Parsnip and Their Cytotoxic Activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, L.V.; Napier, J.A.; Molino, D.; Faure, J.D. Plant sphingolipids: Their importance in cellular organization and adaption. Biochim. Biophys. Acta 2016, 1861, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, Y.; Kronzucker, H.J.; Shi, W. Quantification and enzyme targets of fatty acid amides from duckweed root exudates involved in the stimulation of denitrification. J. Plant Physiol. 2016, 198, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Groenbaek, M.; Tybirk, E.; Neugart, S.; Sundekilde, U.K.; Schreiner, M.; Kristensen, H.L. Flavonoid glycosides and hydroxycinnamic acid derivatives in baby leaf rapeseed from white and yellow flowering cultivars with repeated harvest in a 2-years field study. Front. Plant Sci. 2019, 10, 355. [Google Scholar] [CrossRef]
- Nocedo-Mena, D.; Rivas-Galindo, V.M.; Navarro, P.; Garza-González, E.; González-Maya, L.; Ríos, M.Y.; García, A.; Ávalos-Alanís, F.G.; Rodríguez-Rodríguez, J.; del Rayo Camacho-Corona, M. Antibacterial and cytotoxic activities of new sphingolipids and other constituents isolated from Cissus incisa leaves. Heliyon 2020, 6, e04671. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-Glucoside and Peonidin 3-Glucoside Inhibit Tumor Cell Growth and Induce Apoptosis In Vitro and Suppress Tumor Growth In Vivo. Nutr. Cancer. 2005, 53, 232–243. [Google Scholar] [CrossRef]
- Vuko, E.; Dunkić, V.; Ruščić, M.; Nazlić, M.; Mandić, N.; Soldo, B.; Šprung, M.; Fredotović, Ž. Chemical Composition and New Biological Activities of Essential Oil and Hydrosol of Hypericum perforatum L. ssp. veronense (Schrank) H. Lindb. Plants 2021, 10, 1014. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol Composition of Extracts of the Fruits of Laserpitium Krapffii Crantz and Their Antioxidant and Cytotoxic Activity. Antioxidants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Cinar, A.S.; Bakar-Ates, F.; Onder, A. Seseli petraeum M. Bieb. (Apiaceae) Significantly Inhibited Cellular Growth of A549 Lung Cancer Cells through G0/G1 Cell Cycle Arrest. An. Acad. Bras. Cienc. 2020, 92, 1–11. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, M.M.; Prajapati, V.; Kumar, A.K. Insecticidal Activity of Myristicin from Piper mullesua. Pharm. Biol. 2001, 39, 226–229. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Flora Europaea Search Results. Available online: https://websites.rbge.org.uk/cgi-bin/nph-readbtree.pl/feout?GENUS_XREF=Portenschlagiella&SPECIES_XREF=ramosissima (accessed on 10 November 2022).
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome. Pharmaceuticals 2022, 15, 743. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium × cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef]
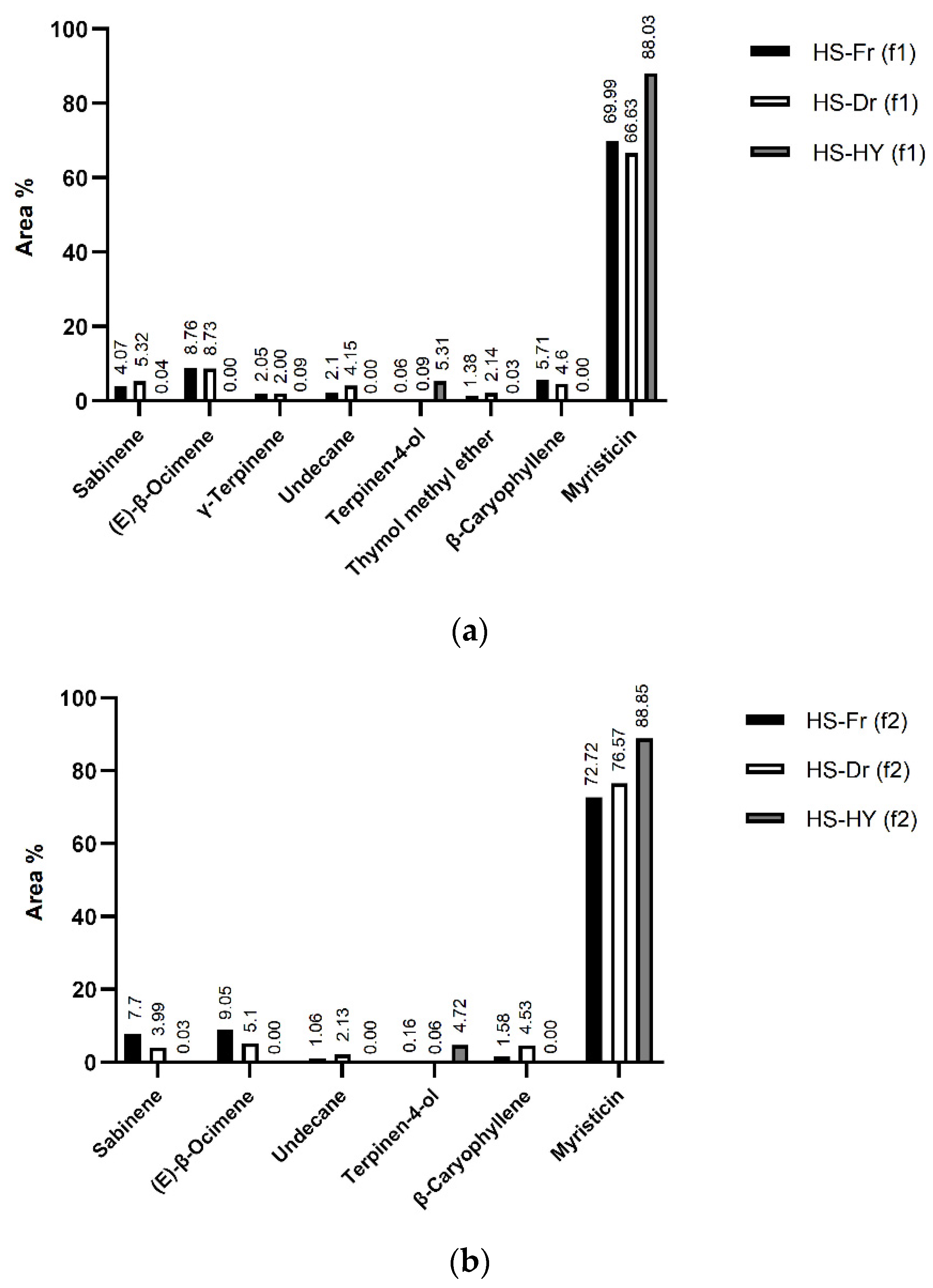

| No. | Compound | RI | Average Area% ± SD | |
|---|---|---|---|---|
| EO | HY | |||
| 1 | Furfural | <900 | - | 0.40 ± 0.08 |
| 2 | (E)-Hex-2-enal | <900 | 0.03 ± 0.03 | 0.11 ± 0.03 |
| 3 | Ethylbenzene | <900 | - | 0.56 ± 0.00 |
| 4 | p-Xylene | <900 | - | 2.24 ± 0.04 |
| 5 | Nonane | 901 | 0.48 ± 0.10 | - |
| 6 | (Z)-Non-2-ene | 917 | 0.02 ± 0.00 | - |
| 7 | α-Thujene | 934 | 0.12 ± 0.03 | - |
| 8 | α-Pinene | 942 | 0.06 ± 0.01 | - |
| 9 | Benzaldehyde | 966 | 0.01 ± 0.00 | 0.18 ± 0.02 |
| 10 | Sabinene | 982 | 10.23 ± 0.33 | - |
| 11 | β-Pinene | 985 | 0.26 ± 0.06 | - |
| 12 | β-Myrcene | 994 | 0.68 ± 0.13 | - |
| 13 | Decane | 1001 | 0.02 ± 0.00 | - |
| 14 | α-Phellanderene | 1009 | 0.01 ± 0.00 | - |
| 15 | δ-3-Carene | 1016 | 0.01 ± 0.00 | - |
| 16 | α-Terpinene | 1023 | 0.34 ± 0.08 | - |
| 17 | p-Cymene | 1031 | 0.85 ± 0.18 | - |
| 18 | β-Phellandrene | 1036 | 0.09 ± 0.02 | - |
| 19 | Eucalyptole | 1038 | 0.01 ± 0.00 | |
| 20 | Benzyl alcohol | 1040 | - | 0.25 ± 0.01 |
| 21 | (E)-β-Ocimene | 1045 | 7.95 ± 0.12 | - |
| 22 | Phenylacetaldehyde | 1051 | 0.03 ± 0.01 | 0.86 ± 0.01 |
| 23 | (Z)-β-Ocimene | 1054 | 0.29 ± 0.06 | - |
| 24 | γ-Terpinene | 1066 | 2.80 ± 0.38 | - |
| 25 | 2-Methyldecane | 1068 | 0.01 ± 0.00 | - |
| 26 | (E)-4-Thujanol | 1073 | 0.17 ± 0.04 | 2.09 ± 0.19 |
| 27 | Linalool oxide | 1078 | - | 0.10 ± 0.01 |
| 28 | α-Terpinolene | 1092 | 0.25 ± 0.05 | 0.18 ± 0.03 |
| 29 | Linalool | 1101 | - | 3.60 ± 0.23 |
| 30 | Undecane | 1102 | 1.72 ± 0.31 | - |
| 31 | 2,6-Dimethylcyclohexan-1-ol | 1113 | - | 0.27 ± 0.05 |
| 32 | 2-Phenylethanol | 1118 | - | 0.41 ± 0.04 |
| 33 | (Z)-p-Menthen-2-en-1-ol | 1127 | 0.07 ± 0.02 | 0.97 ± 0.12 |
| 34 | (Z)-Alloocimene | 1134 | 0.11 ± 0.03 | - |
| 35 | Terpinen-1-ol | 1146 | 0.03 ± 0.01 | 0.68 ± 0.07 |
| 36 | p-Methoxystyrene | 1158 | 0.01 ± 0.00 | - |
| 37 | Sabine ketone | 1164 | - | 0.10 ± 0.02 |
| 38 | Terpinen-4-ol | 1183 | 0.99 ± 0.20 | 11.36 ± 0.59 |
| 39 | p-Cymen-8-ol | 1189 | - | 0.27 ± 0.00 |
| 40 | α-Terpineol | 1194 | 0.04 ± 0.01 | 0.77 ± 0.03 |
| 41 | (Z)-Dec-4-enal | 1197 | 0.06 ± 0.02 | - |
| 42 | (Z)-Piperitol | 1198 | - | 0.14 ± 0.01 |
| 43 | Dodecane | 1202 | 0.04 ± 0.01 | - |
| 44 | (3Z,5Z)-2,6-Dimethylocta-3,5,7-trien-2-ol | 1204 | 0.02 ± 0.01 | 0.12 ± 0.02 |
| 45 | Decanal | 1209 | 0.06 ± 0.01 | - |
| 46 | (3E,5E)-2,6-Dimethylocta-3,5,7-trien-2-ol | 1213 | 0.04 ± 0.01 | - |
| 47 | (E)-Piperitol | 1213 | - | 0.35 ± 0.04 |
| 48 | Benzothiazole | 1228 | - | 0.35 ± 0.03 |
| 49 | Thymol methyl ether | 1241 | 2.32 ± 0.72 | - |
| 50 | Carvacrol methyl ether | 1250 | 0.03 ± 0.01 | - |
| 51 | Dec-4-en-1-ol | 1264 | 0.06 ± 0.03 | - |
| 52 | 2-Phenylbut-2-enal | 1277 | - | 0.11 ± 0.01 |
| 53 | (Z)-Tridec-3-ene | 1295 | 0.01 ± 0.00 | - |
| 54 | Indole | 1296 | - | 0.09 ± 0.01 |
| 55 | Tridecane | 1302 | 0.01 ± 0.01 | - |
| 56 | Thymol | 1306 | 0.01 ± 0.00 | - |
| 57 | Undecanal | 1310 | 0.01 ± 0.01 | - |
| 58 | p-Vinylguaiacol (4-Ethenyl-2-methoxyphenol) | 1318 | 0.01 ± 0.00 | 0.39 ± 0.10 |
| 59 | Cyclosativene | 1372 | 0.02 ± 0.01 | - |
| 60 | α-Copaene | 1380 | 0.03 ± 0.01 | - |
| 61 | β-Elemene | 1395 | 0.02 ± 0.01 | - |
| 62 | Methyleugenol | 1409 | 0.72 ± 0.09 | 0.45 ± 0.03 |
| 63 | Dodecanal | 1413 | 0.27 ± 0.05 | - |
| 64 | β-Funebrene | 1416 | 0.06 ± 0.01 | - |
| 65 | Caryophyllene | 1424 | 2.16 ± 0.36 | - |
| 66 | α-Gurjunene | 1429 | 0.03 ± 0.01 | - |
| 67 | β-Gurjunene | 1434 | 0.01 ± 0.00 | - |
| 68 | α-Humulene | 1458 | 0.22 ± 0.05 | - |
| 69 | β-Farnesene | 1462 | 0.04 ± 0.01 | - |
| 70 | γ-Curcumene | 1471 | 0.01 ± 0.00 | - |
| 71 | γ-Muurolene | 1478 | 0.03 ± 0.00 | - |
| 72 | Germacrene D | 1485 | 0.35 ± 0.09 | 0.06 ± 0.01 |
| 73 | Germacrene C | 1499 | 0.05 ± 0.01 | - |
| 74 | α-Muurolene | 1503 | 0.06 ± 0.02 | - |
| 75 | Myristicin | 1538 | 63.92 ± 3.80 | 66.67 ± 1.45 |
| 76 | Elemicin (1,2,3-Trimethoxy-5-prop-2-enylbenzene) | 1566 | 0.82 ± 0.03 | 5.13 ± 0.04 |
| 77 | Caryophyllene oxide | 1589 | - | 0.06 ± 0.01 |
| 78 | (E)-Isomyristicin | 1621 | 0.04 ± 0.00 | - |
| 79 | Cubenol | 1648 | 0.05 ± 0.01 | - |
| 80 | (E)-Isoelemicin | 1658 | 0.01 ± 0.00 | 0.15 ± 0.02 |
| 81 | α-Cadinol | 1661 | 0.07 ± 0.01 | - |
| 82 | Benzyl benzoate | 1768 | 0.06 ± 0.01 | - |
| 83 | Neophytadiene | 1844 | 0.01 ± 0.00 | - |
| 84 | Phytol | 2120 | 0.13 ± 0.09 | - |
| No. | Compound | RI | Average Area% ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| HS-Fr (f1) | HS-Dr (f1) | HS-HY (f1) | HS-Fr (f2) | HS-Dr (f2) | HS-HY (f2) | |||
| 1 | Pentanal | <900 | - | - | 0.04 ± 0.02 | - | - | 0.1 ± 0.00 |
| 2 | Pyridine | <900 | - | - | 0.04 ± 0.01 | - | - | 0.05 ± 0.01 |
| 3 | Furfural | <900 | - | - | 0.10 ± 0.01 | - | - | 0.16 ± 0.02 |
| 4 | (E)-Hex-2-enal | <900 | - | - | - | 0.03 ± 0.01 | - | - |
| 5 | Hexan-1-ol | <900 | - | - | 0.02 ± 0.00 | - | - | 0.02 ± 0.01 |
| 6 | Nonane | 904 | 0.09 ± 0.00 | 0.15 ± 0.02 | - | 0.14 ± 0.01 | 0.07 ± 0.01 | - |
| 7 | (Z)-Non-2-ene | 920 | - | 0.01 ± 0.00 | - | 0.01 ± 0.00 | - | - |
| 8 | α-Thujene | 937 | 0.24 ± 0.02 | 0.32 ± 0.02 | - | 0.13 ± 0.00 | 0.13 ± 0.03 | - |
| 9 | α-Pinene | 945 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 |
| 10 | Benzaldehyde | 970 | - | 0.01 ± 0.00 | 0.10 ± 0.01 | - | 0.01 ± 0.00 | 0.10 ± 0.03 |
| 11 | Sabinene | 982 | 4.07 ± 0.29 | 5.32 ± 0.27 | 0.04 ± 0.01 | 7.70 ± 0.56 | 3.99 ± 0.33 | 0.03 ± 0.01 |
| 12 | β-Pinene | 986 | 0.27 ± 0.00 | 0.37 ± 0.02 | - | 0.27 ± 0.01 | 0.19 ± 0.05 | - |
| 13 | β-Myrcene | 995 | 0.49 ± 0.04 | 0.46 ± 0.02 | - | 0.50 ± 0.01 | 0.24 ± 0.07 | - |
| 14 | Decane | 1003 | - | 0.04 ± 0.00 | - | - | 0.01 ± 0.00 | - |
| 15 | 2,6-Dimethylcyclohexan-1-ol | 1009 | - | - | 0.03 ± 0.01 | - | - | - |
| 16 | [(Z)-Hex-3-enyl] acetate | 1011 | 0.44 ± 0.07 | - | - | - | - | - |
| 17 | α-Phellanderene | 1011 | - | 0.02 ± 0.00 | 0.08 ± 0.01 | 0.01 ± 0.00 | - | 0.07 ± 0.03 |
| 18 | Hexyl acetate | 1018 | 0.07 ± 0.01 | - | - | - | - | - |
| 19 | α-Terpinene | 1023 | 0.22 ± 0.02 | 0.11 ± 0.00 | 0.10 ± 0.00 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.09 ± 0.03 |
| 20 | p-Cymene | 1032 | 0.54 ± 0.01 | 1.21 ± 0.03 | - | 0.87 ± 0.03 | 0.68 ± 0.32 | - |
| 21 | β-Phellandrene | 1037 | 0.12 ± 0.01 | 0.09 ± 0.00 | 0.12 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.01 | 0.07 ± 0.02 |
| 22 | Benzyl alcohol | 1041 | - | 0.02 ± 0.00 | 0.04 ± 0.00 | - | 0.10 ± 0.08 | 0.08 ± 0.03 |
| 23 | (E)-β-Ocimene | 1045 | 8.76 ± 0.95 | 8.73 ± 0.23 | - | 9.05 ± 0.11 | 5.10 ± 1.78 | - |
| 24 | Phenylacetaldehyde | 1051 | 0.03 ± 0.01 | - | 0.12 ± 0.03 | - | - | 0.16 ± 0.01 |
| 25 | (Z)-β-Ocimene | 1055 | 0.31 ± 0.03 | 0.20 ± 0.01 | - | 0.25 ± 0.00 | 0.11 ± 0.03 | - |
| 26 | γ-Terpinene | 1066 | 2.05 ± 0.24 | 2.00 ± 0.06 | 0.09 ± 0.00 | 1.94 ± 0.00 | 1.10 ± 0.45 | 0.05 ± 0.02 |
| 27 | 2-Methyldecane | 1069 | - | 0.03 ± 0.00 | - | - | 0.02 ± 0.00 | - |
| 28 | (E)-4-Thujanol | 1074 | 0.04 ± 0.01 | 0.18 ± 0.00 | 0.68 ± 0.06 | 0.08 ± 0.00 | 0.12 ± 0.09 | 0.68 ± 0.16 |
| 29 | α-Terpinolene | 1093 | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.05 ± 0.02 | - |
| 30 | Nonanal | 1097 | 0.01 ± 0.00 | 0.03 ± 0.00 | - | - | 0.01 ± 0.00 | - |
| 31 | Methyl benzoate | 1099 | 0.01 ± 0.00 | - | - | - | - | - |
| 32 | Linalool | 1102 | - | - | 0.80 ± 0.08 | - | - | 0.77 ± 0.18 |
| 33 | Undecane | 1103 | 2.10 ± 0.49 | 4.15 ± 0.14 | - | 1.06 ± 0.05 | 2.13 ± 0.68 | - |
| 34 | 2-Phenylethanol | 1119 | - | - | 0.05 ± 0.00 | - | - | - |
| 35 | (3E,5Z)-2,6-Dimethylocta-1,3,5,7-tetraene | 1128 | 0.04 ± 0.00 | 0.03 ± 0.00 | - | 0.04 ± 0.00 | 0.02 ± 0.01 | - |
| 36 | (Z)-p-Menthen-2-en-1-ol | 1128 | - | - | 0.32 ± 0.03 | - | - | 0.33 ± 0.08 |
| 37 | (Z)-Alloocimene | 1134 | 0.24 ± 0.02 | 0.24 ± 0.01 | 0.03 ± 0.00 | 0.19 ± 0.01 | 0.11 ± 0.05 | - |
| 38 | Terpinen-1-ol | 1146 | - | - | 0.21 ± 0.02 | - | - | 0.20 ± 0.06 |
| 39 | 3-Methylundecane | 1175 | 0.04 ± 0.01 | 0.06 ± 0.00 | - | - | 0.04 ± 0.00 | - |
| 40 | Terpinen-4-ol | 1183 | 0.06 ± 0.02 | 0.09 ± 0.00 | 5.31 ± 0.54 | 0.16 ± 0.05 | 0.06 ± 0.04 | 4.72 ± 0.89 |
| 41 | p-Cymen-8-ol | 1190 | - | - | 0.05 ± 0.00 | - | - | 0.07 ± 0.01 |
| 42 | α-Terpineol | 1195 | - | - | 0.25 ± 0.03 | - | - | 0.24 ± 0.06 |
| 43 | (Z)-Dec-4-enal | 1197 | 0.01 ± 0.00 | - | - | 0.05 ± 0.00 | - | - |
| 44 | Salicylic acid | 1198 | - | 0.02 ± 0.00 | - | - | 0.01 ± 0.00 | - |
| 45 | (Z)-Piperitol | 1199 | - | - | 0.09 ± 0.00 | - | - | 0.09 ± 0.00 |
| 46 | Dodecane | 1202 | - | 0.01 ± 0.00 | - | - | 0.01 ± 0.00 | - |
| 47 | (3Z,5Z)-2,6-Dimethylocta-3,5,7-trien-2-ol | 1205 | - | 0.04 ± 0.00 | - | - | 0.03 ± 0.01 | - |
| 48 | Decanal | 1209 | 0.02 ± 0.00 | - | - | 0.06 ± 0.01 | - | - |
| 49 | (3E,5E)-2,6-Dimethylocta-3,5,7-trien-2-ol | 1213 | - | 0.01 ± 0.00 | - | - | 0.02 ± 0.00 | - |
| 50 | (E)-Piperitol | 1213 | - | - | 0.10 ± 0.01 | - | - | 0.10 ± 0.01 |
| 51 | Thymol methyl ether | 1236 | 1.38 ± 0.05 | 2.14 ± 0.00 | 0.03 ± 0.00 | 1.71 ± 0.04 | 1.75 ± 0.53 | 0.03 ± 0.00 |
| 52 | Carvacrol methyl ether | 1250 | 0.02 ± 0.00 | 0.04 ± 0.00 | - | 0.02 ± 0.00 | 0.03 ± 0.01 | - |
| 53 | Dec-4-en-1-ol | 1263 | - | 0.04 ± 0.00 | - | - | 0.03 ± 0.00 | - |
| 54 | 2-Phenylbut-2-enal | 1277 | - | - | 0.05 ± 0.00 | - | - | 0.04 ± 0.00 |
| 55 | (Z)-Tridec-3-ene | 1293 | 0.02 ± 0.00 | 0.03 ± 0.00 | - | - | 0.02 ± 0.01 | - |
| 56 | Tridecane | 1302 | 0.04 ± 0.01 | 0.05 ± 0.00 | - | 0.01 ± 0.00 | 0.03 ± 0.01 | - |
| 57 | Thymol | 1306 | - | - | 0.02 ± 0.00 | - | - | - |
| 58 | p-Vinylguaiacol (4-Ethenyl-2-methoxyphenol) | 1318 | - | - | 0.07 ± 0.00 | - | - | 0.06 ± 0.00 |
| 59 | Cyclosativene | 1372 | 0.01 ± 0.00 | 0.04 ± 0.00 | - | - | 0.03 ± 0.01 | - |
| 60 | α-Copaene | 1380 | 0.02 ± 0.01 | 0.06 ± 0.00 | - | - | 0.05 ± 0.00 | - |
| 61 | β-Elemene | 1392 | - | 0.01 ± 0.00 | 0.11 ± 0.01 | - | 0.01 ± 0.00 | 0.09 ± 0.00 |
| 62 | Methyleugenol | 1409 | 0.29 ± 0.01 | 0.26 ± 0.00 | 0.36 ± 0.00 | 0.22 ± 0.00 | 0.36 ± 0.06 | 0.29 ± 0.01 |
| 63 | Dodecanal | 1413 | 0.15 ± 0.03 | 0.02 ± 0.00 | - | 0.14 ± 0.01 | 0.11 ± 0.09 | - |
| 64 | β-Funebrene | 1416 | 0.16 ± 0.03 | 0.17 ± 0.00 | - | 0.06 ± 0.00 | 0.15 ± 0.03 | - |
| 65 | β-Caryophyllene | 1424 | 5.71 ± 1.03 | 4.60 ± 0.06 | - | 1.58 ± 0.12 | 4.53 ± 0.29 | - |
| 66 | α-Gurjunene | 1429 | 0.04 ± 0.01 | 0.03 ± 0.00 | - | 0.02 ± 0.00 | 0.03 ± 0.00 | - |
| 67 | β-Gurjunene | 1433 | 0.03 ± 0.01 | 0.03 ± 0.00 | - | - | 0.03 ± 0.00 | - |
| 68 | α-Humulene | 1458 | 0.41 ± 0.08 | 0.33 ± 0.00 | - | 0.12 ± 0.02 | 0.32 ± 0.03 | - |
| 69 | β-Farnesene | 1462 | 0.09 ± 0.03 | 0.10 ± 0.00 | - | 0.03 ± 0.00 | 0.10 ± 0.01 | - |
| 70 | γ-Curcumene | 1471 | 0.02 ± 0.00 | 0.02 ± 0.00 | - | - | 0.02 ± 0.00 | - |
| 71 | γ-Muurolene | 1478 | 0.04 ± 0.01 | 0.05 ± 0.00 | - | 0.01 ± 0.00 | 0.04 ± 0.01 | - |
| 72 | α-Curcumene | 1484 | 0.43 ± 0.12 | 0.16 ± 0.00 | - | 0.16 ± 0.00 | 0.27 ± 0.1 | - |
| 73 | Germacrene D | 1487 | - | 0.06 ± 0.00 | - | - | 0.06 ± 0.01 | - |
| 74 | Germacrene C | 1497 | 0.04 ± 0.01 | 0.03 ± 0.00 | - | - | 0.03 ± 0.00 | - |
| 75 | α-Muurolene | 1503 | 0.05 ± 0.01 | 0.08 ± 0.00 | - | 0.02 ± 0.00 | 0.07 ± 0.00 | - |
| 76 | γ-Cadinene | 1518 | 0.04 ± 0.01 | 0.04 ± 0.00 | - | 0.02 ± 0.00 | 0.04 ± 0.01 | - |
| 77 | 2,4-Ditert-butylphenol | 1519 | - | - | 0.08 ± 0.04 | - | - | 0.08 ± 0.03 |
| 78 | Myristicin | 1533 | 69.99 ± 0.19 | 66.63 ± 0.77 | 88.03 ± 1.07 | 72.72 ± 0.54 | 76.57 ± 3.96 | 88.85 ± 1.99 |
| 79 | Elemicin (1,2,3-trimethoxy-5-prop-2-enylbenzene) | 1563 | 0.25 ± 0.00 | 0.20 ± 0.00 | 1.49 ± 0.09 | 0.19 ± 0.01 | 0.23 ± 0.01 | 1.44 ± 0.17 |
| 80 | [(Z)-Hex-3-enyl] benzoate | 1575 | 0.03 ± 0.00 | - | 0.03 ± 0.00 | - | - | - |
| 81 | Caryophyllene oxide | 1587 | 0.04 ± 0.00 | 0.32 ± 0.01 | - | 0.03 ± 0.00 | 0.28 ± 0.09 | - |
| 82 | (E)-Isomyristicin | 1620 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.15 ± 0.02 | - | 0.02 ± 0.01 | 0.12 ± 0.01 |
| 83 | Cubenol | 1647 | - | - | 0.04 ± 0.00 | - | - | 0.05 ± 0.01 |
| 84 | α-Cadinol | 1659 | - | - | 0.17 ± 0.01 | - | - | 0.18 ± 0.02 |
| 85 | Benzyl benzoate | 1768 | - | - | 0.03 ± 0.00 | - | - | 0.04 ± 0.01 |
| tR (min) | Name | Monoisotopic Mass | [M + H]+ | Mass Difference (ppm) | Structure | Area (Counts) | |
|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||
| 1 | 6.24 | Flavonoid glycoside 1 (Isoquercitrin/Hyperoside/Quercetin/Spiraeoside) * | 464.09548 | 465.10275 | 3.0 | C21H20O12 | 600,867 |
| 2 | 6.71 | Flavonoid glycoside 2 (Flavonol O-glycoside) * | 448.10056 | 449.10784 | 3.5 | C21H20O11 | 963,514 |
| 3 | 6.87 | Flavonoid glycoside 3 (Flavone C-glycoside) * | 462.11621 | 463.12349 | 5.0 | C22H22O11 | 2,714,820 |
| 7 | 18.58 | Anthocyanidin glycoside (peonidin 3-rutinoside or peonidin 3-rhamnoside 5-glucoside) | 609.18195 | 610.18922 | 4.5 | C28H33O15 | 2,846,789 |
| Lipids | |||||||
| 4 | 7.68 | Hexadecasphinganine | 273.26678 | 274.27406 | 7.7 | C16H35NO2 | 12,726,415 |
| 5 | 13.14 | Octadecanoid | 308.19876 | 309.20604 | 0.6 | C18H28O4 | 1,505,124 |
| 6 | 16.02 | Erucamide * | 337.33446 | 338.34174 | 6.0 | C22H43NO | 3,888,486 |
| 8 | 19.05 | Phosphoserine (PS (O-18:0/13:0) or PS (O-16:0/15:0) | 707.51012 | 708.51740 | 5.2 | C37H74NO9P | 1,957,720 |
| LLN ± SD | LLN ± SD | ||||
|---|---|---|---|---|---|
| (a) | C | 52.17 ± 14.54 | (b) | Ch | 8.84 ± 0.92 |
| PT-EO | 32.10 ± 9.53 | SI-EO | 4.98 ± 0.81 * | ||
| PT-HY | 16.67 ± 6.81 * | Ch | 14.98 ± 1.89 | ||
| SI-HY | 9.90 ± 1.40 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuko, E.; Radman, S.; Jerković, I.; Kamenjarin, J.; Vrkić, I.; Fredotović, Ž. A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity. Pharmaceuticals 2022, 15, 1454. https://doi.org/10.3390/ph15121454
Vuko E, Radman S, Jerković I, Kamenjarin J, Vrkić I, Fredotović Ž. A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity. Pharmaceuticals. 2022; 15(12):1454. https://doi.org/10.3390/ph15121454
Chicago/Turabian StyleVuko, Elma, Sanja Radman, Igor Jerković, Juraj Kamenjarin, Irena Vrkić, and Željana Fredotović. 2022. "A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity" Pharmaceuticals 15, no. 12: 1454. https://doi.org/10.3390/ph15121454
APA StyleVuko, E., Radman, S., Jerković, I., Kamenjarin, J., Vrkić, I., & Fredotović, Ž. (2022). A Plant Worthy of Further Study—Volatile and Non-Volatile Compounds of Portenschlagiella ramosissima (Port.) Tutin and Its Biological Activity. Pharmaceuticals, 15(12), 1454. https://doi.org/10.3390/ph15121454