Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells
Abstract
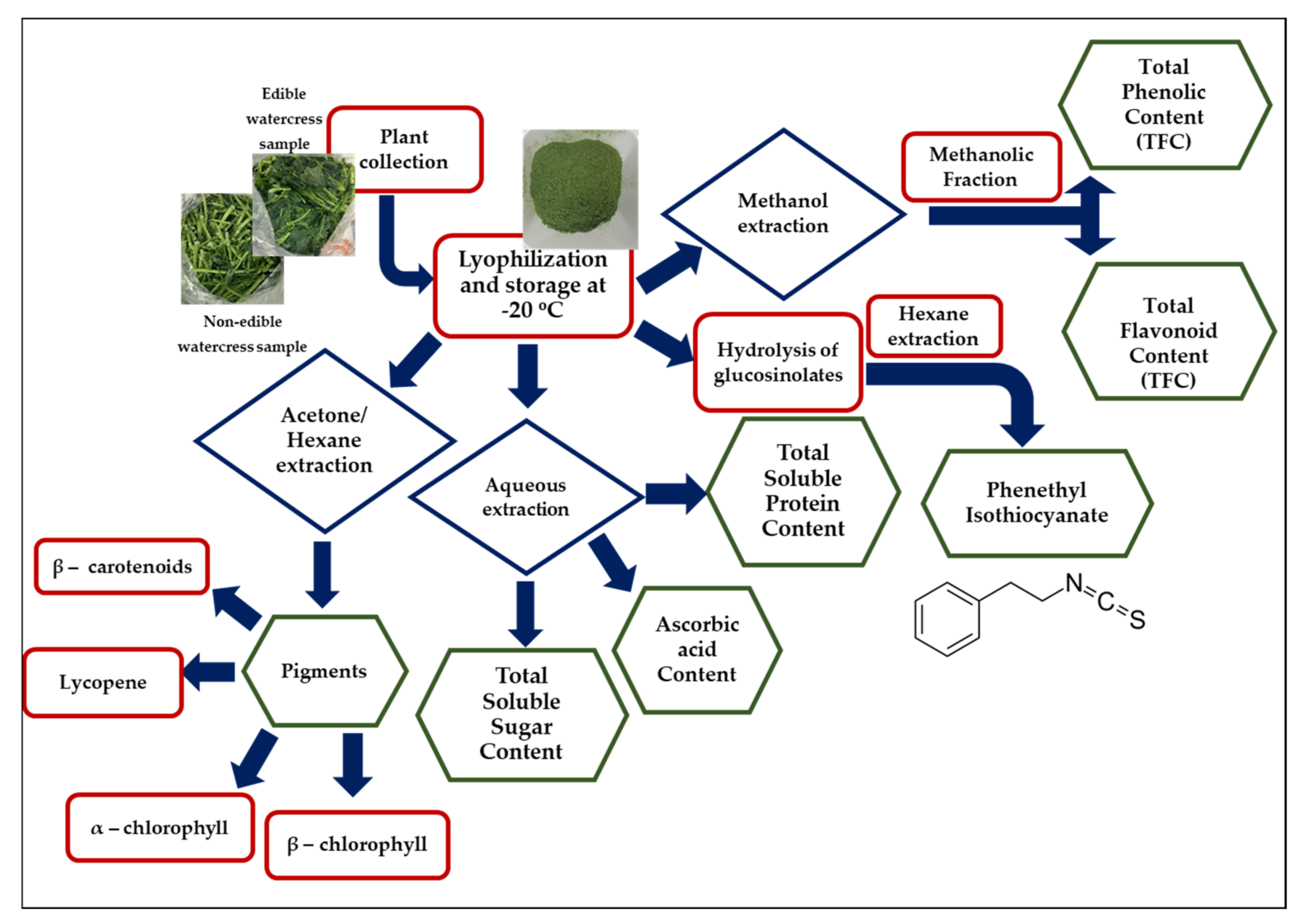
:1. Introduction
2. Results
2.1. Extraction of PEITC from Edible and Non-Edible Watercress Samples
2.2. Determination of Total Polyphenol (TPC) and Flavonoid (TFC) Contents in Edible and Non-Edible Watercress Extracts
2.3. Extraction of Total Soluble Proteins, Sugars and Ascorbic Acid, as Well as Various Pigments from Edible and Non-Edible Watercress Samples
2.4. Antioxidant Evaluation of Edible and Non-Edible Watercress Extracts
2.5. Biological Evaluation of Edible and Non-Edible Watercress Extracts
3. Discussion
4. Materials and Methods
4.1. List of Reagents
4.2. Plant Material Cultivation, Processing and Storage
4.3. Hydrolysis of Glucosinolates, Extraction and Quantification of PEITC
4.4. Determination of Total Flavonoid and Phenolic Contents
4.5. Determination of Pigments
4.6. Determination of Total Soluble Protein Content
4.7. Determination of Total Soluble Sugar Content
4.8. Extraction and Quantification of Ascorbic Acid
4.9. Determination of Antioxidant Activity
4.10. Cell Lines
4.11. Determination of Cell Viability
4.12. Determination of Malondialdehyde and Protein Carbonyl Contents
4.13. Determination of Caspase Activity
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef] [PubMed]
- Wentzell, A.M.; Kliebenstein, D.J. Genotype, age, tissue, and environment regulate the structural outcome of glucosinolate activation. Plant Physiol. 2008, 147, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica oleracea Varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food. 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by aselection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef]
- Mirza, N.; Schulz, A.; Halkier, B.A. Characterization of methylsulfinylalkyl glucosinolate specific polyclonal antibodies. J. Plant Biochem. Biotechnol. 2016, 25, 433–436. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Vo, Q.V.; Rochfort, S.; Nam, P.C.; Nguyen, T.L.; Nguyen, T.T.; Mechler, A. Synthesis of aromatic and indole alpha-glucosinolates. Carbohydr. Res. 2018, 455, 45–53. [Google Scholar] [CrossRef]
- Kristal, A.R.; Lampe, J.W. Brassica Vegetables and Prostate Cancer Risk: A Review of the Epidemiological Evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef]
- Kolonel, L.N.; Hankin, J.H.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P.; Wilkens, L.R.; John, E.M.; Howe, G.R.; Dreon, D.M.; West, D.W.; et al. Vegetables, Fruits, Legumes and Prostate Cancer: A Multiethnic Case-Control Study. Cancer Epidemiol. Biomarkers. Prev. 2000, 9, 795–804. [Google Scholar]
- Dixon, M.J.; Shaw, P.J. Watercress and Water Quality: The Effect of Phenethyl Isothiocyanate on the Mating Behaviour of Gammarus pulex. Int. J. Zool. 2011, 2011, 328749. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Chiang, L.; Chae, K.; Lee, D.-H. Phenethyl isothiocyanate inhibits hypoxia-induced accumulation of HIF-1α and VEGF expression in human glioma cells. Food Chem. 2013, 141, 1841–1846. [Google Scholar] [CrossRef]
- Marynowski, S.W.; Xiao, D.; Lew, K.L.; Zeng, Y.; Dhir, R.; Xiao, H.; Singh, S.V. Phenethyl isothiocyanate, a constituent of processed cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenograftsin vivo in association with induction of Bax and Bid. Cancer Res. 2006, 66, 1316. [Google Scholar]
- Kim, S.H.; Sehrawat, A.; Sakao, K.; Hahm, E.R.; Singh, S.V. Notch activation by phenethyl isothiocyanate attenuates its inhibitory effect on prostate cancer cell migration. PLoS ONE 2011, 6, e26615. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Budhraja, A.; Cheng, S.; Liu, E.H.; Chen, J.; Yang, Z.; Chen, D.; Zhang, Z.; Shi, X. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death Dis. 2011, 2, e140. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Shi, Y.; Dang, U.J.; Di Pasqua, A.J. Phenethyl isothiocyanate and cisplatin co-encapsulated in a liposomal nanoparticle for treatment of non-small cell lung cancer. Molecules 2019, 24, 801. [Google Scholar] [CrossRef] [Green Version]
- Mi, L.; Gan, N.; Chung, F.-L. Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis 2011, 32, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Tusskorn, O.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, U.; Kukongviriyapan, V. Phenethyl isothiocyanate induces calcium mobilization and mitochondrial cell death pathway in cholangiocarcinoma KKU-M214 cells. BMC Cancer 2013, 13, 571. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.C.; Hsu, S.C.; Kuo, C.L.; Ip, S.W.; Yang, J.S.; Hsu, Y.M.; Huang, H.Y.; Wu, S.H.; Chung, J.G. Phenethyl isothiocyanate inhibited tumor migration and invasion via suppressing multiple signal transduction pathways in human colon cancer HT29 cells. J. Agric. Food Chem. 2010, 58, 11148–11155. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Wang, X.; Govind, S.; Hood, B.L.; Veenstra, T.D.; Conrads, T.P.; Saha, D.T.; Goldman, R.; Chung, F.-L. The Role of Protein Binding in Induction of Apoptosis by Phenethyl Isothiocyanate and Sulforaphane in Human Non–Small Lung Cancer Cells. Cancer Res. 2007, 67, 6409–6416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiogianni, M.; Kyriakou, S.; Anestopoulos, I.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; Panayiotidis, M.I. An Evaluation of the Anti-Carcinogenic Response of Major Isothiocyanates in Non-Metastatic and Metastatic Melanoma Cells. Antioxidants 2021, 10, 284. [Google Scholar] [CrossRef]
- Xiao, D.; Lew, K.L.; Zeng, Y.; Xiao, H.; Marynowski, S.W.; Dhir, R.; Singh, S.V. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis 2006, 27, 2223–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, C.I.R.; Haldar, S.; Boyd, L.A.; Bennett, R.; Whiteford, J.; Butler, M.; Pearson, J.R.; Bradbury, I.; Rowland, I.R. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am. J. Clin. Nutr. 2007, 85, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, L.A.; McCann, M.J.; Hashim, Y.; Bennett, R.N.; Gill, C.I.R.; Rowland, I.R. Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr. Cancer. 2006, 55, 232–241. [Google Scholar] [CrossRef]
- Fallah, N.; Ebrahimi, S. The Anti-Cancer Effect of Watercress (Rorripa Nasturtium Aquaticum) Extract on Breast Cancer Cells. Zahedan J. Res. Med. Sci. 2016, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yazdanparast, R.; Bahramikia, S.; Ardestani, A. Nasturtium Officinale Reduces Oxidative Stress and Enhances Antioxidant Capacity in Hypercholesterolaemic Rats. Chem. Biol. Interact. 2008, 172, 176–184. [Google Scholar] [CrossRef]
- Arias-Carmona, M.D.; Romero-Rodríguez, M.Á.; Vasquez-Oderiz, M.L. Determination of Organic Acids in Brassica Rapa L. Leaves (Turnip Greens and Turnip Tops) Regulated by the Protected Geographical Indication “Grelos De Galicia”. J. Food Nutr. Res. 2014, 2, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Aires, A.; Carvalho, R.; Rosa, E.A.S.; Saavedra, M.J. Phytochemical characterization and antioxidant properties of baby-leaf watercress produced under organic production system. CyTA J. Food. 2013, 11, 343–351. [Google Scholar] [CrossRef]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of antioxidant and antiproliferative compounds from watercress using pressurized fluid extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- Aripin, N.F.B.; Surugau, N. Effects of Temperature and pH on Myrosinase Activity and Gluconasturtiin Hydrolysis Products in Watercress. Trans. Sci. Technol. 2016, 3, 449–454. [Google Scholar]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A. 2014, 1327, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A Comparative Study of Flavonoid Compounds, Vitamin C, and Antioxidant Properties of Baby Leaf Brassicaceae Species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J. Med. Plants Res. 2011, 5, 1147–1154. [Google Scholar]
- Franco, D.; Sineiro, J.; Rubilar, M.; Sánchez, M.; Jerez, M.; Pinelo, M.; Costoya, N.; Núñez, M.J. Polyphenols from plant materials: Extraction and antioxidant power. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3210–3216. [Google Scholar]
- Ojha, S.B.; Roy, S.; Das, S.; Dhangadamajhi, G. Phytochemicals Screening, Phenolic Estimation and Evaluation for Anti-Oxidant, Anti-Inflammatory and Anti-Microbial Activities of Sequentially Soxhlet Extracted Coconut Testa. Food Nutr. Sci. 2019, 10, 900–922. [Google Scholar] [CrossRef] [Green Version]
- Abu-Reidah, I.M.; Gil-Izquierdo, Á.; Medina, S.; Ferreres, F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MS(n). Food Res. Int. 2017, 100, 494–500. [Google Scholar] [CrossRef]
- Liu, S.-C.; Lin, J.-T.; Hu, C.-C.; Shen, B.-Y.; Chen, T.-Y.; Chang, Y.-L.; Shih, C.-H.; Yang, D.-J. Phenolic compositions and antioxidant attributes of leaves and stems from three inbred varieties of Lycium chinense Miller harvested at various times. Food Chem. 2017, 215, 284–291. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4, 35234. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.-T.; Yeh, H.; Su, S.-H.; Lin, J.-S.; Lee, K.-J.; Shyu, H.-W.; Chen, Z.-F.; Huang, S.-Y.; Su, S.-J. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic. Biol. Med. 2014, 74, 1–13. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Shang, P. β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 5021983. [Google Scholar] [CrossRef] [Green Version]
- Cunja, V.; Mikulic-Petkovsek, M.; Zupan, A.; Stampar, F.; Schmitzer, V. Frost decreases content of sugars, ascorbic acid and some quercetin glycosides but stimulates selected carotenes in Rosa canina hips. J. Plant Physiol. 2015, 178, 55–63. [Google Scholar] [CrossRef]
- Sasaki, H.; Ichimura, K.; Okada, K.; Oda, M. Freezing tolerance and soluble sugar contents affected by water stress during cold-acclimation and de-acclimation in cabbage seedlings. Sci. Hortic. 1999, 76, 161–169. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Fei, M.L.I.; Tong, L.I.; Wei, L.I.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Anestopoulos, I.; Kyriakou, S.; Trafalis, D.T.; Franco, R.; Pappa, A.; Panayiotidis, M.I. Benzyl and phenethyl isothiocyanates as promising epigenetic drug compounds by modulating histone acetylation and methylation marks in malignant melanoma. Investig. New Drugs 2021, 39, 1460–1468. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Jenkins, N.C.; Grossman, D. Role of Melanin in Melanocyte Dysregulation of Reactive Oxygen Species. Biomed. Res. Int. 2013, 2013, 908797. [Google Scholar] [CrossRef] [PubMed]
- Meierjohann, S. Oxidative stress in melanocyte senescence and melanoma transformation. Eur. J. Cell Biol. 2014, 93, 36–41. [Google Scholar] [CrossRef]
- Becker, T.M.; Rizos, H.; Kefford, R.F.; Mann, G.J. Functional impairment of melanoma-associated p16(INK4a) mutants in melanoma cells despite retention of cyclin-dependent kinase 4 binding. Clin. Cancer Res. 2001, 7, 3282–3288. [Google Scholar]
- Jenkins, N.C.; Liu, T.; Cassidy, P.; Leachman, S.A.; Boucher, K.M.; Goodson, A.G.; Samadashwily, G.; Grossman, D. The p16(INK4A) tumor suppressor regulates cellular oxidative stress. Oncogene 2011, 30, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, N.C.; Jung, J.; Liu, T.; Wilde, M.; Holmen, S.L.; Grossman, D. Familial melanoma-associated mutations in p16 uncouple its tumor-suppressor functions. J. Investig. Dermatol. 2013, 133, 1043–1051. [Google Scholar] [CrossRef] [Green Version]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Mishra, R.; Patel, H.; Alanazi, S.; Wei, X.; Ma, Z.; Garrett, J.T. BRAF Mutant Melanoma Adjusts to BRAF/MEK Inhibitors via Dependence on Increased Antioxidant SOD2 and Increased Reactive Oxygen Species Levels. Cancers 2020, 12, 1661. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef] [Green Version]
- Su, J.-C.; Lin, K.; Wang, Y.; Sui, S.-H.; Gao, Z.-Y.; Wang, Z.-G. In vitro studies of phenethyl isothiocyanate against the growth of LN229 human glioma cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4269–4276. [Google Scholar] [PubMed]
- Wang, Y.; Wei, S.; Wang, J.; Fang, Q.; Chai, Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Mol. Med. Rep. 2014, 10, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Halm, E.-R.; Singh, S.V. Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Mol. Carcinog. 2012, 51, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Govind, S.; Sajankila, S.P.; Mi, L.; Roy, R.; Chung, F.-L. Phenethyl isothiocyanate sensitizes human cervical cancer cells to apoptosis induced by cisplatin. Mol. Nutr. Food Res. 2011, 55, 1572–1581. [Google Scholar] [CrossRef] [Green Version]
- Mantso, T.; Sfakianos, A.P.; Atkinson, A.; Anestopoulos, I.; Mitsiogianni, M.; Botaitis, S.; Perente, S.; Simopoulos, C.; Vasileiadis, S.; Franco, R.; et al. Development of a novel experimental in vitro model of isothiocyanate-induced apoptosis in human malignant melanoma cells. Anticancer Res. 2016, 36, 6303–6309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiogianni, M.; Amery, T.; Franco, R.; Zoumpourlis, V.; Pappa, A.; Panayiotidis, M.I. From chemo-prevention to epigenetic regulation: The role of isothiocyanates in skin cancer prevention. Pharmacol Ther. 2018, 190, 187–201. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). SpringerPlus 2015, 4, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Tian, Y.-X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.-R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [Green Version]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Soran, M.L.; Marutoiu, C. Extraction and HPLC determination of the ascorbic acid content of three indigenous spice plants. J. Anal. Chem. 2014, 69, 998–1002. [Google Scholar] [CrossRef]





| Watercress Sample | Solvent | [PEITC] (μg/g of Dry Extract) |
|---|---|---|
| Edible | Hexane | 1695 ± 100.46 a |
| Chloroform | 895 ± 21.54 b | |
| Ethyl Acetate | 1023 ± 98.61 c | |
| Non-edible | Hexane | 1002 ± 94.21 a |
| Chloroform | 0.12 ± 0.05 b | |
| Ethyl Acetate | 1.23 ± 1.01 c |
| Hydrolysis of GLs—Hexane Fraction | Expression Units | ||
|---|---|---|---|
| Content | Edible Watercress Sample | Non-Edible Watercress Sample | |
| PEITC | 1695 ± 100.46 | 1002 ± 94.21 | μg/g dry watercress |
| Ascorbic acid | 0.6021 ± 0.03 | 0.1015 ± 0.09 | mg of ascorbic acid/g of dry extract |
| TSSC | 212.45 ± 7.71 | 106.26 ± 2.36 | nmol of mannose equivalents/g of dry extract |
| TSPC | 15.98 ± 0.03 | 31.71 ± 0.09 | mg of BSA equivalents/mL/g of dry extract |
| Pigments | 14.22 ± 0.99 | 11.21 ± 2.66 | mg of lycopene/g of dry extract |
| 16.82 ± 0.56 | 17.18 ± 1.69 | mg chlorophyll–a/g of dry extract | |
| 36.02 ± 2.13 | 25.93 ± 2.73 | mg chlorophyll–b/g of dry extract | |
| 0.077 ± 0.01 | 0.053 ± 0.002 | mg β–carotene/g of dry extract | |
| TPC | 47.66 ± 0.63 | 9.31 ± 1.51 | mg of gallic acid equivalent/g of dry extract |
| TFC | 64.52 ± 2.69 | 12.94 ± 0.91 | mg of rutin equivalents/g or dry extract |
| 13.55 ± 2.28 | 19.29 ± 1.88 | mg of catechin equivalents/g of dry extract | |
| EC50 (% v/v) (μΜ PEITC) | ||||
|---|---|---|---|---|
| Cell Line | Time (h) | Synthetic PEITC (μΜ) | Edible Watercress Sample | Non-Edible Watercress Sample |
| A375 | 24 | 28.44 ± 1.12 | 2.31 ± 0.14 | 2.61 ± 0.11 |
| (9.99 ± 0.71 μM) | (24.13 ± 1.99 μM) | |||
| 48 | 11.66 ± 2.26 | 1.31 ± 0.10 | 2.54 ± 0.12 | |
| (2.48 ± 0.11 μM) | (13.48 ± 1.19 μM) | |||
| 72 | 7.23 ± 1.38 | 1.21 ± 0.12 | 2.03 ± 0.15 | |
| (1.78 ± 0.52 μM) | (6.27 ± 1.07 μM) | |||
| A431 | 24 | 23.22 ± 1.11 | 3.45 ± 0.21 | 4.89 ± 1.12 |
| (37.29 ± 2.42μM) | (42.10 ± 2.23 μM) | |||
| 48 | 19.48 ± 2.21 | 2.61 ± 0.13 | 4.82 ± 1.23 | |
| (15.28 ± 1.06 μM) | (37.12 ± 1.77 μM) | |||
| 72 | 16.69 ± 1.37 | 2.64 ± 0.09 | 4.8 ± 1.11 | |
| (17.42 ± 1.13 μM) | (36.01 ± 2.1 μM) | |||
| HaCaT | 24 | 44.27 ± 1.2 | n.d. | n.d. |
| 48 | 33.97 ± 1.27 | 4.96 ± 0.23 | n.d. | |
| (45.42 ± 2.23 μM) | ||||
| 72 | 25.6 ± 2.11 | 4.92 ± 0.14 | n.d. | |
| (38.92 ± 1.25 μM) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, S.; Tragkola, V.; Alghol, H.; Anestopoulos, I.; Amery, T.; Stewart, K.; Winyard, P.G.; Trafalis, D.T.; Franco, R.; Pappa, A.; et al. Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells. Pharmaceuticals 2022, 15, 141. https://doi.org/10.3390/ph15020141
Kyriakou S, Tragkola V, Alghol H, Anestopoulos I, Amery T, Stewart K, Winyard PG, Trafalis DT, Franco R, Pappa A, et al. Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells. Pharmaceuticals. 2022; 15(2):141. https://doi.org/10.3390/ph15020141
Chicago/Turabian StyleKyriakou, Sotiris, Venetia Tragkola, Heba Alghol, Ioannis Anestopoulos, Tom Amery, Kyle Stewart, Paul G. Winyard, Dimitrios T. Trafalis, Rodrigo Franco, Aglaia Pappa, and et al. 2022. "Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells" Pharmaceuticals 15, no. 2: 141. https://doi.org/10.3390/ph15020141
APA StyleKyriakou, S., Tragkola, V., Alghol, H., Anestopoulos, I., Amery, T., Stewart, K., Winyard, P. G., Trafalis, D. T., Franco, R., Pappa, A., & Panayiotidis, M. I. (2022). Evaluation of Bioactive Properties of Lipophilic Fractions of Edible and Non-Edible Parts of Nasturtium officinale (Watercress) in a Model of Human Malignant Melanoma Cells. Pharmaceuticals, 15(2), 141. https://doi.org/10.3390/ph15020141









