New 5-Aryl-1,3,4-Thiadiazole-Based Anticancer Agents: Design, Synthesis, In Vitro Biological Evaluation and In Vivo Radioactive Tracing Studies
Abstract
:1. Introduction
2. Results and Discussion
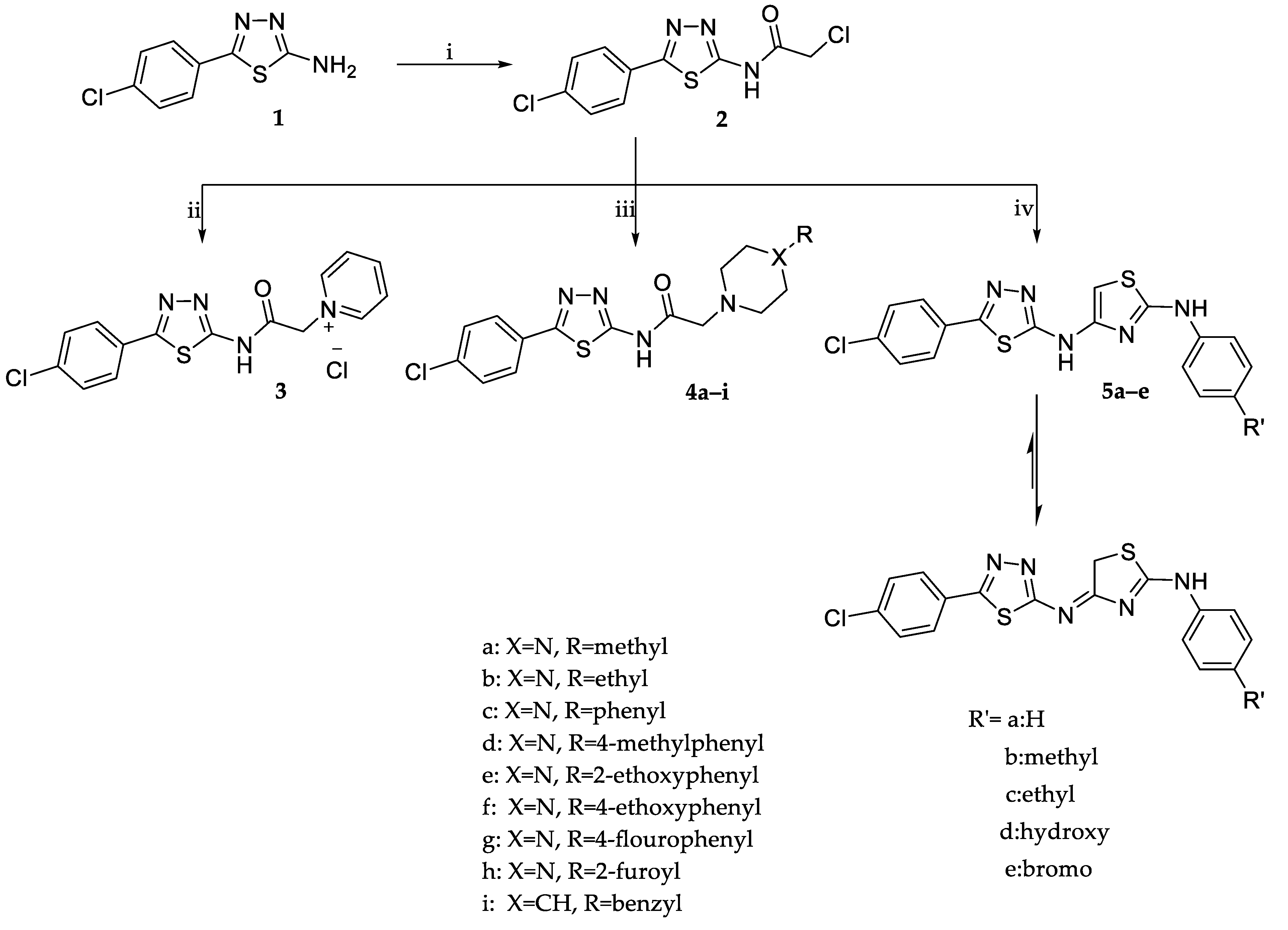
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxic Activity
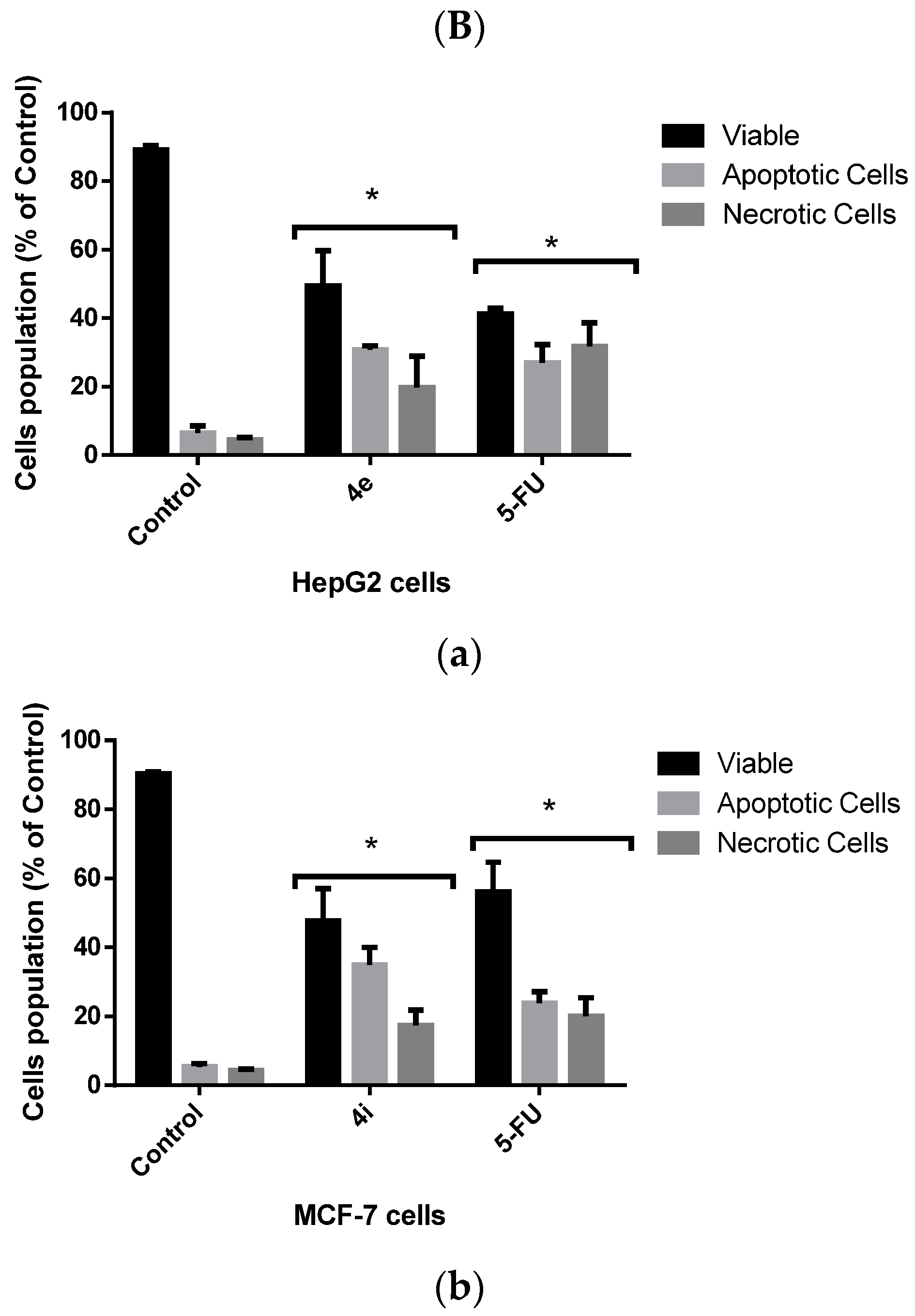
2.2.2. Evaluation of Cellular Death Pattern Induced by 4e and 4i
Morphological Evaluation of Apoptotic and Necrotic Cells Using AO/EB Stain
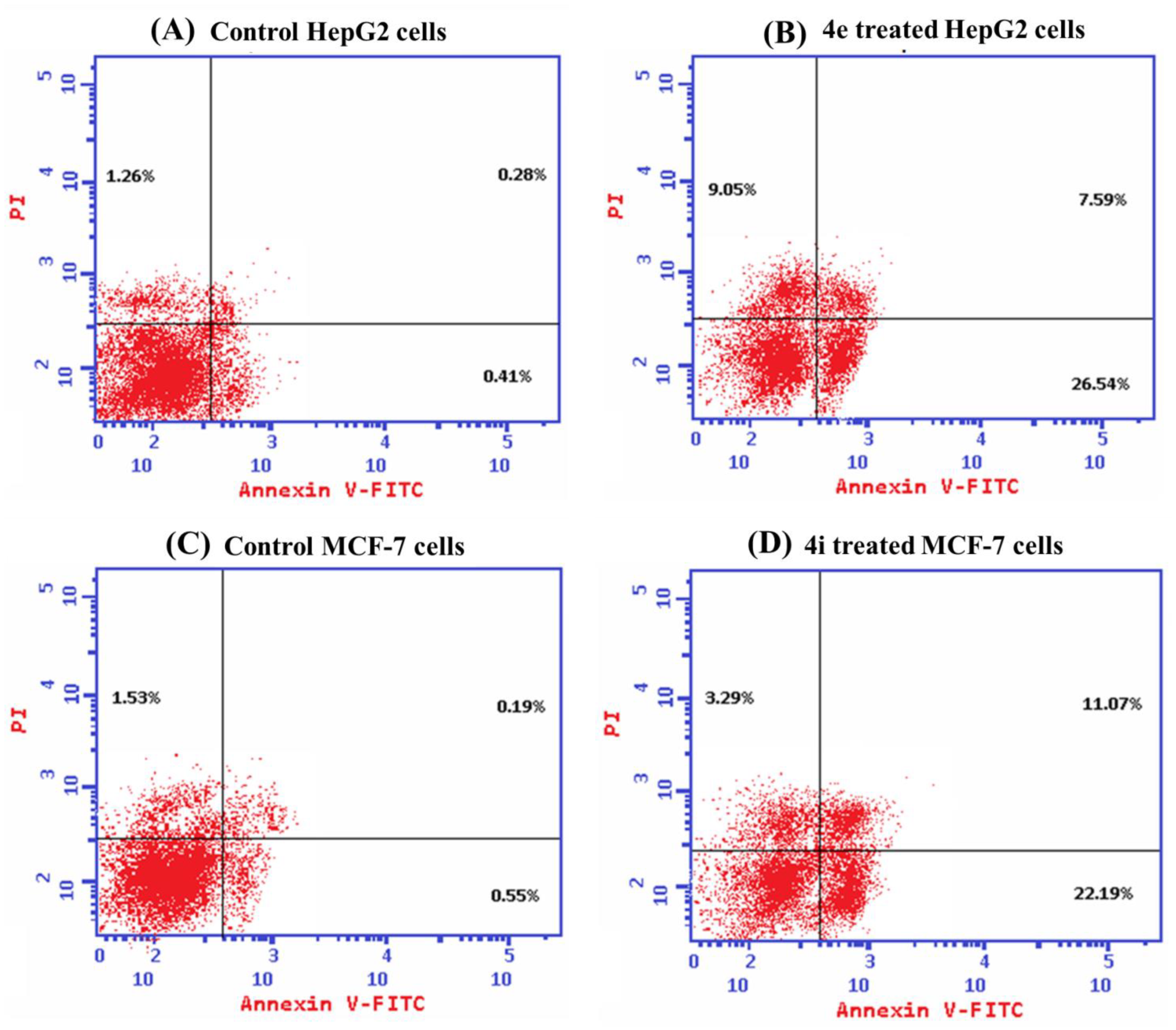
Flow Cytometric Analysis of Apoptotic Cell Death
2.2.3. Cell Cycle Analysis
2.2.4. Effects of 4e and 4i Compounds on the Bax/Bcl2 Ratio, Caspase 9, and VEGF Levels in Cancer Cells
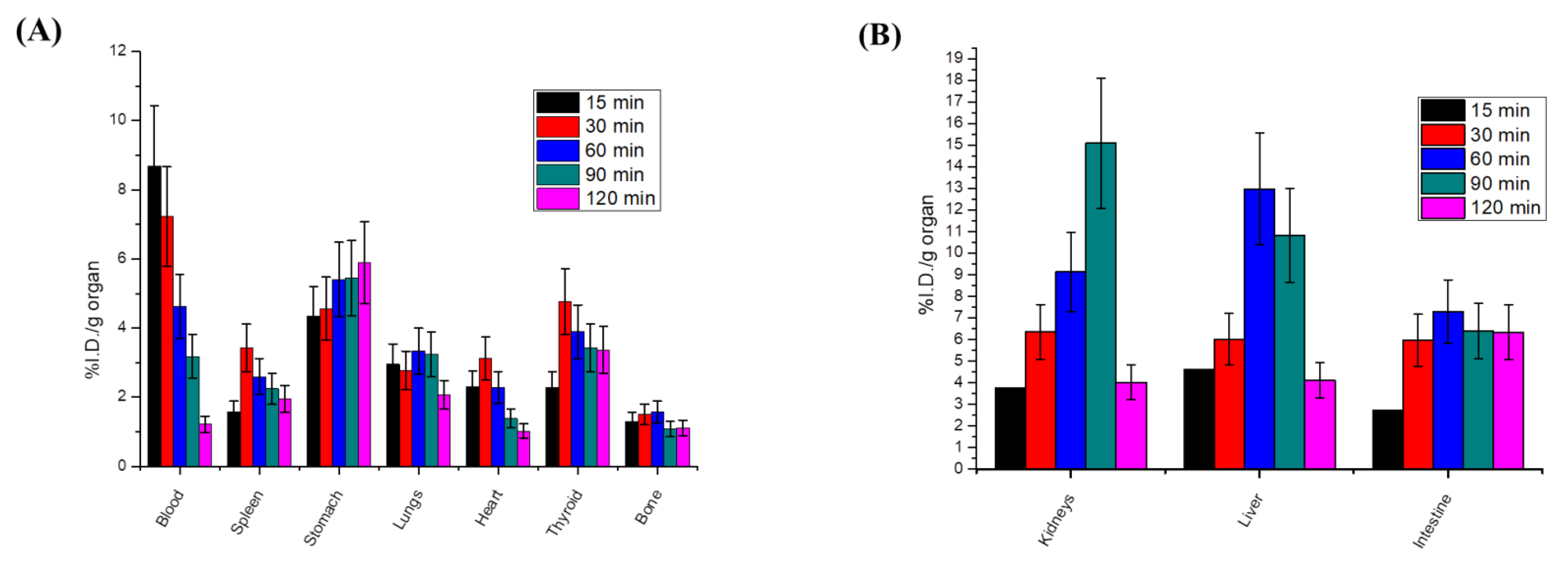
2.2.5. Radiosynthesis and In Vivo Biodistribution of Radioiodinated-4i
Radiosynthesis of Radioiodinated-4i
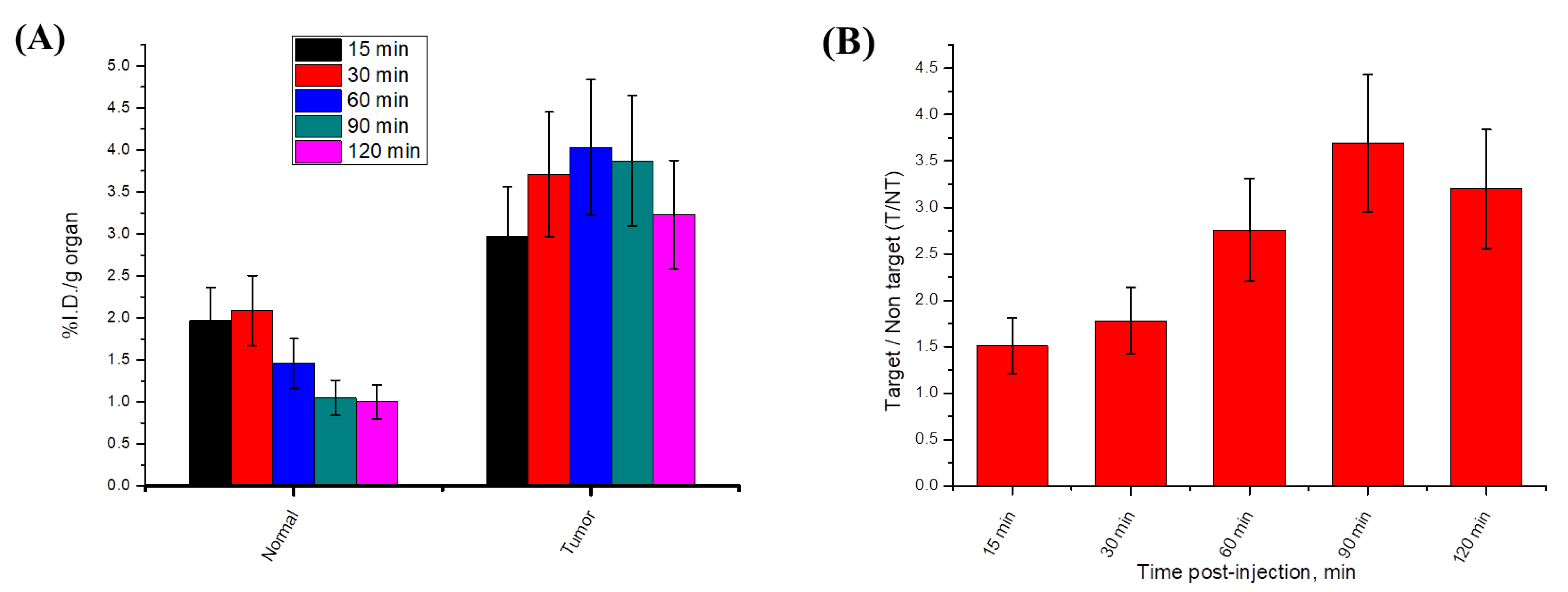
Radioactive In Vivo Tracing Studies of Radioiodinated-4i in a Sarcoma-Bearing Mice Model
3. Materials and Methods
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of 2-Chloro-N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl) Acetamide (2) [29]
3.1.3. Synthesis of 1-(2-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Amino)-2-Oxoethyl)Pyridinium Chloride (3)
3.1.4. General Procedures for the Synthesis of N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(Disubstitutedamino)Acetamide Derivatives (4a–i)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-Methylpiperazin-1-yl)Acetamide (4a)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-Ethylpiperazin-1-yl)Acetamide (4b)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-Phenylpiperazin-1-yl)Acetamide (4c)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-(4-Tolyl)Piperazin-1-yl)Acetamide (4d)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-(2-Ethoxyphenyl)Piperazin-1-yl)Acetamide (4e)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-(4-Ethoxyphenyl)Piperazin-1-yl)Acetamide (4f)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-(4-Fluorophenyl)Piperazin-1-yl)Acetamide (4g)
N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)-2-(4-(Furan-2-Carbonyl)Piperazin-1-yl)Acetamide (4h)
2-(4-Benzylpiperidin-1-yl)-N-(5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Acetamide (4i)
3.1.5. General Procedures for the Synthesis of 4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-N-(4-Substitutedphenyl)-4,5-Dihydrothiazol-2-Amine (5a–e)
4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-N-Phenyl-4,5-Dihydrothiazol-2-Amine (5a)
4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-N-(4-Tolyl)-4,5-Dihydrothiazol-2-Amine (5b)
4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-N-(4-Ethylphenyl)-4,5-Dihydrothiazol-2-Amine (5c)
4-((4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-4,5-Dihydrothiazol-2-yl)Amino)Phenol (5d)
N-(4-Bromophenyl)-4-((5-(4-Chlorophenyl)-1,3,4-Thiadiazol-2-yl)Imino)-4,5-Dihydrothiazol-2-Amine (5e)
3.2. In Vitro Cytotoxicity Screening
3.2.1. Cell Culture
3.2.2. Cytotoxicity Assay
3.3. Acridine Orange/Ethidium Bromide Fluorescent Stain
3.4. Apoptosis Analysis by Flowcytometry
3.5. Cell Cycle Analysis
3.6. ELISA Assays
3.7. Radiosynthesis and In Vivo Biodistribution of Radioiodinated-4i
3.7.1. Radiosynthesis of Radioiodinated-4i
3.7.2. Radioactive In Vivo Tracing Studies of the Radioiodinated-4i Compound in a Sarcoma-Bearing Mice Model
Sarcoma Induction in Mice Model
Biodistribution Studies of Radioiodinated-4i in Sarcoma-Bearing Mice Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Remesh, A. Toxicities of Anticancer Drugs and Its Management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Obakachi, V.A.; Kushwaha, B.; Kushwaha, N.D.; Mokoena, S.; Ganai, A.M.; Pathan, T.K.; van Zyl, W.E.; Karpoormath, R. Synthetic and Anti-Cancer Activity Aspects of 1, 3, 4-Thiadiazole Containing Bioactive Molecules: A Concise Review. J. Sulfur Chem. 2021, 42, 670–691. [Google Scholar] [CrossRef]
- El-Masry, R.M.; Kadry, H.H.; Taher, A.T.; Abou-Seri, S.M. Comparative Study of the Synthetic Approaches and Biological Activities of the Bioisosteres of 1,3,4-Oxadiazoles and 1,3,4-Thiadiazoles over the Past Decade. Molecules 2022, 27, 2709. [Google Scholar] [CrossRef] [PubMed]
- Shahin, R.; Aljamal, S. Kinesin Spindle Protein Inhibitors in Cancer: From High Throughput Screening to Novel Therapeutic Strategies. Future Sci. OA 2022, 8, FSO778. [Google Scholar] [CrossRef]
- Yang, X.H.; Wen, Q.; Zhao, T.T.; Sun, J.; Li, X.; Xing, M.; Lu, X.; Zhu, H.L. Synthesis, Biological Evaluation, and Molecular Docking Studies of Cinnamic Acyl 1,3,4-Thiadiazole Amide Derivatives as Novel Antitubulin Agents. Bioorganic Med. Chem. 2012, 20, 1181–1187. [Google Scholar] [CrossRef]
- Jakovljević, K.; Matić, I.Z.; Stanojković, T.; Krivokuća, A.; Marković, V.; Joksović, M.D.; Mihailović, N.; Nićiforović, M.; Joksović, L. Synthesis, Antioxidant and Antiproliferative Activities of 1,3,4-Thiadiazoles Derived from Phenolic Acids. Bioorganic Med. Chem. Lett. 2017, 27, 3709–3715. [Google Scholar] [CrossRef]
- Durand, C.; Szostak, M. Recent Advances in the Synthesis of Piperazines: Focus on C–H Functionalization. Organics 2021, 2, 337–347. [Google Scholar] [CrossRef]
- İbiş, K.; Nalbat, E.; Çalışkan, B.; Kahraman, D.C.; Cetin-Atalay, R.; Banoglu, E. Synthesis and Biological Evaluation of Novel Isoxazole-Piperazine Hybrids as Potential Anti-Cancer Agents with Inhibitory Effect on Liver Cancer Stem Cells. Eur. J. Med. Chem. 2021, 221, 113489. [Google Scholar] [CrossRef]
- Al-Ghorbani, M.; Gouda, M.A.; Baashen, M.; Alharbi, O.; Almalki, F.A.; Ranganatha, L.V. Piperazine Heterocycles as Potential Anticancer Agents: A Review. Pharm. Chem. J. 2022, 56, 29–37. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Moghimi, S.; Foroumadi, A. Thiazole in the Targeted Anticancer Drug Discovery. Future Med. Chem. 2019, 11, 1929–1952. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, R.M.; Amin, M.A.; Korany, M.; Giovannuzzi, S.; Sakr, T.M.; Kadry, H.H.; Supuran, C.T.; Abou-Seri, S.M.; Taher, A.T. Elaborating 5-(4-Chlorophenyl)-1,3,4-Thiadiazole Scaffold with a p-Tolyl Sulfonamide Moiety Enhances Cytotoxic Activity: Design, Synthesis, in Vitro Cytotoxicity Evaluation, Radiolabelling, and in Vivo Pharmacokinetic Study. Egypt. J. Chem. 2022, in press. [CrossRef]
- Bangade, V.M.; Mali, P.R.; Meshram, H.M. Synthesis of Potent Anticancer Substituted 5-Benzimidazol-2-Amino Thiazoles Controlled by Bifunctional Hydrogen Bonding under Microwave Irradiations. J. Org. Chem. 2021, 86, 6056–6065. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Zhang, S.J.; Ge, Q.F.; Guo, D.W.; Cai, C.M.; Hu, W.X. Synthesis and Anticancer Evaluation of Thiazolyl-Chalcones. Bioorgan. Med. Chem. Lett. 2010, 20, 6555–6559. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [Green Version]
- Bezabeh, T.; Mowat, M.R.A.; Jarolim, L.; Greenberg, A.H.; Smith, I.C.P. Detection of Drug-Induced Apoptosis and Necrosis in Human Cervical Carcinoma Cells Using 1H NMR Spectroscopy. Cell Death Differ. 2001, 8, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Boise, L.; Shanmugam, M. Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef] [Green Version]
- Arbiser, J.L.; Bonner, M.Y.; Gilbert, L.C. Targeting the Duality of Cancer. NPJ Precis. Oncol. 2017, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Jin, F.; Wu, W.; Kumar, S.K. Cell Cycle Regulation and Hematologic Malignancies. Blood Sci. 2019, 1, 34–43. [Google Scholar] [CrossRef]
- Pan, Z.; Luo, Y.; Xia, Y.; Zhang, X.; Qin, Y.; Liu, W.; Li, M.; Liu, X.; Zheng, Q.; Li, D. Cinobufagin Induces Cell Cycle Arrest at the S Phase and Promotes Apoptosis in Nasopharyngeal Carcinoma Cells. Biomed. Pharmacother. 2020, 122, 109763. [Google Scholar] [CrossRef]
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahagh, M.H.; Dehghan, G.; Mehdipour, M.; Teimuri-Mofrad, R.; Payami, E.; Sheibani, N.; Ghaffari, M.; Asadi, M. Synthesis, Characterization, Anti-Proliferative Properties and DNA Binding of Benzochromene Derivatives: Increased Bax/Bcl-2 Ratio and Caspase-Dependent Apoptosis in Colorectal Cancer Cell Line. Bioorg. Chem. 2019, 93, 103329. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; AL-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer Combination Therapies by Angiogenesis Inhibitors; a Comprehensive Review. Cell Commun. Signal. 2022, 20, 1–23. [Google Scholar] [CrossRef]
- Prager, G.W.; Poettler, M.; Unseld, M.; Zielinski, C.C. Angiogenesis in Cancer: Anti-VEGF Escape Mechanisms. Transl. Lung Cancer Res. 2012, 1, 14–25. [Google Scholar] [CrossRef]
- Isin, E.M.; Elmore, C.S.; Nilsson, G.N.; Thompson, R.A.; Weidolf, L. Use of Radiolabeled Compounds in Drug Metabolism and Pharmacokinetic Studies. Chem. Res. Toxicol. 2012, 25, 532–542. [Google Scholar] [CrossRef]
- Mahmoud, A.F.; Aboumanei, M.H.; Abd-Allah, W.H.; Swidan, M.M.; Sakr, T.M. New Frontier Radioiodinated Probe Based on In-Silico Resveratrol Repositioning for Microtubules Dynamic Targeting. Int. J. Radiat. Biol. 2022. accepted. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS Depressant Activity of Some Novel 3-[5-Substituted 1,3,4-Thiadiazole-2-Yl]-2-Styryl Quinazoline-4(3H)-Ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ahuja, P.; Malik, S.; Arya, S.K. Design of Benzothiazole-1,3,4-Thiadiazole Conjugates: Synthesis and Anticonvulsant Evaluation. Arch. Pharm. 2013, 346, 819–831. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB Staining to Detect Apoptosis in Osteosarcoma Cells Compared with Flow Cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.B.; Alaraby Salem, M.; Fasih, T.W.; Brown, A.; Sakr, T.M. Radioiodinated Doxorubicin as a New Tumor Imaging Model: Preparation, Biological Evaluation, Docking and Molecular Dynamics. J. Radioanal. Nucl. Chem. 2018, 317, 1243–1252. [Google Scholar] [CrossRef]
- Sakr, T.M.; Sanad, M.H.; Abd-Alla, W.H.; Salama, D.H.; Saleh, G.M. Radioiodinated Esmolol as a Highly Selective Radiotracer for Myocardial Perfusion Imaging: In Silico Study and Preclinical Evaluation. Appl. Radiat. Isot. 2018, 137, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; Ibrahim, I.T.; Abd-Alla, W.H. Molecular Modeling and Preclinical Evaluation of Radioiodinated Tenoxicam for Inflammatory Disease Diagnosis. J. Radioanal. Nucl. Chem. 2018, 316, 233–246. [Google Scholar] [CrossRef]
- Swidan, M.M.; Sakr, T.M.; Motaleb, M.A.; El-Bary, A.A.; El-Kolaly, M.T. Radioiodinated Acebutolol as a New Highly Selective Radiotracer for Myocardial Perfusion Imaging. J. Label. Compd. Radiopharm. 2014, 57, 593–599. [Google Scholar] [CrossRef] [PubMed]
- El-Safoury, D.M.; Ibrahim, A.B.; El-Setouhy, D.A.; Khowessah, O.M.; Motaleb, M.A.; Sakr, T.M. Amelioration of Tumor Targeting and In Vivo Biodistribution of 99mTc-Methotrexate-Gold Nanoparticles (99mTc-Mex-AuNPs). J. Pharm. Sci. 2021, 110, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Syam, Y.M.; Anwar, M.M.; Abd El-Karim, S.S.; El-Seginy, S.A.; Essa, B.M.; Sakr, T.M. New Quinoxaline Compounds as DPP-4 Inhibitors and Hypoglycemics: Design, Synthesis, Computational and Bio-Distribution. RSC Adv. 2021, 11, 36989–37010. [Google Scholar] [CrossRef]








 | |||
| Compound | Ar/R’ | IC50 (µg/mL) | |
| MCF-7 | HepG2 | ||
| 3 |  | 7.56 ± 1.20 | 13.00 ± 0.11 |
| 4a |  | 51.56 ± 0.48 | 26.30 ± 5.76 |
| 4b |  | 25.21 ± 1.30 | 3.55 ± 0.05 |
| 4c |  | 49.40 ± 3.50 | 27.94 ± 0.93 |
| 4d |  | 21.75 ± 0.43 | 44.87 ± 1.54 |
| 4e | 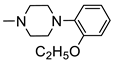 | 5.36 ± 0.36 | 3.13 ± 0.20 |
| 4f | 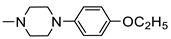 | 18.39 ± 0.85 | 38.10 ± 0.14 |
| 4g | 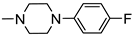 | 10.10 ± 1.20 | 13.87 ± 0.59 |
| 4h |  | 3.21 ± 0.30 | 8.35 ± 0.15 |
| 4i |  | 2.32 ± 0.21 | 6.51 ± 0.03 |
| 5a | H | 24.79 ± 2.30 | 26.12 ± 2.02 |
| 5b | CH3 | 12.64 ± 0.47 | 8.81 ± 0.64 |
| 5c | CH2CH3 | 5.72 ± 0.17 | 11.25 ± 0.44 |
| 5d | OH | 6.12 ± 0.51 | 14.12 ± 0.01 |
| 5e | Br | 3.77 ± 0.13 | 25.77 ± 0.64 |
| 5-FU | _ | 6.80 ± 0.90 | 8.40 ± 1.20 |
| Compound | IC50 (µg/mL) | SI Values | |||
| MCF-7 | HepG2 | Vero | MCF-7 | HepG2 | |
| 4e | 5.36 ± 0.36 | 3.13 ± 0.20 | 154.30 ± 32.20 | 28.79 | 49.20 |
| 4i | 2.32 ± 0.21 | 6.51 ± 0.03 | 85.29 ± 0.52 | 36.70 | 13.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Masry, R.M.; Essa, B.M.; Selim, A.A.; El-Emam, S.Z.; Mohamed, K.O.; Sakr, T.M.; Kadry, H.H.; Taher, A.T.; Abou-Seri, S.M. New 5-Aryl-1,3,4-Thiadiazole-Based Anticancer Agents: Design, Synthesis, In Vitro Biological Evaluation and In Vivo Radioactive Tracing Studies. Pharmaceuticals 2022, 15, 1476. https://doi.org/10.3390/ph15121476
El-Masry RM, Essa BM, Selim AA, El-Emam SZ, Mohamed KO, Sakr TM, Kadry HH, Taher AT, Abou-Seri SM. New 5-Aryl-1,3,4-Thiadiazole-Based Anticancer Agents: Design, Synthesis, In Vitro Biological Evaluation and In Vivo Radioactive Tracing Studies. Pharmaceuticals. 2022; 15(12):1476. https://doi.org/10.3390/ph15121476
Chicago/Turabian StyleEl-Masry, Rana M., Basma M. Essa, Adli A. Selim, Soad Z. El-Emam, Khaled O. Mohamed, Tamer M. Sakr, Hanan H. Kadry, Azza T. Taher, and Sahar M. Abou-Seri. 2022. "New 5-Aryl-1,3,4-Thiadiazole-Based Anticancer Agents: Design, Synthesis, In Vitro Biological Evaluation and In Vivo Radioactive Tracing Studies" Pharmaceuticals 15, no. 12: 1476. https://doi.org/10.3390/ph15121476





