Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats
Abstract
:1. Introduction
2. Results
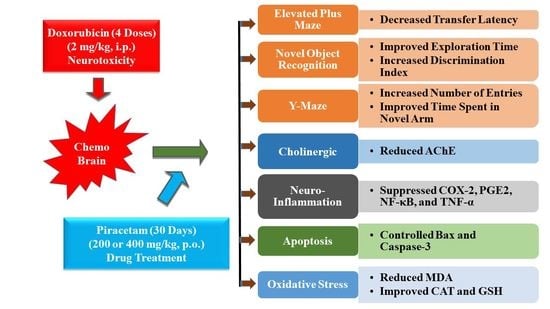
2.1. Effect of PIRA on DOX-Induced Cognitive Impairment Parameters Using an Elevated Plus-Maze (EPM) Test
2.2. Effect of PIRA on DOX-Induced Cognitive Impairment Parameters Using Novel Object Recognition (NOR) Test
2.3. Effect of PIRA on DOX-Induced Cognitive Impairment Parameters Using Y-Maze Test
2.4. Effect of PIRA on Acetylcholinesterase Level in the Brain Homogenate of DOX-Treated Animals
2.5. Effect of PIRA on Neuro-Inflammatory Mediators in the Brain Homogenate of DOX-Treated Animals
2.6. Effect of PIRA on Apoptosis Parameters in the Brain Homogenate of DOX-Treated Animals
2.7. Effect of PIRA on Oxidative Parameters in the Brain Homogenate of DOX-Treated Animals
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Groups and Drug Treatment
4.3. Assessment of Spatial Memory
4.3.1. Elevated Plus-Maze (EPM) Test
4.3.2. Novel Object Recognition (NOR) Test
4.3.3. Y-Maze Test
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) Using Brain Homogenate
4.4.1. Brain Samples Collection
4.4.2. Acetylcholinesterase (AChE)
4.4.3. Inflammatory Markers
4.4.4. Apoptotic Proteins
4.4.5. Oxidative Parameters
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winblad, B. Piracetam: A review of pharmacological properties and clinical uses. CNS Drug Rev. 2005, 11, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Malykh, A.G.; Sadaie, M.R. Piracetam and piracetam-like drugs: From basic science to novel clinical applications to CNS disorders. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Gupta, S.; Biswas, J.; Joshi, N.; Singh, A.; Gupta, P.; Tiwari, S.; Sivarama Raju, K.; Chaturvedi, S.; Wahajuddin, M.; et al. New therapeutic activity of metabolic enhancer piracetam in treatment of neurodegenerative disease: Participation of caspase independent death factors, oxidative stress, inflammatory responses and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2078–2096. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Gupta, S.; Biswas, J.; Joshi, N.; Sivarama Raju, K.; Wahajuddin, M.; Singh, S. Metabolic enhancer piracetam attenuates the translocation of mitochondrion-specific proteins of caspase-independent pathway, poly [ADP-Ribose] polymerase 1 up-regulation and oxidative DNA fragmentation. Neurotox. Res. 2018, 34, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Stockburger, C.; Miano, D.; Pallas, T.; Friedland, K.; Müller, W.E. Enhanced neuroplasticity by the metabolic enhancer piracetam associated with improved mitochondrial dynamics and altered permeability transition pore function. Neural Plast. 2016, 2016, 8075903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.; Mahajan, A.; Chen, W.N.; Chowbay, B. Pharmacogenetics of target genes across doxorubicin disposition pathway: A review. Curr. Drug Metab. 2010, 11, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Ramalingayya, G.V.; Cheruku, S.P.; Nayak, P.G.; Kishore, A.; Shenoy, R.; Rao, C.M.; Krishnadas, N. Rutin protects against neuronal damage in vitro and ameliorates doxorubicin-induced memory deficits in vivo in Wistar rats. Drug Des. Devel. Ther. 2017, 11, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Eide, S.; Feng, Z.P. Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef]
- Salas-Ramirez, K.Y.; Bagnall, C.; Frias, L.; Abdali, S.A.; Ahles, T.A.; Hubbard, K. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav. Brain Res. 2015, 292, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Morean, D.F.; O’Dwyer, L.; Cherney, L.R. Therapies for cognitive deficits associated with chemotherapy for breast cancer: A systematic review of objective outcomes. Arch. Phys. Med. Rehabil. 2015, 96, 1880–1897. [Google Scholar] [CrossRef]
- Ren, X.; St Clair, D.K.; Butterfield, D.A. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res. 2017, 117, 267–273. [Google Scholar] [CrossRef]
- Walczak, H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008698. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, B.; Zhou, C.; Bi, Y. Matrine induces apoptosis in angiotensin II-stimulated hyperplasia of cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3 activation. Basic Clin. Pharmacol. Toxicol. 2007, 101, 1–8. [Google Scholar] [CrossRef]
- Shaker, F.H.; El-Derany, M.O.; Wahdan, S.A.; El-Demerdash, E.; El-Mesallamy, H.O. Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 2021, 269, 119078. [Google Scholar] [CrossRef]
- Lyu, W.; Ouyang, M.; Ma, X.; Han, T.; Pi, D.; Qiu, S. Kai-Xin-San attenuates doxorubicin-induced cognitive impairment by reducing inflammation, oxidative stress, and neural degeneration in 4T1 breast cancer mice. Evid. Based Complementary Altern. Med. 2021, 2021, 5521739. [Google Scholar] [CrossRef]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin ameliorates doxorubicin-induced cognitive impairment (chemobrain) in experimental rat model: Impact on oxidative, inflammatory, and apoptotic machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef]
- Kuzu, M.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Caglayan, C.; Turk, E. Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018, 106, 443–453. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P. Levetiracetam ameliorates doxorubicin-induced chemobrain by enhancing cholinergic transmission and reducing neuroinflammation using an experimental rat model and molecular docking study. Molecules 2022, 27, 7364. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, A.F.; El-Sayed, Y.S. All-trans-retinoic acid ameliorates doxorubicin-induced cardiotoxicity: In vivo potential involvement of oxidative stress, inflammation, and apoptosis via caspase-3 and p53 down-expression. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-induced cognitive impairment: The mechanistic insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, Y.A.; Hosny, E.N.; Mohammed, H.S. Protective effect of nanocurcumin against neurotoxicity induced by doxorubicin in rat’s brain. Neurotoxicology 2021, 85, 1–9. [Google Scholar] [CrossRef]
- Mani, V. Betahistine protects doxorubicin-induced memory deficits via cholinergic and anti-inflammatory pathways in mouse brain. Int. J. Pharmacol. 2021, 17, 584–595. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Chigurupati, S.; Sajid, S.; Mani, V. Ameliorative effect of metformin on cyclophosphamide-induced memory impairment in mice. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9660–9666. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Mohammed, H.A.; Elsisi, H.A.; Sajid, S.; Almogbel, Y.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharm. J. 2022, 30, 750–763. [Google Scholar] [CrossRef]
- Silvers, J.M.; Harrod, S.B.; Mactutus, C.F.; Booze, R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods 2007, 166, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Liet, C.; Amenouche, F.; Freret, T.; Boulouard, M.; Mauvieux, B.; Lelong-Boulouard, V.; Bocca, M.L. Effects of acute administration of melatonin on attentional, executive, and working memory processes in rats. Fundam. Clin. Pharmacol. 2015, 29, 472–477. [Google Scholar] [CrossRef]
- Tripathi, A.; Paliwal, P.; Krishnamurthy, S. Piracetam attenuates LPS-induced neuroinflammation and cognitive impairment in rats. Cell Mol. Neurobiol. 2017, 37, 1373–1386. [Google Scholar] [CrossRef]
- Poimenova, A.; Markaki, E.; Rahiotis, C.; Kitraki, E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 2010, 167, 741–749. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Santos, B.; González-Fraile, E.; Zabala, A.; Guillén, V.; Rueda, J.R.; Ballesteros, J. Cognitive improvement of acetylcholinesterase inhibitors in schizophrenia. J. Psychopharmacol. 2018, 32, 1155–1166. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Ali, M.A.; Menze, E.T.; Tadros, M.G.; Tolba, M.F. Caffeic acid phenethyl ester counteracts doxorubicin-induced chemobrain in Sprague-Dawley rats: Emphasis on the modulation of oxidative stress and neuroinflammation. Neuropharmacology 2020, 181, 108334. [Google Scholar] [CrossRef]
- Sivalingam, K.; Samikkannu, T. Neuroprotective effect of piracetam against cocaine-induced neuro epigenetic modification of DNA methylation in astrocytes. Brain Sci. 2020, 10, 611. [Google Scholar] [CrossRef]
- Kikuchi, D.S.; Campos, A.C.P.; Qu, H.; Forrester, S.J.; Pagano, R.L.; Lassègue, B.; Sadikot, R.T.; Griendling, K.K.; Hernandes, M.S. Poldip2 mediates blood-brain barrier disruption in a model of sepsis-associated encephalopathy. J. Neuroinflamm. 2019, 16, 241. [Google Scholar] [CrossRef]
- Nassar, A.; Sharon-Granit, Y.; Azab, A.N. Psychotropic drugs attenuate lipopolysaccharide-induced hypothermia by altering hypothalamic levels of inflammatory mediators in rats. Neurosci. Lett. 2016, 626, 59–67. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, J.; Cao, J.; Wang, G.; Bai, H. Geniposide alleviates traumatic brain injury in rats via anti-inflammatory effect and MAPK/NF-κB inhibition. Cell Mol. Neurobiol. 2020, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.H.; Karam, R.A.; Amer, M.G. Epicatechin attenuates doxorubicin-induced brain toxicity: Critical role of TNF-α, iNOS and NF-κB. Brain Res. Bull. 2011, 86, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Sig. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xu, J.; Xu, J.; Zheng, W.; Chen, Q.; Jiao, D. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol. Med. Rep. 2018, 17, 5007–5012. [Google Scholar] [CrossRef] [Green Version]
- Keeney, J.T.; Miriyala, S.; Noel, T.; Moscow, J.A.; St Clair, D.K.; Butterfield, D.A. Superoxide induces protein oxidation in plasma and TNF-α elevation in macrophage culture: Insights into mechanisms of neurotoxicity following doxorubicin chemotherapy. Cancer Lett. 2015, 367, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Keeney, J.; Ren, X.; Warrier, G.; Noel, T.; Powell, D.K.; Brelsfoard, J.M.; Sultana, R.; Saatman, K.E.; Clair, D.; Butterfield, D.A. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: Protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget 2018, 9, 30324–30339. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, J.; Esposti, M.D.; Gilmore, A.P. Bcl-2 proteins and mitochondria—Specificity in membrane targeting for death. Biochim. Biophys. Acta. 2011, 1813, 532–539. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Li, J.; Li, E. Dexmedetomidine reduces the inflammation and apoptosis of doxorubicin-induced myocardial cells. Exp. Mol. Pathol. 2020, 113, 104371. [Google Scholar] [CrossRef]
- Tangpong, J.; Miriyala, S.; Noel, T.; Sinthupibulyakit, C.; Jungsuwadee, P.; St Clair, D.K. Doxorubicin-induced central nervous system toxicity and protection by xanthone derivative of Garcinia mangostana. Neurosci. 2011, 175, 292–299. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P.; Arfeen, M. Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats. Pharmaceuticals 2022, 15, 1563. https://doi.org/10.3390/ph15121563
Mani V, Rabbani SI, Shariq A, Amirthalingam P, Arfeen M. Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats. Pharmaceuticals. 2022; 15(12):1563. https://doi.org/10.3390/ph15121563
Chicago/Turabian StyleMani, Vasudevan, Syed Imam Rabbani, Ali Shariq, Palanisamy Amirthalingam, and Minhajul Arfeen. 2022. "Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats" Pharmaceuticals 15, no. 12: 1563. https://doi.org/10.3390/ph15121563
APA StyleMani, V., Rabbani, S. I., Shariq, A., Amirthalingam, P., & Arfeen, M. (2022). Piracetam as a Therapeutic Agent for Doxorubicin-Induced Cognitive Deficits by Enhancing Cholinergic Functions and Reducing Neuronal Inflammation, Apoptosis, and Oxidative Stress in Rats. Pharmaceuticals, 15(12), 1563. https://doi.org/10.3390/ph15121563