Behavioral, Anti-Inflammatory, and Neuroprotective Effects of a Novel FPR2 Agonist in Two Mouse Models of Autism
Abstract
:1. Introduction
2. Results
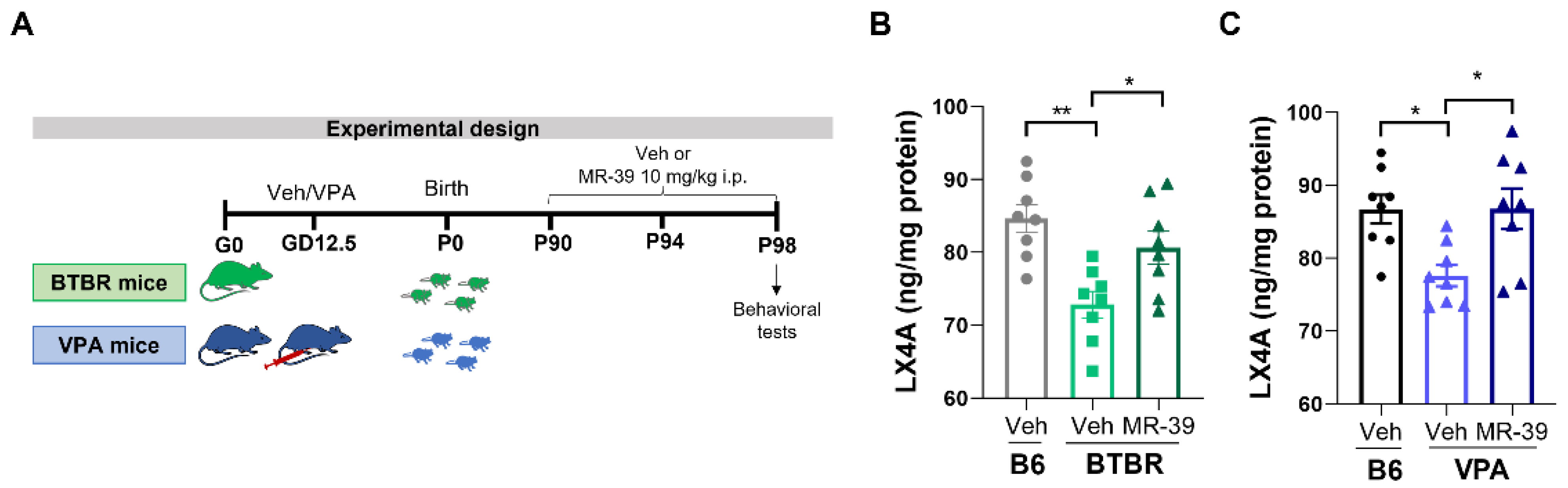
2.1. MR-39 Modulates the Expression of Lipoxin A4 and Its Receptor FPR2 in ASD Mouse Models
2.2. The FPR2 Agonist MR-39 Modulates Hippocampal Cytokines in ASD Mouse Models
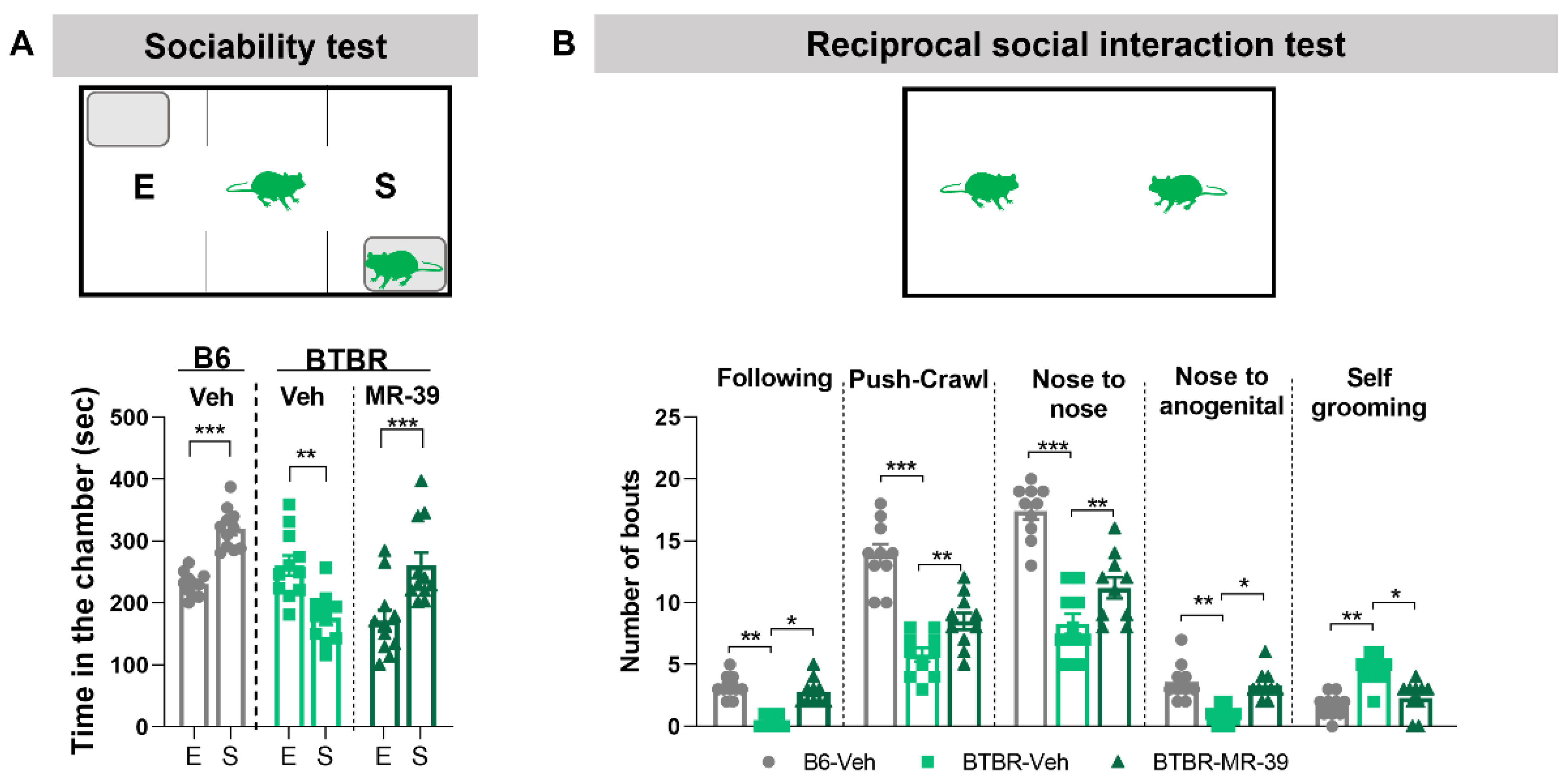
2.3. The FPR2 Agonist MR-39 Increased Sociability of BTBR Mice
2.4. The FPR2 Agonist MR-39 Increased Sociability of VPA Mice
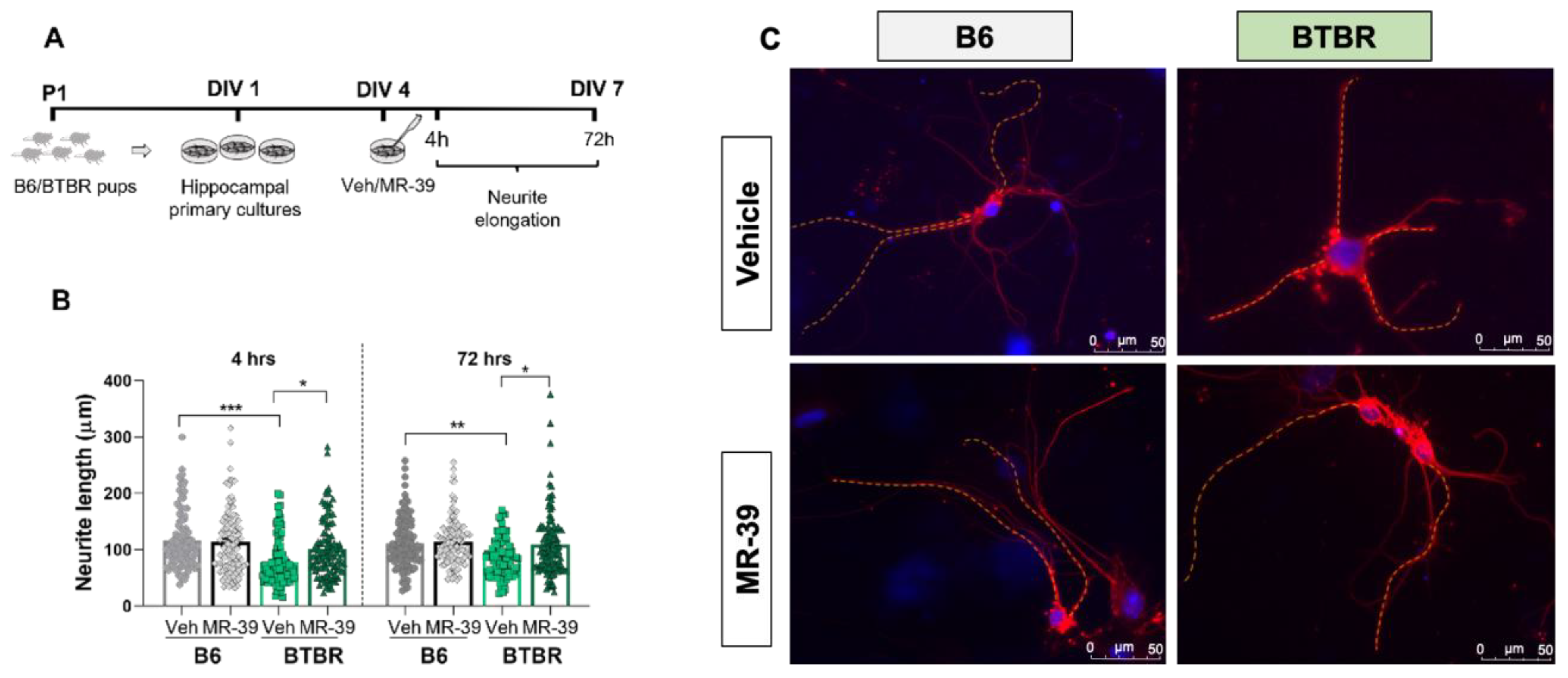
2.5. The FPR2 Agonist MR-39 Selectively Stimulates Neurite Outgrowth in Hippocampal Neurons of BTBR Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.1.1. BTBR Mouse Strain
4.1.2. VPA Mouse Model
4.2. Drug and Treatment
4.3. Behavioral Tests
4.3.1. Social Approach Test
4.3.2. Reciprocal Social Interaction Test
4.4. Sample Collection
4.5. RNA Extraction and Quantification of Gene Expression Using RT-PCR
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Culturing of Hippocampal Neurons
4.8. Immunofluorescence and Morphological Analyses
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, C.; Risi, S.; DiLavore, P.S.; Shulman, C.; Thurm, A.; Pickles, A. Autism From 2 to 9 Years of Age. Arch. Gen. Psychiatry 2006, 63, 694. [Google Scholar] [CrossRef] [PubMed]
- Losh, M.; Piven, J. Social-Cognition and the Broad Autism Phenotype: Identifying Genetically Meaningful Phenotypes. J. Child Psychol. Psychiatry 2007, 48, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, F.R.; Lord, C.; Bailey, A.; Schultz, R.T.; Klin, A. Autism and Pervasive Developmental Disorders. J. Child Psychol. Psychiatry 2004, 45, 135–170. [Google Scholar] [CrossRef]
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). CoDAS 2013, 25, 190–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, E.B.; Neale, B.M.; Hyman, S.E. Genetic Research in Autism Spectrum Disorders. Curr. Opin. Pediatr. 2015, 27, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depino, A.M. Peripheral and Central Inflammation in Autism Spectrum Disorders. Mol. Cell. Neurosci. 2013, 53, 69–76. [Google Scholar] [CrossRef]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients with Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated Plasma Cytokines in Autism Spectrum Disorders Provide Evidence of Immune Dysfunction and Are Associated with Impaired Behavioral Outcome. Brain. Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Ashwood, P.; Corbett, B.A.; Kantor, A.; Schulman, H.; Van de Water, J.; Amaral, D.G. In Search of Cellular Immunophenotypes in the Blood of Children with Autism. PLoS ONE 2011, 6, e19299. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated Immune Response in the Brain of Autistic Patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Sadeq, A.; Sasse, A.; Sadek, B. Role of Neuroinflammation in Autism Spectrum Disorder and the Emergence of Brain Histaminergic System. Lessons Also for BPSD? Front. Pharmacol. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.H.; Kelley, K.W. Immune–Neural Connections: How the Immune System’s Response to Infectious Agents Influences Behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [Green Version]
- Yirmiya, R.; Goshen, I. Immune Modulation of Learning, Memory, Neural Plasticity and Neurogenesis. Brain. Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Ifert-Miller, F.; Najib, U.; Bashir, S.; Gonzalez-Heydrich, J.; Picker, J.; Rotenberg, A.; Pascual-Leone, A. Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. J. Child Adolesc. Psychopharmacol. 2016, 26, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.; Man, H.-Y. Fundamental Elements in Autism: From Neurogenesis and Neurite Growth to Synaptic Plasticity. Front. Cell. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Penna, E.; Pizzella, A.; Cimmino, F.; Trinchese, G.; Cavaliere, G.; Catapano, A.; Allocca, I.; Chun, J.T.; Campanozzi, A.; Messina, G.; et al. Neurodevelopmental Disorders: Effect of High-Fat Diet on Synaptic Plasticity and Mitochondrial Functions. Brain Sci. 2020, 10, 805. [Google Scholar] [CrossRef]
- Nam, S.M.; Kim, J.W.; Kwon, H.J.; Yoo, D.Y.; Jung, H.Y.; Kim, D.W.; Hwang, I.K.; Seong, J.K.; Yoon, Y.S. Differential Effects of Low- and High-Dose Zinc Supplementation on Synaptic Plasticity and Neurogenesis in the Hippocampus of Control and High-Fat Diet-Fed Mice. Neurochem. Res. 2017, 42, 3149–3159. [Google Scholar] [CrossRef]
- Hall, J.M.; Gomez-Pinilla, F.; Savage, L.M. Nerve Growth Factor Is Responsible for Exercise-Induced Recovery of Septohippocampal Cholinergic Structure and Function. Front. Neurosci. 2018, 12, 773. [Google Scholar] [CrossRef]
- Abookasis, D.; Lerman, D.; Roth, H.; Tfilin, M.; Turgeman, G. Optically Derived Metabolic and Hemodynamic Parameters Predict Hippocampal Neurogenesis in the BTBR Mouse Model of Autism. J. Biophotonics 2018, 11, e201600322. [Google Scholar] [CrossRef]
- Cai, Y.; Zhong, H.; Li, X.; Xiao, R.; Wang, L.; Fan, X. The Liver X Receptor Agonist TO901317 Ameliorates Behavioral Deficits in Two Mouse Models of Autism. Front. Cell. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Moieni, M.; Eisenberger, N.I. Effects of Inflammation on Social Processes and Implications for Health: Effect of Inflammation on Social Processes. Ann. N. Y. Acad. Sci. 2018, 1428, 5–13. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.G.; Kusek, G.K.; Yang, M.; Phoenix, J.L.; Bolivar, V.J.; Crawley, J.N. Autism-like Behavioral Phenotypes in BTBR T+tf/J Mice. Genes Brain Behav. 2008, 7, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Onore, C.E.; Careaga, M.; Babineau, B.A.; Schwartzer, J.J.; Berman, R.F.; Ashwood, P. Inflammatory Macrophage Phenotype in BTBR T+tf/J Mice. Front. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Schneider, T.; Przewłocki, R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology 2005, 30, 80–89. [Google Scholar] [CrossRef]
- Roullet, F.I.; Lai, J.K.Y.; Foster, J.A. In Utero Exposure to Valproic Acid and Autism—A Current Review of Clinical and Animal Studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Podestá, M.F.; Uccelli, N.A.; Reinés, A. Differential Local Connectivity and Neuroinflammation Profiles in the Medial Prefrontal Cortex and Hippocampus in the Valproic Acid Rat Model of Autism. Dev. Neurosci. 2015, 37, 215–231. [Google Scholar] [CrossRef]
- Wagner, G.C.; Reuhl, K.R.; Cheh, M.; McRae, P.; Halladay, A.K. A New Neurobehavioral Model of Autism in Mice: Pre- and Postnatal Exposure to Sodium Valproate. J. Autism Dev. Disord. 2006, 36, 779–793. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism Spectrum Disorder: Neuropathology and Animal Models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Juliandi, B.; Tanemura, K.; Igarashi, K.; Tominaga, T.; Furukawa, Y.; Otsuka, M.; Moriyama, N.; Ikegami, D.; Abematsu, M.; Sanosaka, T.; et al. Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments Following Prenatal Treatment of the Antiepileptic Drug Valproic Acid. Stem Cell Rep. 2015, 5, 996–1009. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, D.T.; O’Neill, S.M.; Narayan, S.; Tiwari, A.; Arnold, E.; Samaroo, H.D.; Du, F.; Ring, R.H.; Campbell, B.; Pletcher, M.; et al. Histopathologic Characterization of the BTBR Mouse Model of Autistic-like Behavior Reveals Selective Changes in Neurodevelopmental Proteins and Adult Hippocampal Neurogenesis. Mol. Autism 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, N.; Serhan, C.N.; Dahlén, S.-E.; Drazen, J.M.; Hay, D.W.P.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The Lipoxin Receptor ALX: Potent Ligand-Specific and Stereoselective Actions in Vivo. Pharmacol. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Powell, W.S.; Dahlén, S.-E.; Drazen, J.M.; Evans, J.F.; Serhan, C.N.; Shimizu, T.; Yokomizo, T.; Rovati, G.E. Update on Leukotriene, Lipoxin and Oxoeicosanoid Receptors: IUPHAR Review 7: IUPHAR Update on LT Receptors. Br. J. Pharmacol. 2014, 171, 3551–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, C.I.; Zattoni, M.; Serhan, C.N. Lipoxins and Aspirin-Triggered Lipoxin Inhibit Inflammatory Pain Processing. J. Exp. Med. 2007, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lima-Garcia, J.; Dutra, R.; da Silva, K.; Motta, E.; Campos, M.; Calixto, J. The Precursor of Resolvin D Series and Aspirin-Triggered Resolvin D1 Display Anti-Hyperalgesic Properties in Adjuvant-Induced Arthritis in Rats: RvD Series Precursor and AT-RvD1 in Arthritis. Br. J. Pharmacol. 2011, 164, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.-Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.-R. Resolvins RvE1 and RvD1 Attenuate Inflammatory Pain via Central and Peripheral Actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Tian, Y.; Wang, Z.-F.; Liu, S.-B.; Mi, W.-L.; Ma, H.-J.; Wu, G.-C.; Wang, J.; Yu, J.; Wang, Y.-Q. Involvement of the Spinal NALP1 Inflammasome in Neuropathic Pain and Aspirin-Triggered-15-Epi-Lipoxin A4 Induced Analgesia. Neuroscience 2013, 254, 230–240. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Ryan, A.; Godson, C. Lipoxins: Regulators of Resolution. Curr. Opin. Pharmacol. 2010, 10, 166–172. [Google Scholar] [CrossRef]
- Chiang, N.; Arita, M.; Serhan, C.N. Anti-Inflammatory Circuitry: Lipoxin, Aspirin-Triggered Lipoxins and Their Receptor ALX. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Romano, M. Lipoxin and Aspirin-Triggered Lipoxins. Sci. World J. 2010, 10, 1048–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A4 Suppresses the Development of Endometriosis in an ALX Receptor-Dependent Manner via the P38 MAPK Pathway: Role of Lipoxin A4 in Endometriosis. Br. J. Pharmacol. 2014, 171, 4927–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, T.; Stables, M.; Colville-Nash, P.; Newson, J.; Bellingan, G.; de Souza, P.M.; Gilroy, D.W. Dichotomy in Duration and Severity of Acute Inflammatory Responses in Humans Arising from Differentially Expressed Proresolution Pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 8842–8847. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-H.; Wang, H.-M.; Cai, Z.-Y.; Xu, F.-Y.; Zhou, X.-Y. Lipoxin A4 Inhibits NF-ΚB Activation and Cell Cycle Progression in RAW264.7 Cells. Inflammation 2014, 37, 1084–1090. [Google Scholar] [CrossRef]
- Fiore, S.; Antico, G.; Aloman, M.; Sodin-Semrl, S. Lipoxin A4 Biology in the Human Synovium. Role of the ALX Signaling Pathways in Modulation of Inflammatory Arthritis. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 189–196. [Google Scholar] [CrossRef]
- Machado, F.S.; Johndrow, J.E.; Esper, L.; Dias, A.; Bafica, A.; Serhan, C.N.; Aliberti, J. Anti-Inflammatory Actions of Lipoxin A4 and Aspirin-Triggered Lipoxin Are SOCS-2 Dependent. Nat. Med. 2006, 12, 330–334. [Google Scholar] [CrossRef]
- N. Serhan, C.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel Anti-Inflammatory-Pro-Resolving Mediators and Their Receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Becker, E.L.; Forouhar, F.A.; Grunnet, M.L.; Boulay, F.; Tardif, M.; Bormann, B.-J.; Sodja, D.; Ye, R.D.; Woska Jr, J.R.; Murphy, P.M. Broad Immunocytochemical Localization of the Formylpeptide Receptor in Human Organs, Tissues, and Cells. Cell Tissue Res. 1998, 292, 129–135. [Google Scholar] [CrossRef]
- Le, Y.; Oppenheim, J.; Wang, J. Pleiotropic Roles of Formyl Peptide Receptors. Cytokine Growth Factor Rev. 2001, 12, 91–105. [Google Scholar] [CrossRef]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl Peptide Receptors: A Promiscuous Subfamily of G Protein-Coupled Receptors Controlling Immune Responses. Cytokine Growth Factor Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Song, J.Y.; Kim, Y.; He, R.; Ye, R.D.; Kwak, J.-Y.; Suh, P.-G.; Ryu, S.H. Differential Activation of Formyl Peptide Receptor Signaling by Peptide Ligands. Mol. Pharmacol. 2003, 64, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-L.; Zhang, J.; Hou, Y. Decreased Plasma Levels of Lipoxin A4 in Children with Autism Spectrum Disorders. Neuroreport 2015, 26, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Tylek, K.; Leśkiewicz, M.; Lasoń, W.; Brandenburg, L.-O.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Exhibits Anti-Inflammatory Activity in LPS-Stimulated Organotypic Hippocampal Cultures. Cells 2021, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Bryniarska, N.; Leśkiewicz, M.; Regulska, M.; Chamera, K.; Szuster-Głuszczak, M.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The Contribution of Formyl Peptide Receptor Dysfunction to the Course of Neuroinflammation: A Potential Role in the Brain Pathology. Curr. Neuropharmacol. 2020, 18, 229–249. [Google Scholar] [CrossRef]
- Mastromarino, M.; Lacivita, E.; Colabufo, N.A.; Leopoldo, M. G-Protein Coupled Receptors Involved in the Resolution of Inflammation: Ligands and Therapeutic Perspectives. Mini-Rev. Med. Chem. 2021, 20, 2090–2103. [Google Scholar] [CrossRef]
- Stama, M.L.; Ślusarczyk, J.; Lacivita, E.; Kirpotina, L.N.; Schepetkin, I.A.; Chamera, K.; Riganti, C.; Perrone, R.; Quinn, M.T.; Basta-Kaim, A.; et al. Novel Ureidopropanamide Based N-Formyl Peptide Receptor 2 (FPR2) Agonists with Potential Application for Central Nervous System Disorders Characterized by Neuroinflammation. Eur. J. Med. Chem. 2017, 141, 703–720. [Google Scholar] [CrossRef]
- Trojan, E.; Tylek, K.; Schröder, N.; Kahl, I.; Brandenburg, L.-O.; Mastromarino, M.; Leopoldo, M.; Basta-Kaim, A.; Lacivita, E. The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Improves Ex Vivo and In Vivo Amyloid Beta (1–42)-Induced Neuroinflammation in Mouse Models of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 6203–6221. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on MRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Eisenberger, N.I.; Inagaki, T.K.; Mashal, N.M.; Irwin, M.R. Inflammation and Social Experience: An Inflammatory Challenge Induces Feelings of Social Disconnection in Addition to Depressed Mood. Brain. Behav. Immun. 2010, 24, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Speranza, L.; Giuliano, T.; Volpicelli, F.; De Stefano, M.E.; Lombardi, L.; Chambery, A.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; et al. Activation of 5-HT7 Receptor Stimulates Neurite Elongation through MTOR, Cdc42 and Actin Filaments Dynamics. Front. Behav. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Speranza, L.; Chambery, A.; Di Domenico, M.; Crispino, M.; Severino, V.; Volpicelli, F.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; Perrone-Capano, C. The Serotonin Receptor 7 Promotes Neurite Outgrowth via ERK and Cdk5 Signaling Pathways. Neuropharmacology 2013, 67, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Labus, J.; Volpicelli, F.; Guseva, D.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; Bijata, M.; Perrone-Capano, C.; et al. Serotonin 5-HT7 Receptor Increases the Density of Dendritic Spines and Facilitates Synaptogenesis in Forebrain Neurons. J. Neurochem. 2017, 141, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant Immune Responses in a Mouse with Behavioral Disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic Diseases and Inflammation of the Brain in the Pathogenesis of Autism Spectrum Disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental Risk Factors for Autism: An Evidence-Based Review of Systematic Reviews and Meta-Analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Geier, M.R. Relevance of Neuroinflammation and Encephalitis in Autism. Front. Cell. Neurosci. 2016, 9, 519. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, C.; Lama, A.; Lembo, F.; Mollica, M.P.; Calignano, A.; Mattace Raso, G. Interplay Between Peripheral and Central Inflammation in Autism Spectrum Disorders: Possible Nutritional and Therapeutic Strategies. Front. Physiol. 2018, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.F.-Y.; Ismail, N.B.; Koh, J.K.-Z.; Gunaseelan, S.; Low, Y.-H.; Ng, Y.-K.; Chua, J.J.-E.; Ong, W.-Y. Localisation of Formyl-Peptide Receptor 2 in the Rat Central Nervous System and Its Role in Axonal and Dendritic Outgrowth. Neurochem. Res. 2018, 43, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, K.E.; DeMars, K.M.; Alexander, J.C.; Leon, L.G.; Pacheco, S.C.; Graves, C.; Yang, C.; McCrea, A.O.; Frankowski, J.C.; Garrett, T.J.; et al. Targeting Resolution of Neuroinflammation after Ischemic Stroke with a Lipoxin A4 Analog: Protective Mechanisms and Long-term Effects on Neurological Recovery. Brain Behav. 2017, 7, e00688. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Bakheet, S.A.; Ibrahim, K.E.; Alqahtani, F.; Alqinyah, M. Nrf2 Activator, Sulforaphane Ameliorates Autism-like Symptoms through Suppression of Th17 Related Signaling and Rectification of Oxidant-Antioxidant Imbalance in Periphery and Brain of BTBR T+tf/J Mice. Behav. Brain Res. 2019, 364, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, C.; Pirozzi, C.; Coretti, L.; Cavaliere, G.; Lama, A.; Russo, R.; Lembo, F.; Mollica, M.P.; Meli, R.; Calignano, A.; et al. Palmitoylethanolamide Counteracts Autistic-like Behaviours in BTBR T+tf/J Mice: Contribution of Central and Peripheral Mechanisms. Brain. Behav. Immun. 2018, 74, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Witt, N.A.; Lee, B.; Ghent, K.; Zhang, W.Q.; Pehrson, A.L.; Sánchez, C.; Gould, G.G. Vortioxetine Reduces Marble Burying but Only Transiently Enhances Social Interaction Preference in Adult Male BTBR T+ Itpr3tf /J Mice. ACS Chem. Neurosci. 2019, 10, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Oliver, C.F.; Karras, M.N.; Gastrell, P.T.; Crawley, J.N. AMPAKINE Enhancement of Social Interaction in the BTBR Mouse Model of Autism. Neuropharmacology 2013, 64, 268–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Simone, R.; Butera, A.; Armida, M.; Pezzola, A.; Boirivant, M.; Potenza, R.L.; Ricceri, L. Beneficial Effects of Fingolimod on Social Interaction, CNS and Peripheral Immune Response in the BTBR Mouse Model of Autism. Neuroscience 2020, 435, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, L.; Nalvarte, I.; Xiao, R.; Li, X.; Fan, X. Citalopram Attenuates Social Behavior Deficits in the BTBR T+Itpr3tf/J Mouse Model of Autism. Brain Res. Bull. 2019, 150, 75–85. [Google Scholar] [CrossRef]
- Kim, J.-W.; Park, K.; Kang, R.J.; Gonzales, E.L.T.; Kim, D.G.; Oh, H.A.; Seung, H.; Ko, M.J.; Kwon, K.J.; Kim, K.C.; et al. Pharmacological Modulation of AMPA Receptor Rescues Social Impairments in Animal Models of Autism. Neuropsychopharmacology 2019, 44, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Hidema, S.; Kikuchi, S.; Takata, R.; Yanai, T.; Shimomura, K.; Horie, K.; Nishimori, K. Single Administration of Resveratrol Improves Social Behavior in Adult Mouse Models of Autism Spectrum Disorder. Biosci. Biotechnol. Biochem. 2020, 84, 2207–2214. [Google Scholar] [CrossRef]
- Lee, G.A.; Lin, Y.-K.; Lai, J.-H.; Lo, Y.-C.; Yang, Y.-C.S.H.; Ye, S.-Y.; Lee, C.-J.; Wang, C.-C.; Chiang, Y.-H.; Tseng, S.-H. Maternal Immune Activation Causes Social Behavior Deficits and Hypomyelination in Male Rat Offspring with an Autism-Like Microbiota Profile. Brain Sci. 2021, 11, 1085. [Google Scholar] [CrossRef]
- Bakos, J.; Bacova, Z.; Grant, S.G.; Castejon, A.M.; Ostatnikova, D. Are Molecules Involved in Neuritogenesis and Axon Guidance Related to Autism Pathogenesis? Neuromolecular Med. 2015, 17, 297–304. [Google Scholar] [CrossRef]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The Influence of Neuroinflammation in Autism Spectrum Disorder. Brain. Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Bagni, C.; Zukin, R.S. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevalye, H.; Yorek, M.S.; Coppey, L.J.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of Enriching the Diet with Menhaden Oil or Daily Treatment with Resolvin D1 on Neuropathy in a Mouse Model of Type 2 Diabetes. J. Neurophysiol. 2015, 114, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, S.; Takuma, K.; Hara, Y.; Maeda, Y.; Ago, Y.; Matsuda, T. Autism-like Behaviours with Transient Histone Hyperacetylation in Mice Treated Prenatally with Valproic Acid. Int. J. Neuropsychopharmacol. 2013, 16, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Moy, S.; Nadler, J.; Young, N.; Perez, A.; Holloway, L.; Barbaro, R.; Barbaro, J.; Wilson, L.; Threadgill, D.; Lauder, J. Mouse Behavioral Tasks Relevant to Autism: Phenotypes of 10 Inbred Strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Pobbe, R.L.H.; Pearson, B.L.; Defensor, E.B.; Bolivar, V.J.; Blanchard, D.C.; Blanchard, R.J. Expression of Social Behaviors of C57BL/6J versus BTBR Inbred Mouse Strains in the Visible Burrow System. Behav. Brain Res. 2010, 214, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, F.; Speranza, L.; Pulcrano, S.; De Gregorio, R.; Crispino, M.; De Sanctis, C.; Leopoldo, M.; Lacivita, E.; di Porzio, U.; Bellenchi, G.C.; et al. The MicroRNA-29a Modulates Serotonin 5-HT7 Receptor Expression and Its Effects on Hippocampal Neuronal Morphology. Mol. Neurobiol. 2019, 56, 8617–8627. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiano, C.; Volpicelli, F.; Crispino, M.; Lacivita, E.; Russo, R.; Leopoldo, M.; Calignano, A.; Perrone-Capano, C. Behavioral, Anti-Inflammatory, and Neuroprotective Effects of a Novel FPR2 Agonist in Two Mouse Models of Autism. Pharmaceuticals 2022, 15, 161. https://doi.org/10.3390/ph15020161
Cristiano C, Volpicelli F, Crispino M, Lacivita E, Russo R, Leopoldo M, Calignano A, Perrone-Capano C. Behavioral, Anti-Inflammatory, and Neuroprotective Effects of a Novel FPR2 Agonist in Two Mouse Models of Autism. Pharmaceuticals. 2022; 15(2):161. https://doi.org/10.3390/ph15020161
Chicago/Turabian StyleCristiano, Claudia, Floriana Volpicelli, Marianna Crispino, Enza Lacivita, Roberto Russo, Marcello Leopoldo, Antonio Calignano, and Carla Perrone-Capano. 2022. "Behavioral, Anti-Inflammatory, and Neuroprotective Effects of a Novel FPR2 Agonist in Two Mouse Models of Autism" Pharmaceuticals 15, no. 2: 161. https://doi.org/10.3390/ph15020161
APA StyleCristiano, C., Volpicelli, F., Crispino, M., Lacivita, E., Russo, R., Leopoldo, M., Calignano, A., & Perrone-Capano, C. (2022). Behavioral, Anti-Inflammatory, and Neuroprotective Effects of a Novel FPR2 Agonist in Two Mouse Models of Autism. Pharmaceuticals, 15(2), 161. https://doi.org/10.3390/ph15020161









