Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model
Abstract
:1. Introduction
2. Results
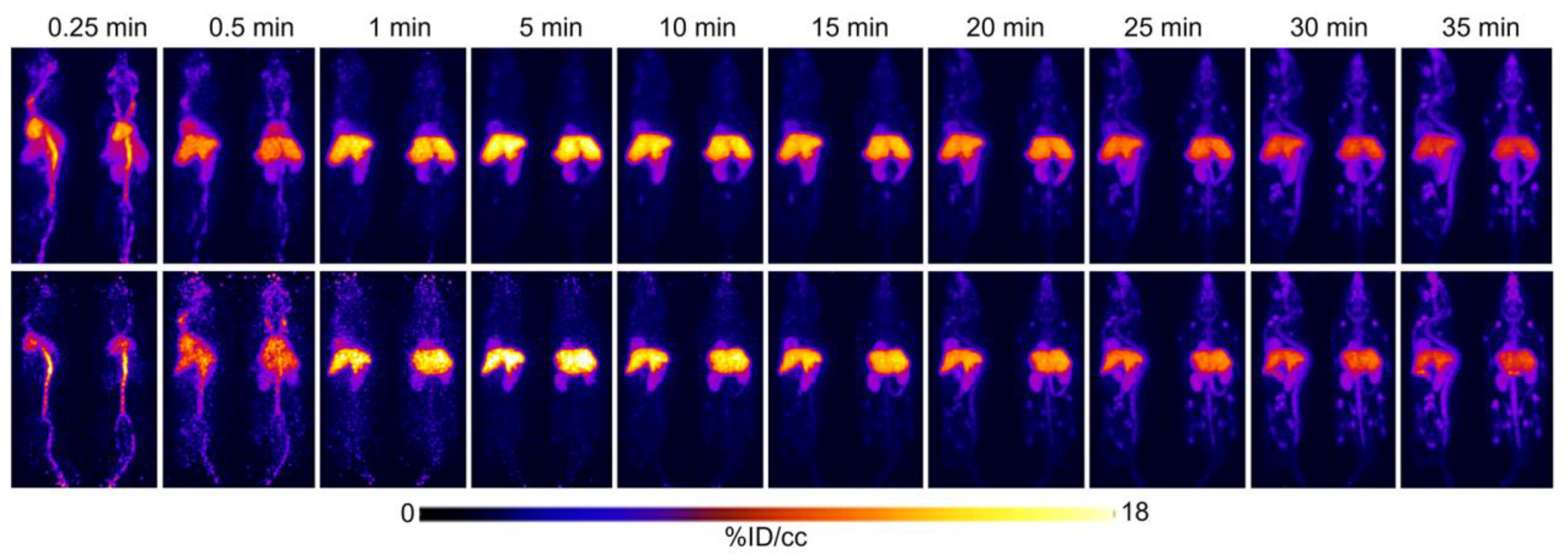
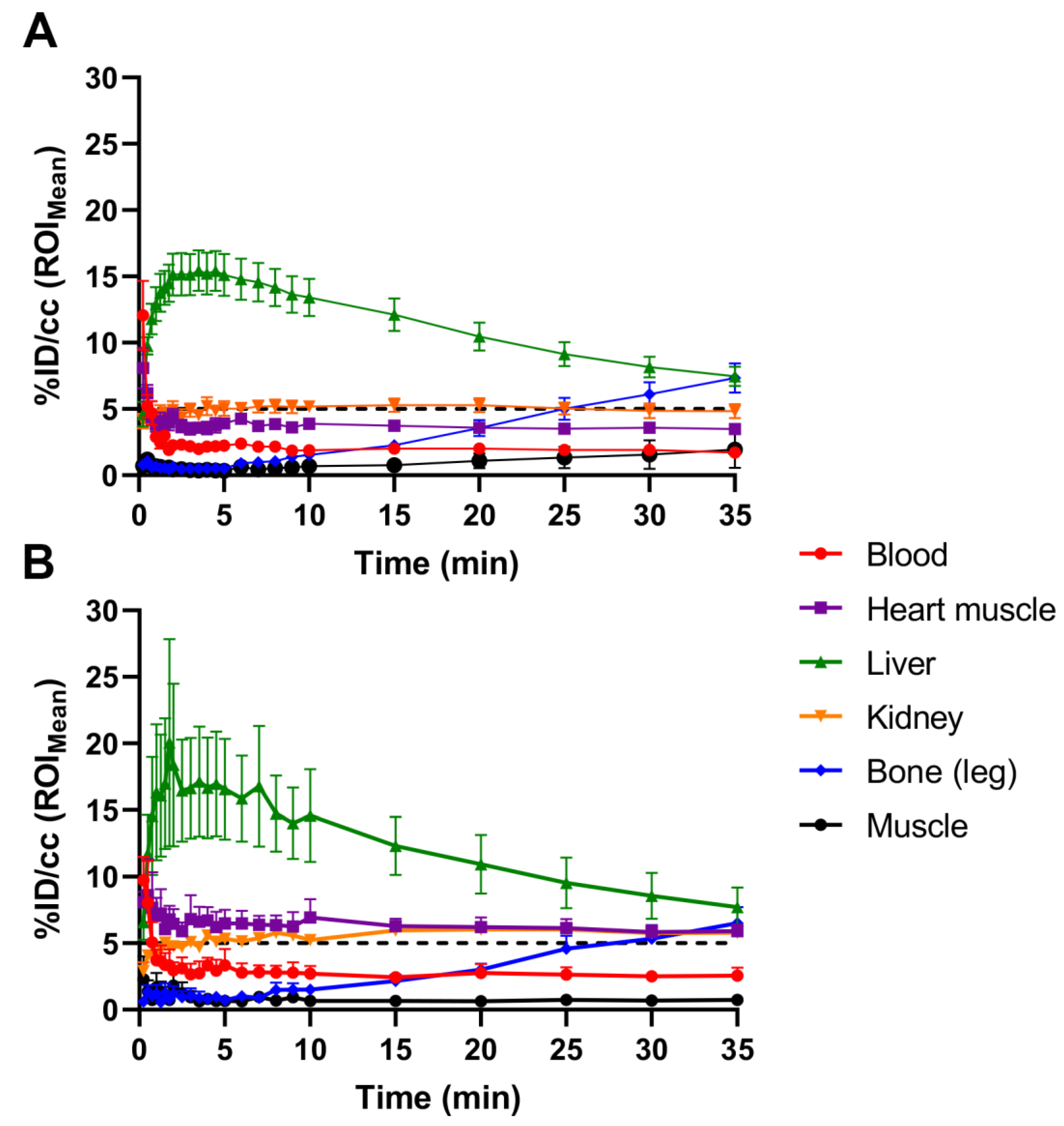
2.1. Initial [18F]FTO Uptake Studies in WT Male Mice
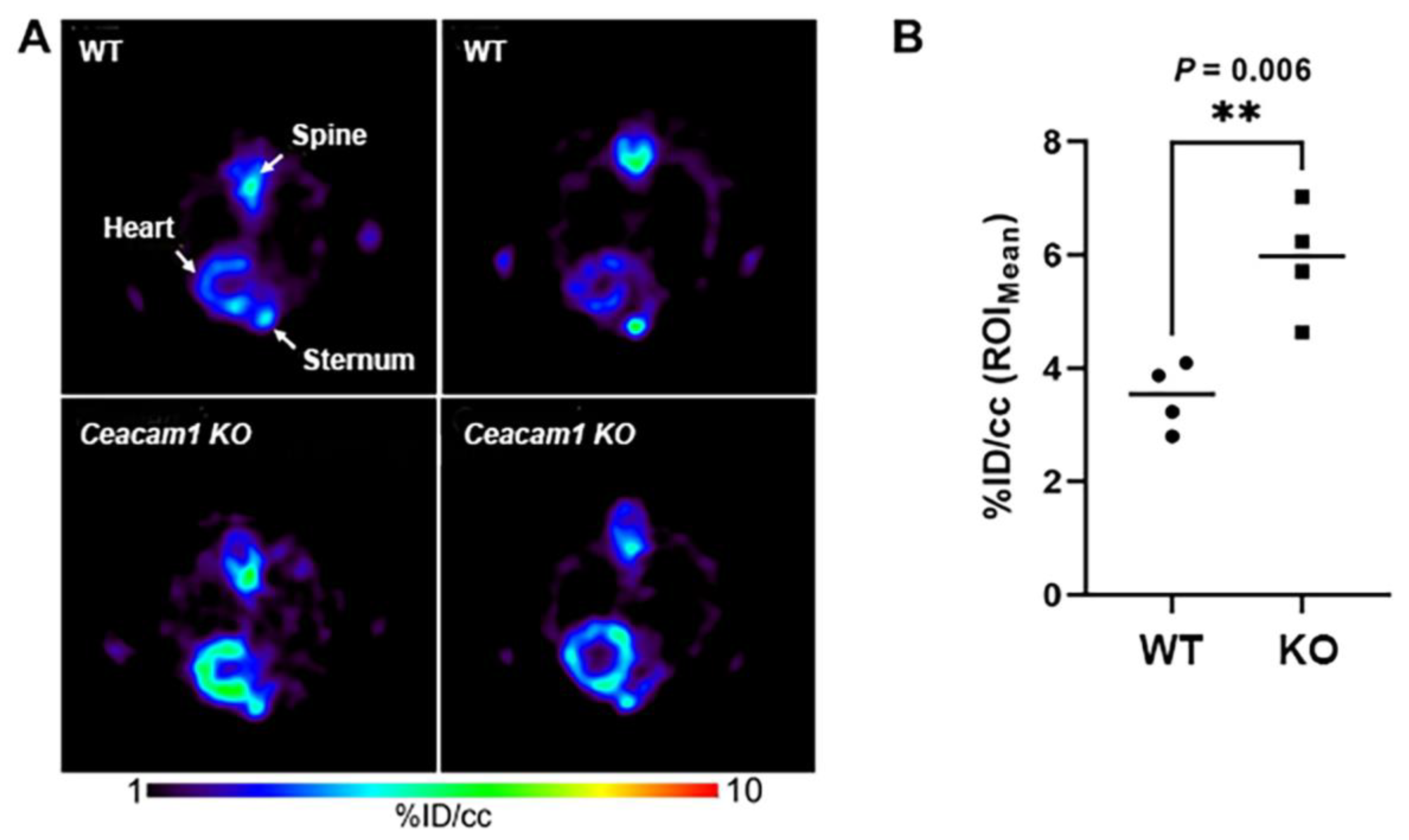
2.2. [18F]FTO Uptake Studies in WT vs. Ceacam1−/− Male Mice
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.1.1. Radiosynthesis
4.1.2. PET/MR Imaging and Biodistributions
4.1.3. Image Analysis
4.1.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasper, P.; Martin, A.; Lang, S.; Kutting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Tomeno, W.; Imajo, K.; Takayanagi, T.; Ebisawa, Y.; Seita, K.; Takimoto, T.; Honda, K.; Kobayashi, T.; Nogami, A.; Kato, T.; et al. Complications of Non-Alcoholic Fatty Liver Disease in Extrahepatic Organs. Diagnostics 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef]
- Lauridsen, B.K.; Stender, S.; Kristensen, T.S.; Kofoed, K.F.; Kober, L.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279 013 individuals. Eur. Heart J. 2018, 39, 385–393. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life. Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nagata, C.; Takeda, J.; Sarui, H.; Kawahito, Y.; Yoshida, N.; Suetsugu, A.; Kato, T.; et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J. Gastroenterol. 2007, 13, 1579–1584. [Google Scholar] [CrossRef] [Green Version]
- Wojcik-Cichy, K.; Koslinska-Berkan, E.; Piekarska, A. The influence of NAFLD on the risk of atherosclerosis and cardiovascular diseases. Clin. Exp. Hepatol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar]
- Francque, S.; Verrijken, A.; Mertens, I.; Hubens, G.; Van Marck, E.; Pelckmans, P.; Michielsen, P.; Van Gaal, L. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int. J. Obes. 2011, 35, 270–278. [Google Scholar] [CrossRef]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006, 87, 1–16. [Google Scholar] [CrossRef]
- Lai, M.; Chandrasekera, P.C.; Barnard, N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes. 2014, 4, e135. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Kaw, M.; Patel, P.R.; Ledford, K.J.; Bowman, T.A.; McInerney, M.F.; Erickson, S.K.; Bourey, R.E.; Najjar, S.M. Mice with null mutation of Ceacam I develop nonalcoholic steatohepatitis. Hepat. Med. 2010, 2010, 69–78. [Google Scholar]
- Heinrich, G.; Ghadieh, H.E.; Ghanem, S.S.; Muturi, H.T.; Rezaei, K.; Al-Share, Q.Y.; Bowman, T.A.; Zhang, D.; Garofalo, R.S.; Yin, L.; et al. Loss of Hepatic CEACAM1: A Unifying Mechanism Linking Insulin Resistance to Obesity and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Poy, M.N.; Yang, Y.; Rezaei, K.; Fernstrom, M.A.; Lee, A.D.; Kido, Y.; Erickson, S.K.; Najjar, S.M. CEACAM1 regulates insulin clearance in liver. Nat. Genet. 2002, 30, 270–276. [Google Scholar] [CrossRef]
- Russo, L.; Muturi, H.T.; Ghadieh, H.E.; Ghanem, S.S.; Bowman, T.A.; Noh, H.L.; Dagdeviren, S.; Dogbey, G.Y.; Kim, J.K.; Heinrich, G.; et al. Liver-specific reconstitution of CEACAM1 reverses the metabolic abnormalities caused by its global deletion in male mice. Diabetologia 2017, 60, 2463–2474. [Google Scholar] [CrossRef]
- Russo, L.; Muturi, H.T.; Ghadieh, H.E.; Wisniewski, A.M.; Morgan, E.E.; Quadri, S.S.; Landesberg, G.P.; Siragy, H.M.; Vazquez, G.; Scalia, R.; et al. Liver-specific rescuing of CEACAM1 reverses endothelial and cardiovascular abnormalities in male mice with null deletion of Ceacam1 gene. Mol. Metab. 2018, 9, 98–113. [Google Scholar] [CrossRef]
- Gray-Owen, S.D.; Blumberg, R.S. CEACAM1: Contact-dependent control of immunity. Nat. Rev. Immunol. 2006, 6, 433–446. [Google Scholar] [CrossRef]
- Chean, J.; Chen, C.J.; Gugiu, G.; Wong, P.; Cha, S.; Li, H.; Nguyen, T.; Bhatticharya, S.; Shively, J.E. Human CEACAM1-LF regulates lipid storage in HepG2 cells via fatty acid transporter CD36. J. Biol. Chem. 2021, 297, 101311. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.X.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An immune regulatory 3D-printed alginate-pectin construct for immunoisolation of insulin producing cells. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 123, 112009. [Google Scholar] [CrossRef] [PubMed]
- Janah, L.; Kjeldsen, S.; Galsgaard, K.D.; Winther-Sorensen, M.; Stojanovska, E.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Wewer Albrechtsen, N.J. Glucagon Receptor Signaling and Glucagon Resistance. Int. J. Mol. Sci. 2019, 20, 3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, S.; Zhang, W.; Zheng, Y.; Liberman, Z.; Rhodes, C.J.; Eldar-Finkelman, H.; Sun, X.J. Glycogen synthase kinase 3 beta mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J. Endocrinol. 2010, 206, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; La Placa, D.; Gugiu, G.; Thunen, A.; Le, K.; Shively, J.E. Reversal of obesity development in Ceacam1−/− male mice by bone marrow transplantation or introduction of the human CEACAM1 gene. Obesity 2022, 30, 1351–1356. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to beta-oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Najjar, S.M.; Ledford, K.J.; Abdallah, S.L.; Paus, A.; Russo, L.; Kaw, M.K.; Ramakrishnan, S.K.; Muturi, H.T.; Raphael, C.K.; Lester, S.G.; et al. Ceacam1 deletion causes vascular alterations in large vessels. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E519–E529. [Google Scholar] [CrossRef] [Green Version]
- Pahk, K.; Kim, E.J.; Lee, Y.J.; Kim, S.; Seo, H.S. Characterization of glucose uptake metabolism in visceral fat by 18 F-FDG PET/CT reflects inflammatory status in metabolic syndrome. PLoS ONE 2020, 15, e0228602. [Google Scholar] [CrossRef] [Green Version]
- Rosamond, T.L.; Abendschein, D.R.; Sobel, B.E.; Bergmann, S.R.; Fox, K.A. Metabolic fate of radiolabeled palmitate in ischemic canine myocardium: Implications for positron emission tomography. J. Nucl. Med. 1987, 28, 1322–1329. [Google Scholar]
- Christensen, N.L.; Jakobsen, S.; Schacht, A.C.; Munk, O.L.; Alstrup, A.K.O.; Tolbod, L.P.; Harms, H.J.; Nielsen, S.; Gormsen, L.C. Whole-Body Biodistribution, Dosimetry, and Metabolite Correction of [(11)C]Palmitate: A PET Tracer for Imaging of Fatty Acid Metabolism. Mol. Imaging 2017, 16, 1536012117734485. [Google Scholar] [CrossRef] [Green Version]
- DeGrado, T.R.; Coenen, H.H.; Stocklin, G. 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid (FTHA): Evaluation in mouse of a new probe of myocardial utilization of long chain fatty acids. J. Nucl. Med. 1991, 32, 1888–1896. [Google Scholar]
- DeGrado, T.R.; Bhattacharyya, F.; Pandey, M.K.; Belanger, A.P.; Wang, S. Synthesis and preliminary evaluation of 18-(18)F-fluoro-4-thia-oleate as a PET probe of fatty acid oxidation. J. Nucl. Med. 2010, 51, 1310–1317. [Google Scholar] [CrossRef] [Green Version]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Wang, S.; Holden, J.E.; Nickles, R.J.; Taylor, M.; Stone, C.K. Synthesis and preliminary evaluation of (18)F-labeled 4-thia palmitate as a PET tracer of myocardial fatty acid oxidation. Nucl. Med. Biol. 2000, 27, 221–231. [Google Scholar] [CrossRef]
- Kawashita, E.; Ishihara, K.; Nomoto, M.; Taniguchi, M.; Akiba, S. A comparative analysis of hepatic pathological phenotypes in C57BL/6J and C57BL/6N mouse strains in non-alcoholic steatohepatitis models. Sci. Rep. 2019, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Faasse, S.; Braun, H.; Vos, M. The role of NAFLD in cardiometabolic disease: An update. F1000Res 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Aby, E.; Saab, S. Nonobese nonalcoholic fatty liver disease. Clin. Liver. Dis. 2017, 10, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Johnston, T.P.; Waxman, D.J. Circulating free fatty acids are increased independently of PPARgamma activity after administration of poloxamer 407 to mice. Can. J. Physiol. Pharmacol. 2008, 86, 643–649. [Google Scholar] [CrossRef]
- Nishikawa, S.; Doi, K.; Nakayama, H.; Uetsuka, K. The effect of fasting on hepatic lipid accumulation and transcriptional regulation of lipid metabolism differs between C57BL/6J and BALB/cA mice fed a high-fat diet. Toxicol. Pathol. 2008, 36, 850–857. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, A.M.; Heinrich, G.; Dai, T.; Bowman, T.A.; Patel, P.R.; Lee, S.J.; Hong, E.G.; Jung, D.Y.; Assmann, A.; Kulkarni, R.N.; et al. Carcinoembryonic antigen-related cell adhesion molecule 1: A link between insulin and lipid metabolism. Diabetes 2008, 57, 2296–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, E.; Dubois, M.J.; Leung, N.; Charbonneau, A.; Turbide, C.; Avramoglu, R.K.; DeMarte, L.; Elchebly, M.; Streichert, T.; Levy, E.; et al. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology 2009, 150, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, G.T.; Shrestha, U.M.; Seo, Y. PET imaging of glucose and fatty acid metabolism for NAFLD patients. J. Nucl. Cardiol. 2020, 27, 1689–1697. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Cohney, S.; De Michieli, F.; Pinach, S.; Saba, F.; Gambino, R. Fatty Liver and Chronic Kidney Disease: Novel Mechanistic Insights and Therapeutic Opportunities. Diabetes Care 2016, 39, 1830–1845. [Google Scholar] [CrossRef] [Green Version]
- Leung, N.; Turbide, C.; Olson, M.; Marcus, V.; Jothy, S.; Beauchemin, N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene 2006, 25, 5527–5536. [Google Scholar] [CrossRef] [Green Version]
- Loening, A.M.; Gambhir, S.S. AMIDE: A free software tool for multimodality medical image analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef]
| Organ/Tissue | WT Mice | Ceacam1−/− Mice | p Value |
|---|---|---|---|
| Heart 2 | 1.66 ± 0.45 | 2.82 ± 0.45 | <0.01 |
| Lung | 0.32 ± 0.08 | 0.45 ± 0.06 | 0.02 |
| Pancreas | 0.25 ± 0.02 | 0.52 ± 0.24 | 0.04 |
| Spleen | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.71 |
| Kidneys | 5.19 ± 0.49 | 8.76 ± 1.81 | <0.01 |
| Liver 3 | 15.33 ± 2.80 | 15.23 ± 4.17 | 0.96 |
| Liver/gallbladder | 15.55 ± 2.73 | 15.49 ± 4.24 | 0.98 |
| Stomach | 0.33 ± 0.08 | 0.45 ± 0.10 | 0.07 |
| Duodenum | 3.34 ± 0.52 | 2.96 ± 0.51 | 0.28 |
| Small intestine | 1.52 ± 0.03 | 2.17 ± 0.20 | <0.01 |
| Cecum | 0.22 ± 0.04 | 0.24 ± 0.06 | 0.41 |
| Large intestine | 0.36 ± 0.07 | 0.45 ± 0.20 | 0.39 |
| Gallbladder | 0.22 ± 0.13 | 0.26 ± 0.23 | 0.71 |
| Brown fat | 0.31 ± 0.03 | 0.27 ± 0.08 | 0.33 |
| Brain | 0.42 ± 0.07 | 0.32 ± 0.09 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, W.; Peng, J.; Wong, P.; Kandeel, F.; Olafsen, T.; Shively, J.E. Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model. Pharmaceuticals 2022, 15, 1577. https://doi.org/10.3390/ph15121577
Li J, Hu W, Peng J, Wong P, Kandeel F, Olafsen T, Shively JE. Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model. Pharmaceuticals. 2022; 15(12):1577. https://doi.org/10.3390/ph15121577
Chicago/Turabian StyleLi, Junfeng, Weidong Hu, Jiangling Peng, Patty Wong, Fouad Kandeel, Tove Olafsen, and John E. Shively. 2022. "Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model" Pharmaceuticals 15, no. 12: 1577. https://doi.org/10.3390/ph15121577
APA StyleLi, J., Hu, W., Peng, J., Wong, P., Kandeel, F., Olafsen, T., & Shively, J. E. (2022). Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model. Pharmaceuticals, 15(12), 1577. https://doi.org/10.3390/ph15121577







