Development and Optimization of Tamarind Gum-β-Cyclodextrin-g-Poly(Methacrylate) pH-Responsive Hydrogels for Sustained Delivery of Acyclovir
Abstract
1. Introduction
2. Results and Discussion
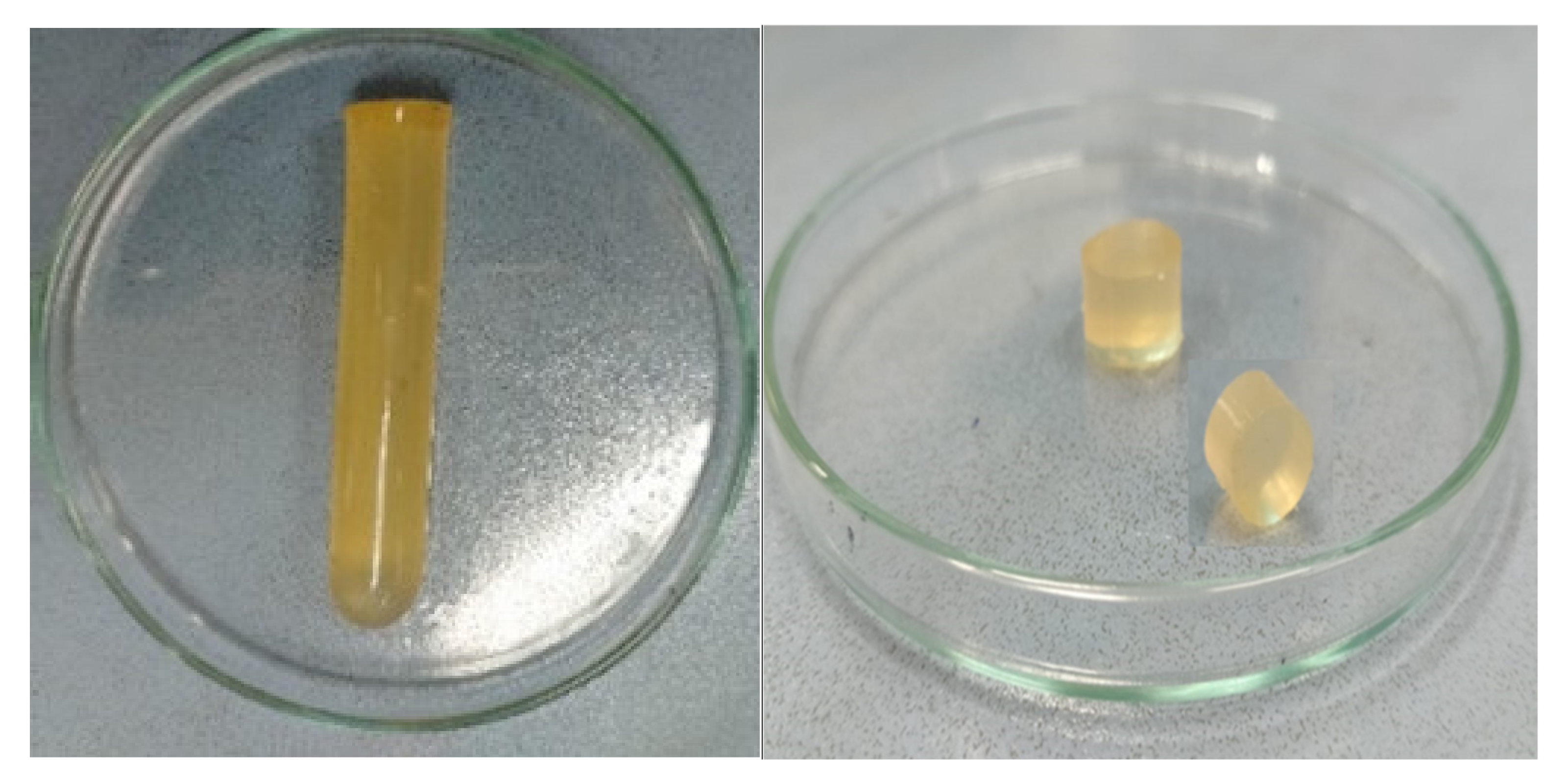
2.1. Physical Appearance
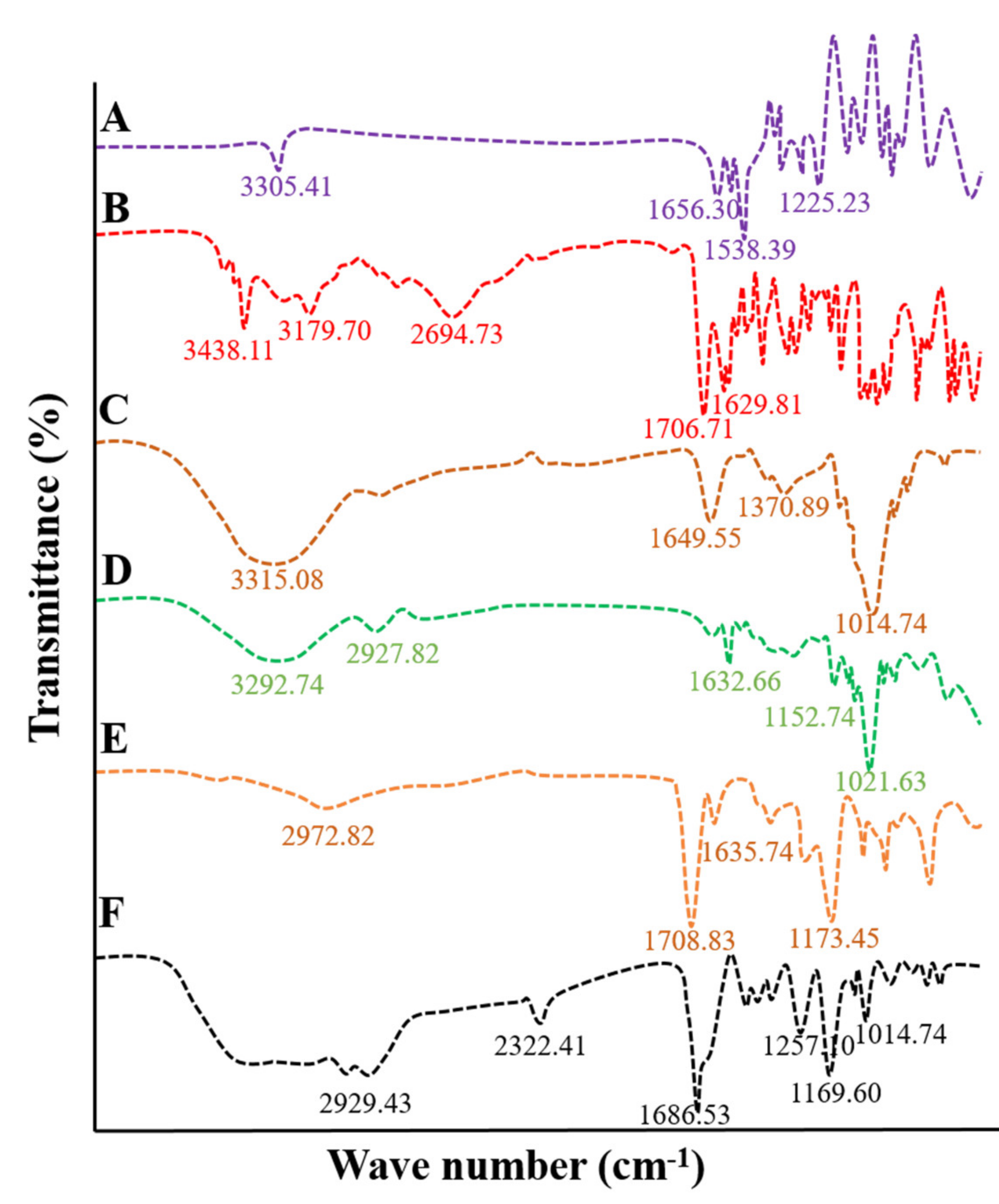
2.2. Fourier Transforms Infrared Spectroscopy
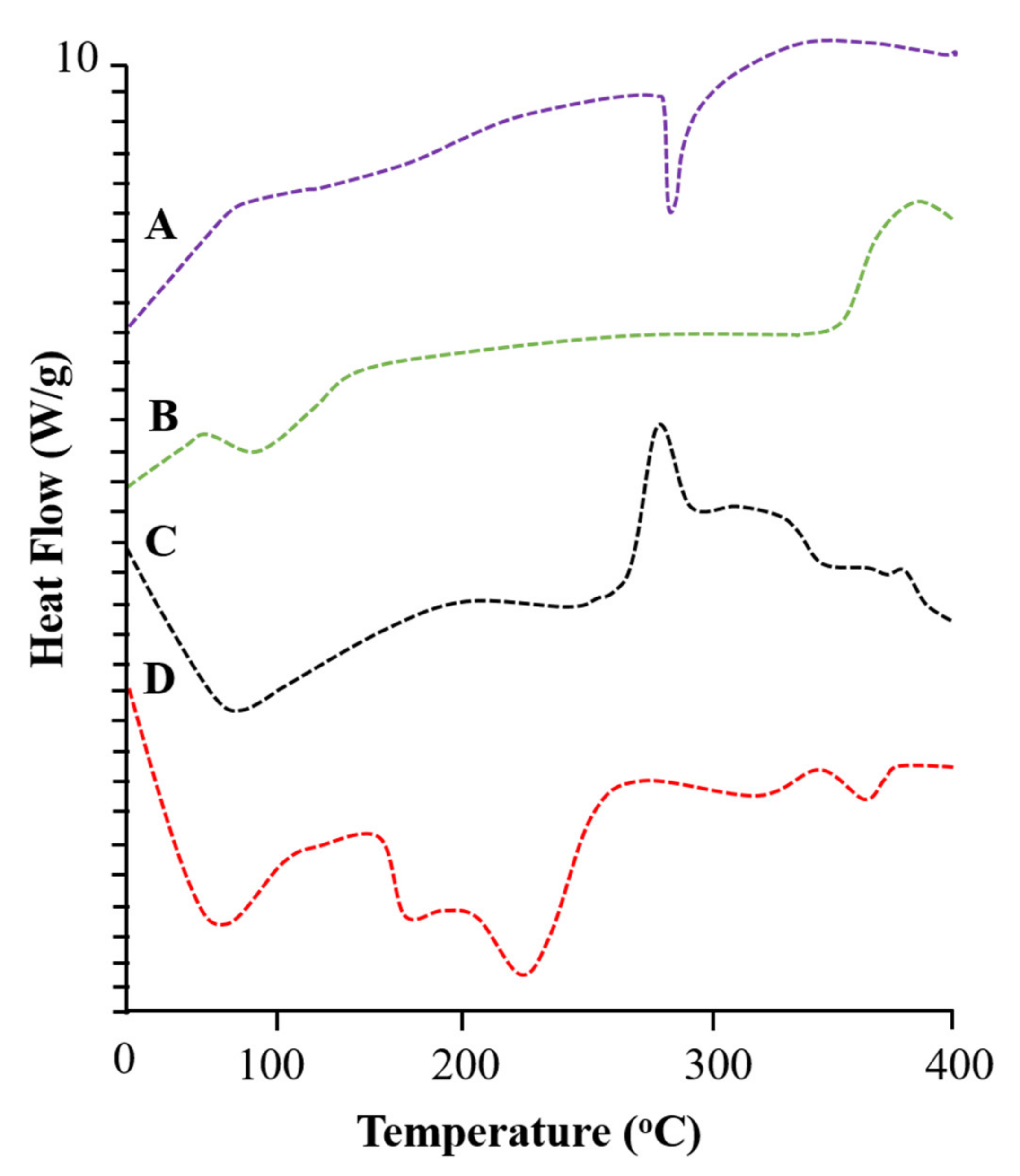
2.3. Differential Scanning Calorimetric Analysis
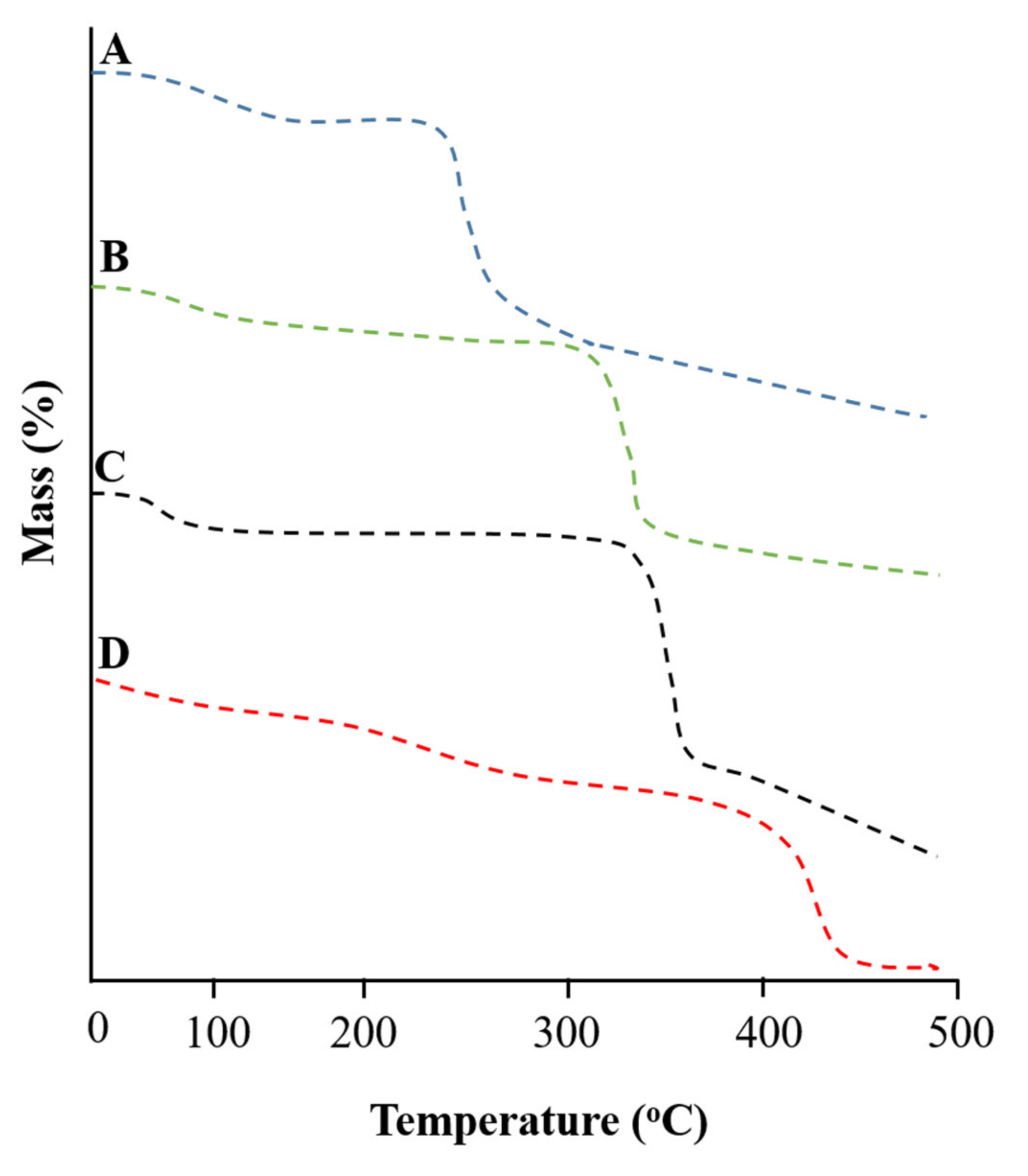
2.4. Thermogravimetric Analysis
2.5. XRD Diffraction Analysis
2.6. Energy-Dispersive X-ray Spectroscopy
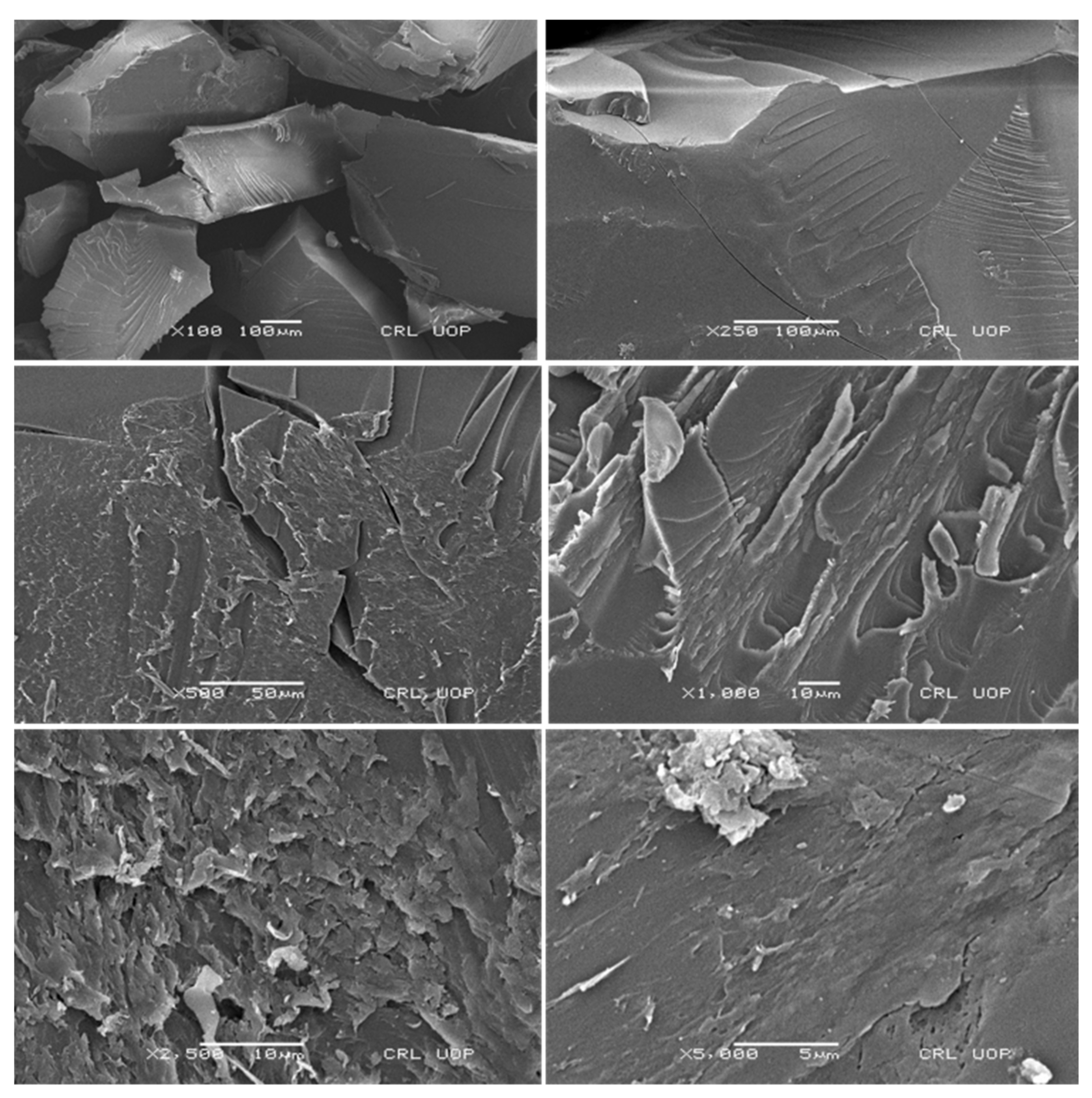
2.7. Scanning Electron Microscopy
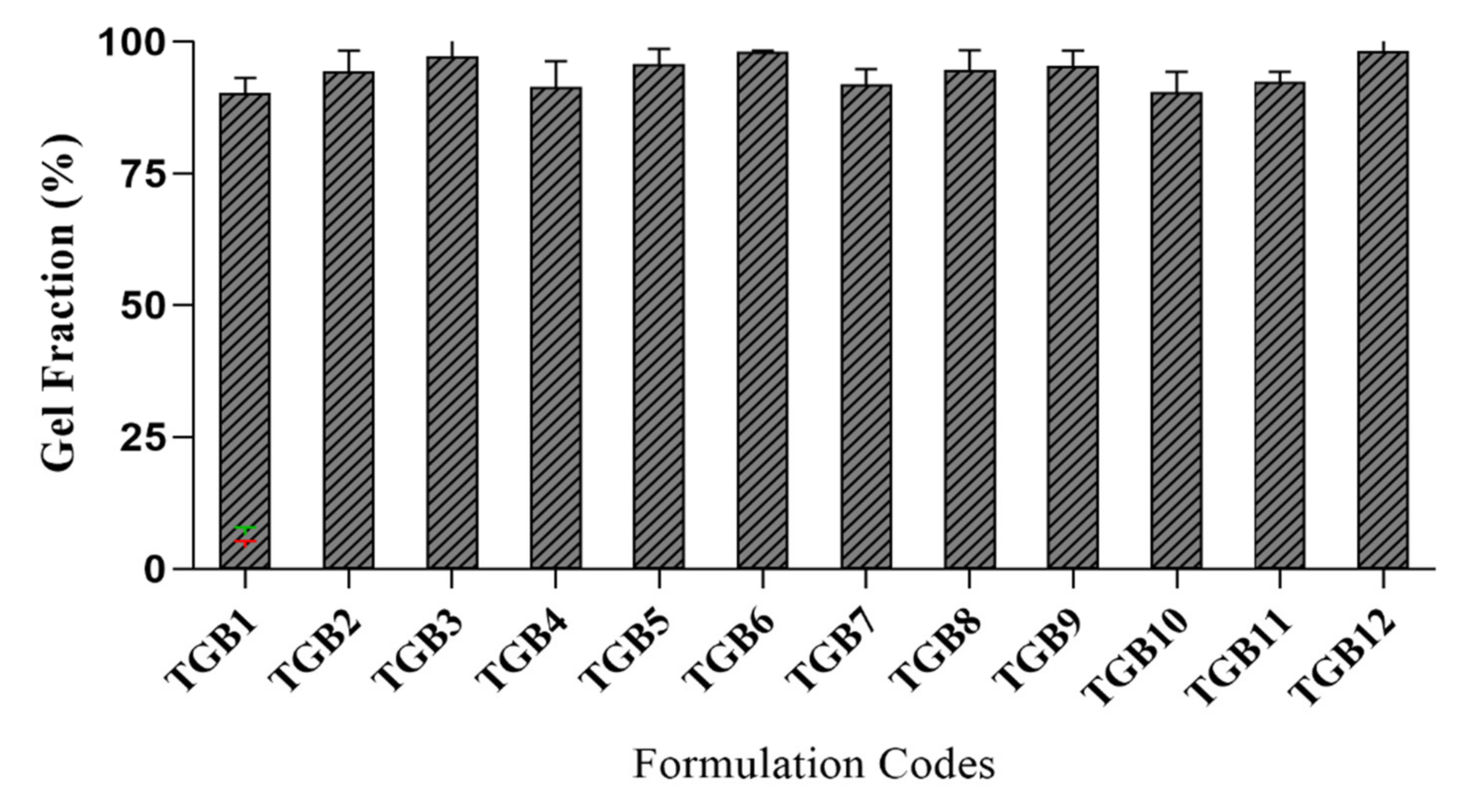
2.8. Determination of the Sol-Gel Fraction
2.9. Equilibrium Swelling Studies
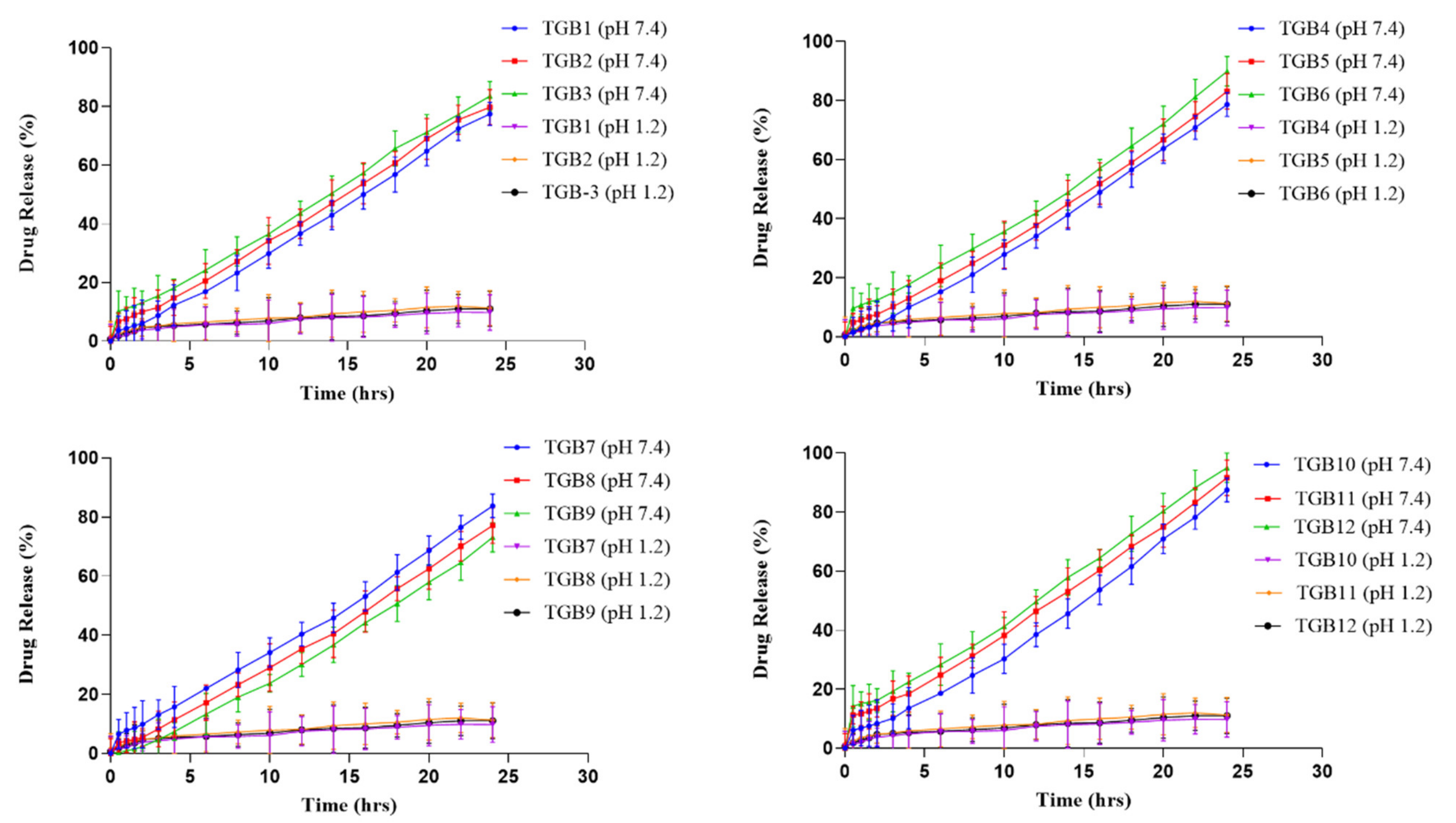
2.10. Effect of Feed Content on Acyclovir Release
2.11. Drug Release Kinetics
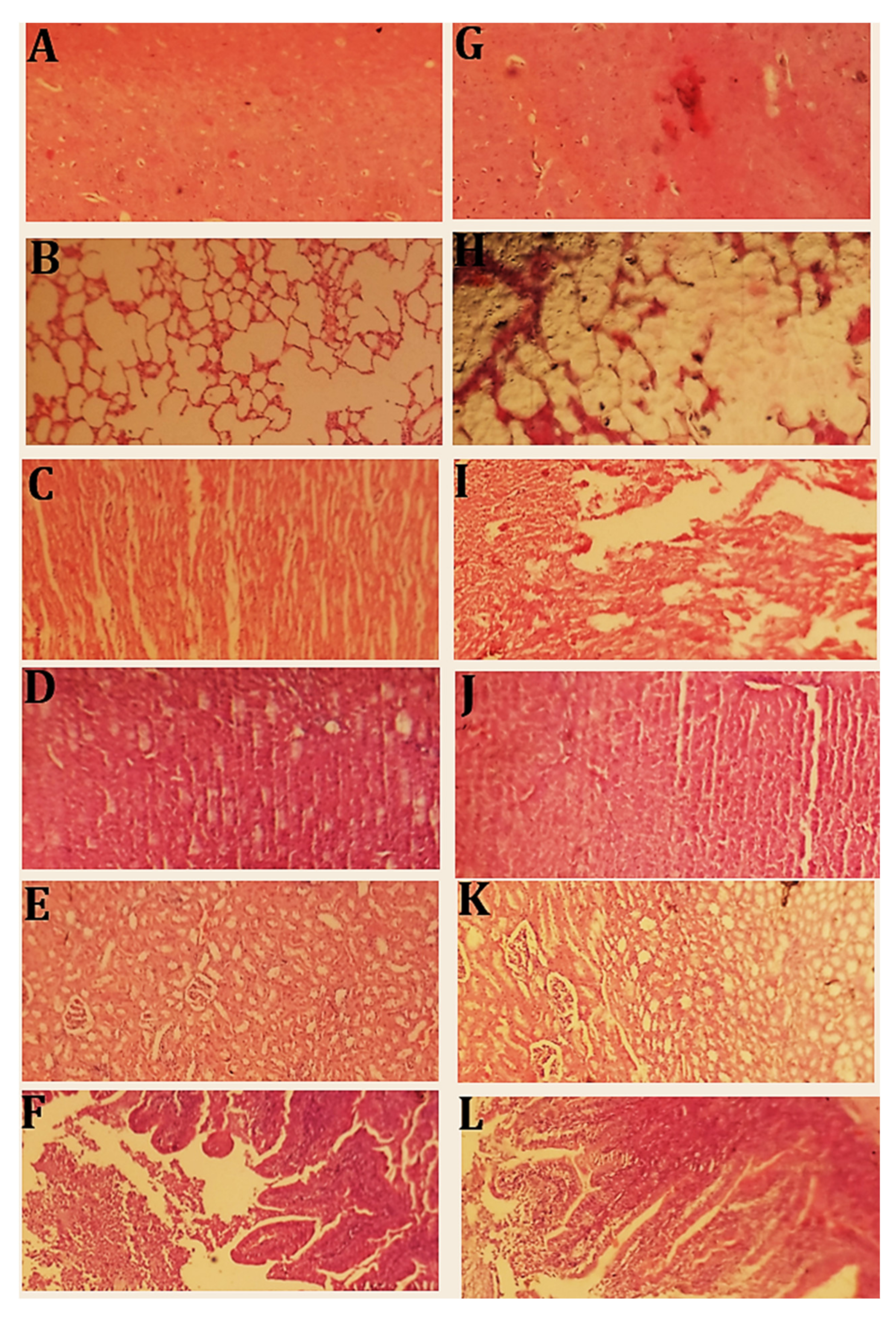
2.12. Toxicity Studies
2.12.1. Clinical Manifestations
2.12.2. Blood Analysis
2.12.3. Histopathological Evaluation
3. Materials and Methods
3.1. Methods
3.1.1. Method of Extraction of Tamarind Mucilage
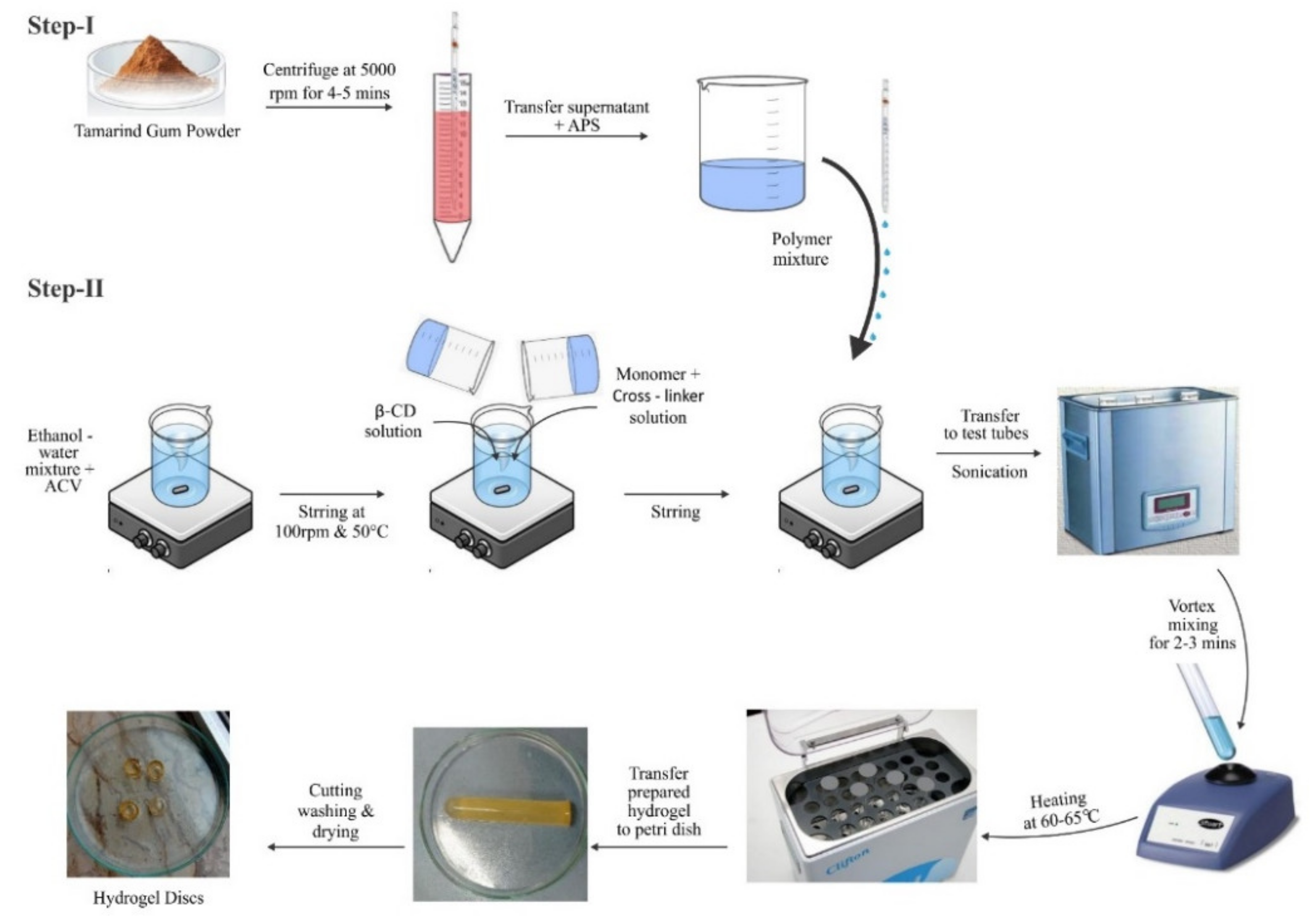
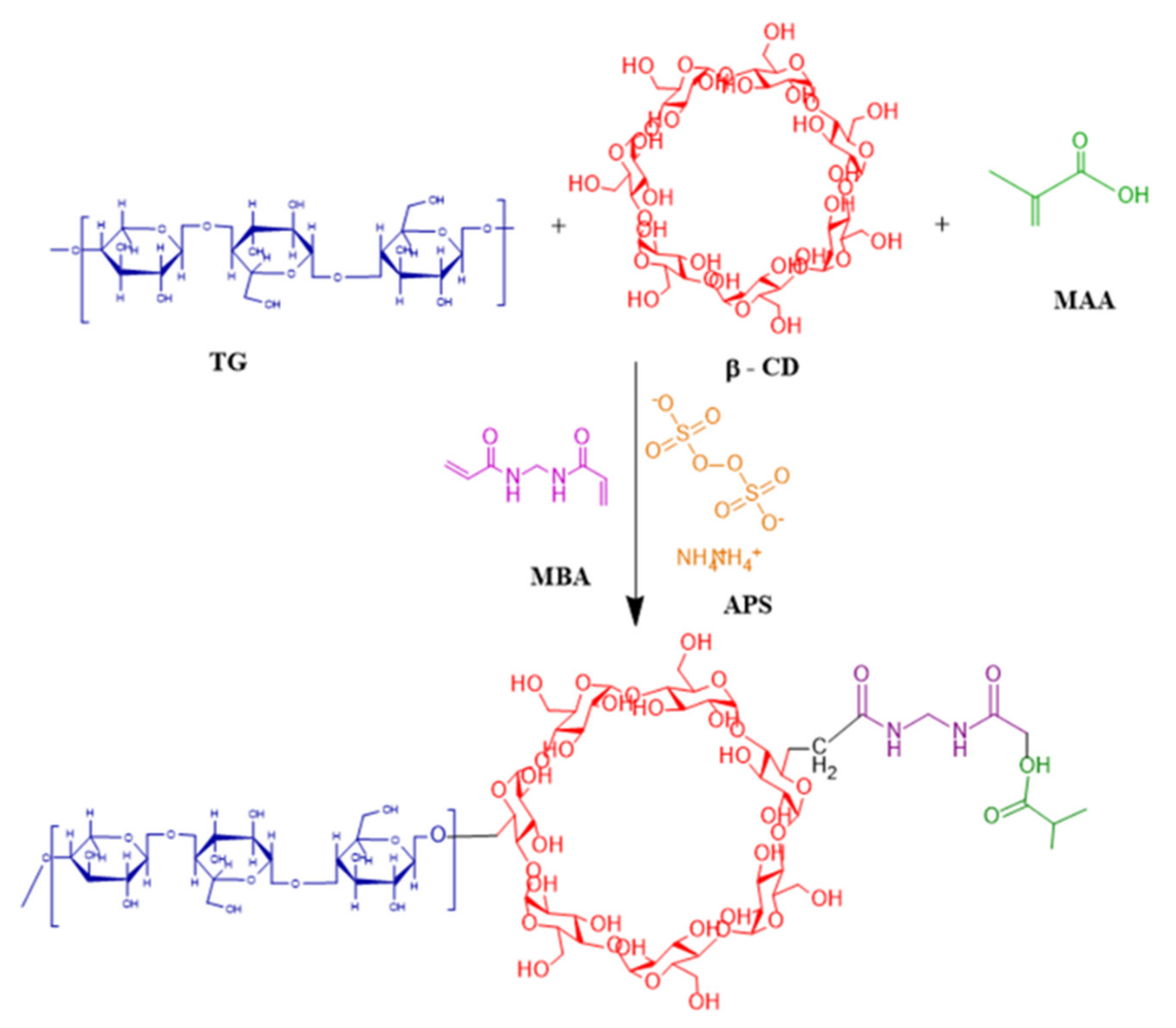
3.1.2. Development of Tamarind-β-CD-co-Poly (Methacrylate) Hydrogels
3.2. Characterization
3.2.1. Fourier Transform Infrared Spectroscopy
3.2.2. Differential Scanning Calorimetry
3.2.3. Thermogravimetric Analysis
3.2.4. Scanning Electron Microscopy
3.2.5. X-ray Diffraction Studies
3.2.6. Energy-Dispersive X-ray Spectroscopy
3.2.7. Sol-Gel Fraction
3.2.8. Swelling Studies
3.2.9. In Vitro Drug Release Studies
3.2.10. Kinetic Modeling
3.2.11. Oral Acute Toxicity Studies
Sampling
Clinical Manifestations
Blood Analysis
Histopathological Examination
4. Conclusions
4.1. Limitations of the Study
4.2. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiti, S.; Sen, K.K. Introductory chapter: Drug delivery concepts. Adv. Technol. Deliv. Ther. 2017, 11, 1–2. [Google Scholar]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.N.; Oliveira, R.R.; Castellano, L.R.; Bonan, P.R.; Carvalho, O.V.; Pena, L.; Souza, J.R.; Oliveira, J.E.; Medeiros, E.S. Controlled release and anti-viral activity of acyclovir-loaded PLA/PEG nanofibers produced by solution blow spinning. Biomat. Adv. 2022, 136, 212785. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumder, P.; Joshi, M.; Joshi, C.; Dalvi, S.V.; Kumar, M. Biomedical application, drug delivery and metabolic pathway of anti-viral nanotherapeutics for combating viral pandemic: A review. Envir. Res. 2020, 191, 110119. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Al-Dhubiab, B.E. Preparation and evaluation of niosome gel containing Acyclovir for enhanced dermal deposition. J. Liposome Res. 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Murtaza, G.; Khalid, Q. Polysaccharide hydrogels for controlled release of Acyclovir: Development, characterization and in vitro evaluation studies. Polym. Bull. 2017, 74, 4311–4328. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. J. Pharm. 2021, 14, 234. [Google Scholar] [CrossRef]
- Djekic, L.; Janković, J.; Rašković, A.; Primorac, M. Semisolid self-microemulsifying drug delivery systems (SMEDDSs): Effects on pharmacokinetics of Acyclovir in rats. Eur. J. Pharm. Sci. 2018, 121, 287–292. [Google Scholar] [CrossRef]
- Nair, A.B.; Attimarad, M.; Al-Dhubiab, B.E.; Wadhwa, J.; Harsha, S.; Ahmed, M. Enhanced oral bioavailability of Acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Deliv. 2014, 21, 540–547. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Ghorpade, V.S. delivery of drugs using tamarind gum and modified tamarind gum: A review. Bull. Fac. Pharm. Cairo Univ. 2019, 57, 1–24. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D. Functionalization of tamarind gum for drug delivery. Funct. Biopolym. 2018, 25–56. [Google Scholar] [CrossRef]
- Mahmood, A.; Amara, S.F.M.; Sarfraz, R.M.; Abrar, M.A.; Qaisar, M.N.; Anwer, N.; Amjad, M.W.; Zaman, M. Development and in vitro evaluation of (β-cyclodextrin-g-methacrylic acid)/Na+-montmorillonite nanocomposite hydrogels for controlled delivery of lovastatin. Int. J. Nanomed. 2019, 14, 5397. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Kurcok, P. α-cyclodextrin-based polypseudorotaxane hydrogels. J. Mater. 2020, 13, 133. [Google Scholar] [CrossRef]
- Hosseini, S.; Azari, P.; Farahmand, E.; Gan, S.N.; Rothan, H.A.; Yusof, R.; Koole, L.H.; Djordjevic, I.; Ibrahim, F. Polymethacrylate coated electrospun PHB fibers: An exquisite outlook for fabrication of paper-based biosensors. Biosens. Bioelectron. 2015, 69, 257–264. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peppas, N.A. pH Responsive poly (itaconic acid-co-N-vinylpyrrolidone) hydrogels with reduced ionic strength loading solutions offer improved oral delivery potential for high isoelectric point-exhibiting therapeutic proteins. Int. J. Pharm. 2014, 471, 83–91. [Google Scholar] [CrossRef]
- Li, N.; Ma, X.; Zha, Q.; Kim, K.; Chen, Y.; Song, C. Maximizing the number of oxygen containing functional groups on activated carbon by using ammonium persulfate and improving the temperature-programmed desorption characterization of carbon surface chemistry. Carbon 2011, 49, 5002–5013. [Google Scholar] [CrossRef]
- Varma, R.S.; Meshra, H.M. Solid state cleavage of semicarbazones and phenylhydrazoneswith ammonium persulfate-clay using microwave or ultrasonic irradiation. Tetrahedron Lett. 1997, 38, 7973–7976. [Google Scholar] [CrossRef]
- Suedee, R.; Seechamnanturakit, V.; Canyuk, B.; Ovatlarnporn, C.; Martin, G.P. Temperature sensitive dopamine-imprinted (N, N-methylene-bis-acrylamide cross-linked) polymer and its potential application to the selective extraction of adrenergic drugs from urine. J. Chromatogr. A. 2006, 1114, 239–249. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, A.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva, J.O., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.; Cardoso, V.M.; Boni, F.I.; Souza, A.L.; Gremião, M.P. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Kumar, L.S.; Selvin, P.C.; Selvasekarapandian, S.; Manjuladevi, R.; Monisha, S.; Perumal, P. Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Solid State Ion. 2018, 24, 3793–3803. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. Development of Acyclovir loaded β-cyclodextrin-g-poly methacrylic acid hydrogel microparticles: An in vitro characterization. Adv. Polym. Technol. 2018, 37, 697–705. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, D.; Mukesh, C.; Prasad, K. Preparation of tamarind gum based soft ion gels having thixotropic properties. Carbohydr. Polym. 2014, 102, 467–471. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Okpozo, O.P.; Onyekwere, O.S.; Daoud, W.A. Citric acid cross-linked natural bi-polymer-based composite hydrogels: Effect of polymer ratio and beta-cyclodextrin on hydrogel microstructure. React. Funct. Polym. 2020, 154, 104682. [Google Scholar] [CrossRef]
- Garcıa, D.M.; Escobar, J.L.; Bada, N.; Casquero, J.; Hernáez, E.; Katime, I. Synthesis and characterization of poly (methacrylic acid) hydrogels for metoclopramide delivery. Eur. Polym. J. 2004, 40, 1637–1643. [Google Scholar] [CrossRef]
- Mali, K.K. Development of vancomycin-loaded polysaccharide-based hydrogel wound dressings: In vitro and in vivo evaluation. Asian Journal of Pharmaceutics (AJP). Asian J. Pharm. 2018, 12. [Google Scholar] [CrossRef]
- Bashir, S.; Zafar, N.; Lebaz, N.; Mahmood, A.; Elaissari, A. Hydroxypropyl Methylcellulose-Based Hydrogel Copolymeric for Controlled Delivery of Galantamine Hydrobromide in Dementia. J. Manuf. Process. 2020, 8, 1350. [Google Scholar] [CrossRef]
- Khan, J.A.; Pervaiz, F.; Ranjha, N.M.; Naeem, M.; Khalid, N.; Javaid, Z. Design and Characterization of PVA–Methacrylic Acid Based Smart Polymeric System for Controlled Release of Metoprolol. J. Polym. Environ. 2017, 25, 556–568. [Google Scholar] [CrossRef]
- Asghar, S.; Akhtar, N.; Minhas, M.U.; Khan, K.U. Bi-polymeric Spongy Matrices through Cross-linking Polymerization: Synthesized and Evaluated for Solubility Enhancement of Acyclovir. AAPS PharmSciTech. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- Malviya, R.; Srivastava, P.; Bansal, M.; Sharma, P.K. Formulation, evaluation, and comparison of sustained release matrix tablets of diclofenac sodium using tamarind gum as release modifier. Asian J. Pharm. Clin. Res. 2010, 3, 238–241. [Google Scholar]
- Singh, R.; Malviya, R.; Sharma, P.K. Extraction and characterization of tamarind seed polysaccharide as a pharmaceutical excipient. Pharmacogn. Mag. 2011, 3, 17–19. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Razak, K.A.; Akil, H.M. Synthesis and physicochemical investigation of chitosan-PMAA-based dual-responsive hydrogels. J. Polym. Res. 2013, 20, 273. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. β-CD based hydrogel microparticulate system to improve the solubility of Acyclovir: Optimization through in-vitro, in-vivo, and toxicological evaluation. J. Drug Deliv. Sci. Technol. 2016, 36, 75–88. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Mahmood, A. pH-responsive CAP-co-poly (methacrylic acid)-based hydrogel as an efficient platform for controlled gastrointestinal delivery: Fabrication, characterization, in vitro and in vivo toxicity evaluation. Drug Deliv. Transl. Res. 2019, 9, 555–577. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH-sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Akram, M.R.; Abrar, A. Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: An in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2017, 11, 3083. [Google Scholar] [CrossRef]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS Pharm. Sci. Tech. 2007, 8, E142–E146. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.; Sarfraz, R.M.; Mahmood, A.; Zafar, N.; Ashraf, M.U. Chitosan/Agarose-g-poly (methacrylate) pH-responsive polymeric blend: A dais for controlled delivery of Capecitabine. Polym. Adv. Technol. 2021, 32, 3782–3794. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Yan, L.; Yu, Q.; Xia, H.; Liu, Y.; Yan, C. Multi-stimuli-responsive poly (hydroxyethyl methacrylate-co-N-vinyl pyrrolidone-co-methacrylic acid-co-N-isopropylacryl amide) hydrogel: Synthesis, characterization, and application in drug release. Iran. Polym. J. 2019, 28, 957–967. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PloS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Jadhav, K.; Dhamecha, D.; Tate, A.; Tambe, H.; Patil, M.B. Application of UV spectrophotometric method for easy and rapid estimation of lafutidine in bulk and pharmaceutical formulation. Pharm. Methods 2011, 2, 264–267. [Google Scholar] [CrossRef]
- Jana, S.; Sharma, R.; Maiti, S.; Sen, K.K. Interpenetrating hydrogels of O-carboxymethyl Tamarind gum and alginate for monitoring delivery of Acyclovir. Int. J. Biol. Macromol. 2016, 92, 1034–1039. [Google Scholar] [CrossRef]
- El-Gizawy, S.A.; El-Maghraby, G.M.; Hedaya, A.A. Formulation of acyclovir-loaded solid lipid nanoparticles: Design, optimization, and in-vitro characterization. Pharm. Dev. Technol. 2019, 24, 1287–1298. [Google Scholar] [CrossRef]

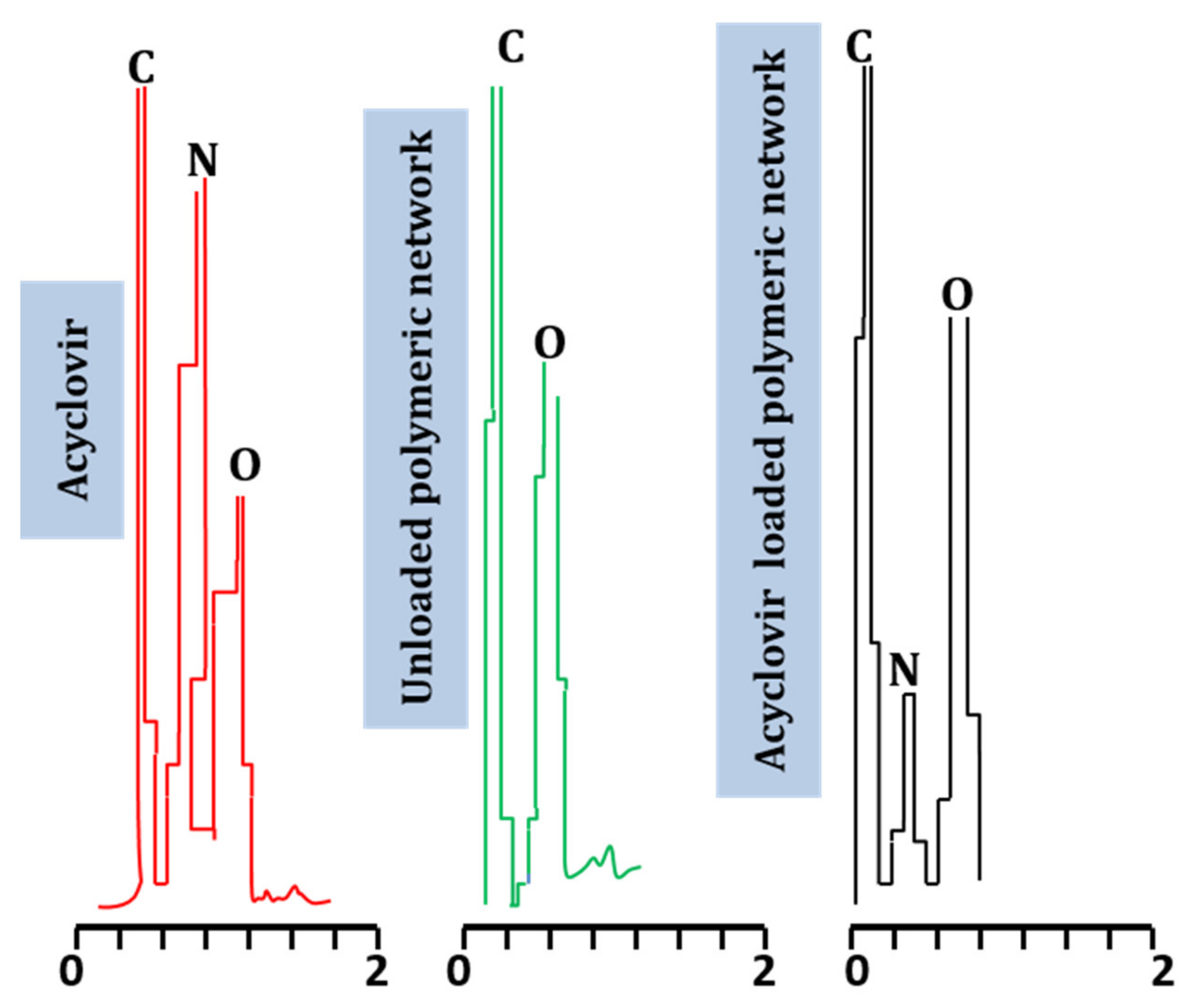


| Material Type | Element | Weight (%) | Atomic (%) |
|---|---|---|---|
| Acyclovir | C | 39.85 | 45.39 |
| N | 30.98 | 30.26 | |
| O | 28.25 | 24.15 | |
| Unloaded hydrogels (TGB12) | C | 58.81 | 65.54 |
| O | 41.19 | 34.46 | |
| Acyclovir-loaded hydrogels (TGB12) | C | 61.16 | 67.80 |
| N | 10.39 | 11.47 | |
| O | 38.45 | 32.00 |
| Kinetic Model | Parameter | Mean |
|---|---|---|
| Zero-order | R2 | 0.99 |
| Ko | 3.38 | |
| t25 | 7.46 | |
| t50 | 14.93 | |
| t75 | 22.40 | |
| First-order | R2 | 0.95 |
| K1 | 0.05 | |
| t25 | 5.86 | |
| t50 | 14.13 | |
| t75 | 28.27 | |
| Higuchi | R2 | 0.86 |
| kH | 13.23 | |
| t25 | 3.70 | |
| t50 | 14.81 | |
| t75 | 33.33 | |
| Korsemeyer–Peppas | R2 | 0.99 |
| Kkp | 3.56 | |
| t25 | 7.53 | |
| t50 | 14.81 | |
| t75 | 22.08 | |
| n | 1.02 |
| Clinical Monitoring | Group I (Control) | Group II (Treated) |
|---|---|---|
| Signs of illness | None | None |
| Body weight (kg) | ||
| 1st Day | 2.05 ± 0.05 | 2.06 ± 0.05 |
| 14th Day | 2.07 ± 0.04 | 2.07 ± 0.04 |
| Food consumption (g) | ||
| 1st Day | 75.49 ± 1.52 | 73.93 ± 1.52 |
| 14th Day | 73.66 ± 2.08 | 75.48 ± 3.01 |
| Water intake (mL) | ||
| 1st Day | 190.48 ± 3.05 | 195.59 ± 1.52 |
| 14th Day | 204.26 ± 2.51 | 200.65 ± 1.15 |
| Dermal Toxicity | None | None |
| Ocular Toxicity | None | None |
| Mortality | None | None |
| Parameter | Group I (Control) | Group II (Treated) | ||
|---|---|---|---|---|
| 1st Day | 14th Day | 1st Day | 14th Day | |
| Hb (g/dL) (10–15 g/dL) | 13.23 ± 0.55 | 13.13 ± 0.45 | 13.66 ± 0.50 | 13.33 ± 1.59 |
| TLC (8.1–21.5 × 103) | 6.83 ± 0.05 | 6.91 ± 0.45 | 7.18 ± 0.28 | 7.12 ± 0.42 |
| RBCs (3.8–7.9 × 106/µL) | 6.46 ± 0.25 | 6.36 ± 0.23 | 6.13 ± 0.35 | 6.25 ± 0.79 |
| Platelets (per µL) (250–650)× 103 | 339 ± 0.30 | 337 ± 0.36 | 325 ± 0.17 | 321 ± 0.05 |
| Monocytes (0–3%) | 3.46 ± 0.40 | 3.53 ± 0.15 | 3.53 ± 0.25 | 3.62 ± 0.17 |
| Lymphocytes (30–70%) | 65.03 ± 4.01 | 65.66 ± 4.16 | 64.66 ± 3.05 | 64.36 ± 2.08 |
| MCV (50–75 fl) | 63.31 ± 2.51 | 65.73 ± 1.58 | 65.31 ± 3.5 | 68.32 ± 1.52 |
| MCH (18–24 pg) | 22.91 ± 0.52 | 23.01 ± 0.62 | 21.86 ± 1.34 | 22.03 ± 0.52 |
| MCHC (27–34 g/dL) | 32.78 ± 2.74 | 33.04 ± 0.79 | 32.64 ± 0.30 | 31.94 ± 0.98 |
| HCT (PCV)% (33–50) | 43.11 ± 0.05 | 43.91 ± 0.17 | 49.31 ± 0.25 | 48.67 ± 0.36 |
| Parameter | Group I (Control) | Group II (Treated) | ||
|---|---|---|---|---|
| 1st Day | 14th Day | 1st Day | 14th Day | |
| ALT (µ/L) | 157.39 ± 3.41 | 125.48 ± 34.80 | 168.67 ± 14.61 | 102.43 ± 0.36 |
| AST (µ/L) | 72.23 ± 2.53 | 68.37 ± 0.51 | 87.36 ± 8.09 | 64.65 ± 1.24 |
| Total proteins (g/dL) | 5.82 ± 2.01 | 5.9 ± 3.08 | 7.13 ± 0.73 | 6.4 ± 3.02 |
| Albumin (g/dL) | 3.7 ± 0.05 | 4.2 ± 2.13 | 3.82 ± 0.05 | 4.42 ± 2.24 |
| Globulin (g/dL) | 2.4 ± 0.12 | 2.3 ± 0.10 | 2.49 ± 0.06 | 2.55 ± 0.08 |
| A/G ratio (%) | 1.5 ± 0.16 | 1.5 ± 0.51 | 0.83 ± 0.84 | 1.11 ± 0.39 |
| Creatinine (mg/dL) | 1.03 ± 0.11 | 0.87 ± 0.02 | 1.22 ± 0.44 | 0.98 ± 0.11 |
| Uric acid (mg/dL) | 3.27 ± 0.12 | 3.36 ± 0.15 | 2.58 ± 0.37 | 2.38 ± 0.53 |
| Urea (mmol/L) | 15.96 ± 3.67 | 19.21 ± 3.25 | 17.66 ± 0.52 | 16.98 ± 0.68 |
| BUN (mg/dL) | 16.5 ± 0.51 | 17 ± 0.43 | 12.39 ± 0.58 | 11.81 ± 1.10 |
| Cholesterol (mg/dL) | 36.10 ± 2.69 | 35.51 ± 2.89 | 65.33 ± 5.09 | 63.32 ± 4.04 |
| Triglycerides (mg/dL) | 64.59 ± 7.76 | 63.10 ± 0.06 | 67.33 ± 5.85 | 66.32 ± 0.21 |
| HDL (mg/dL) | 46.2 ± 0.80 | 47 ± 0.62 | 52.36 ± 1.64 | 54 ± 1.12 |
| LDL (mg/dL) | 64.11 ± 3.09 | 61.02 ± 3.01 | 80.27 ± 4.75 | 85.02 ± 4.78 |
| VLDL (mg/dL) | 14.23 ± 5.08 | 20.03 ± 5.04 | 25.34 ± 2.09 | 28.06 ± 2.72 |
| Formulation Code | Tamarind Gum (g) | β-Cyclodextrin (g) | N, N Methylene Bis-Acrylamide (g) | Methacrylic Acid (g) | Ammonium Persulfate (g) |
|---|---|---|---|---|---|
| TGB1 | 0.2 | 0.5 | 0.03 | 3.0 | 0.02 |
| TGB2 | 0.3 | 0.5 | 0.03 | 3.0 | 0.02 |
| TGB3 | 0.4 | 0.5 | 0.03 | 3.0 | 0.02 |
| TGB4 | 0.2 | 0.5 | 0.03 | 3.0 | 0.02 |
| TGB5 | 0.2 | 0.75 | 0.03 | 3.0 | 0.02 |
| TGB6 | 0.2 | 1.0 | 0.03 | 3.0 | 0.02 |
| TGB7 | 0.2 | 0.5 | 0.05 | 3.0 | 0.02 |
| TGB8 | 0.2 | 0.5 | 0.075 | 3.0 | 0.02 |
| TGB9 | 0.2 | 0.5 | 0.1 | 3.0 | 0.02 |
| TGB10 | 0.2 | 0.5 | 0.03 | 3.0 | 0.02 |
| TGB11 | 0.2 | 0.5 | 0.03 | 6.0 | 0.02 |
| TGB12 | 0.2 | 0.5 | 0.03 | 9.0 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiq, K.; Mahmood, A.; Salem-Bekhit, M.M.; Sarfraz, R.M.; Algarni, A.S.; Taha, E.I.; Mansour, A.A.; Al Zahrani, S.; Benguerba, Y. Development and Optimization of Tamarind Gum-β-Cyclodextrin-g-Poly(Methacrylate) pH-Responsive Hydrogels for Sustained Delivery of Acyclovir. Pharmaceuticals 2022, 15, 1527. https://doi.org/10.3390/ph15121527
Shafiq K, Mahmood A, Salem-Bekhit MM, Sarfraz RM, Algarni AS, Taha EI, Mansour AA, Al Zahrani S, Benguerba Y. Development and Optimization of Tamarind Gum-β-Cyclodextrin-g-Poly(Methacrylate) pH-Responsive Hydrogels for Sustained Delivery of Acyclovir. Pharmaceuticals. 2022; 15(12):1527. https://doi.org/10.3390/ph15121527
Chicago/Turabian StyleShafiq, Kanza, Asif Mahmood, Mounir M. Salem-Bekhit, Rai Muhammad Sarfraz, Alanood S. Algarni, Ehab I. Taha, Ahd A. Mansour, Sami Al Zahrani, and Yacine Benguerba. 2022. "Development and Optimization of Tamarind Gum-β-Cyclodextrin-g-Poly(Methacrylate) pH-Responsive Hydrogels for Sustained Delivery of Acyclovir" Pharmaceuticals 15, no. 12: 1527. https://doi.org/10.3390/ph15121527
APA StyleShafiq, K., Mahmood, A., Salem-Bekhit, M. M., Sarfraz, R. M., Algarni, A. S., Taha, E. I., Mansour, A. A., Al Zahrani, S., & Benguerba, Y. (2022). Development and Optimization of Tamarind Gum-β-Cyclodextrin-g-Poly(Methacrylate) pH-Responsive Hydrogels for Sustained Delivery of Acyclovir. Pharmaceuticals, 15(12), 1527. https://doi.org/10.3390/ph15121527







