Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structural Analysis of Cu(I), Ru(II), and Ir(III) Complexes
2.3. Oxidative Plasmid DNA Degradation
2.4. In Vitro Anticancer Investigation
2.4.1. Determination of IC50 and Partition Coefficients (LogP) Values
2.4.2. Cellular Accumulation
2.4.3. Cell Death Mechanisms
2.4.4. Induction of Cell Cycle Arrest
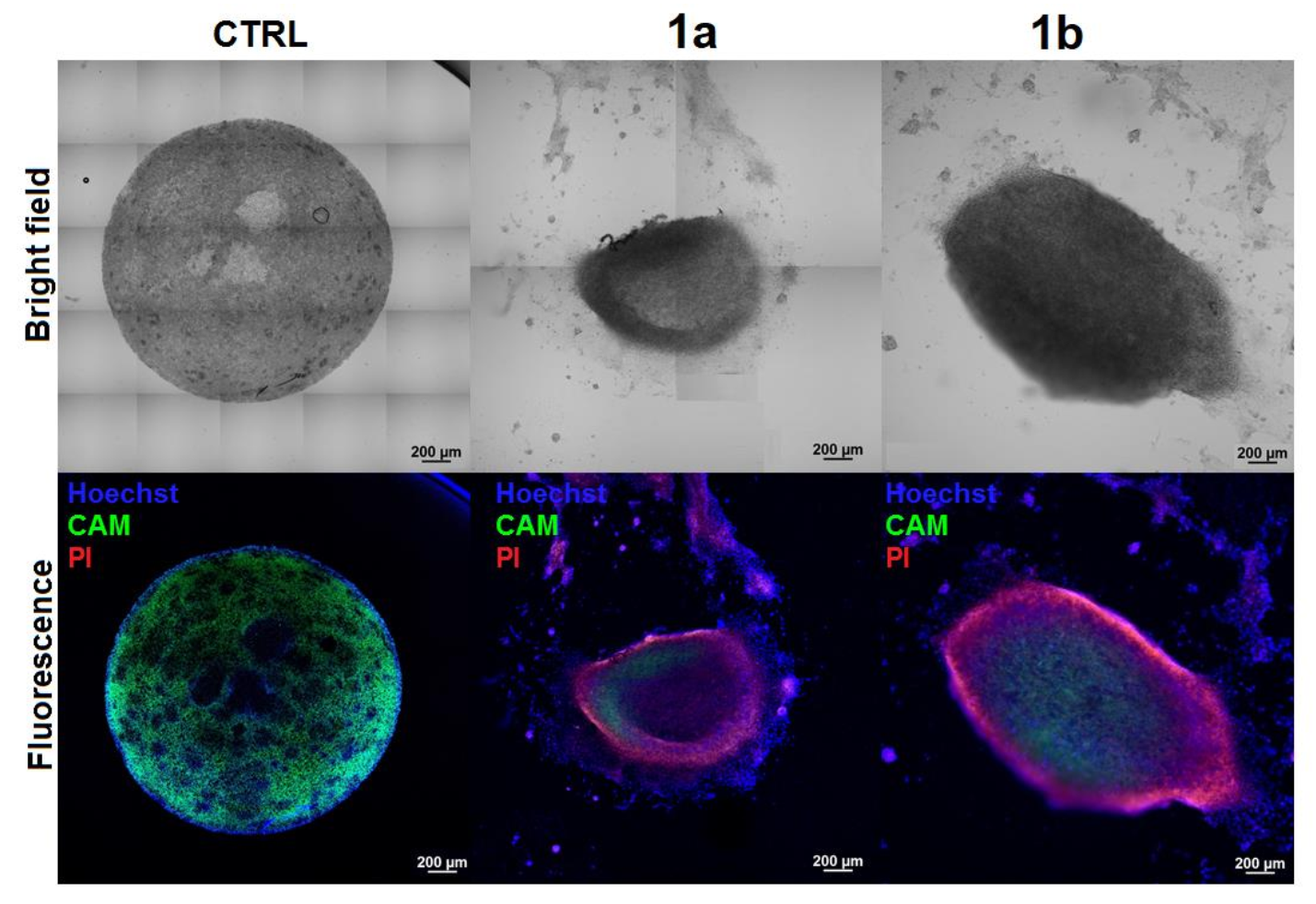
2.4.5. Anticancer Activity in 3D Tumor Spheroids
3. Materials and Methods
3.1. Reagents
3.2. Methods
3.3. Synthesis
3.3.1. Preparation of Ph2PCH2N(CH2CH3)2 (1)
3.3.2. Preparation of Ph2PCH2N(CH2CH2CH2CH3)2 (2)
3.3.3. Preparation of Ir(η5-Cp*)Cl2Ph2PCH2N(CH2CH3)2 (1a)
3.3.4. Preparation of Ru(η6-p-cymene)Cl2Ph2PCH2N(CH2CH3)2(1b)
3.3.5. Preparation of [Cu(NCCH3)2(Ph2PCH2N(CH2CH3)2)2]+BF4− (1c)
3.3.6. Preparation of Ir(η5-Cp*)Cl2Ph2PCH2N(CH2CH2CH2CH3)2 (2a)
3.3.7. Preparation of Ru(η6-p-cymene)Cl2Ph2PCH2N(CH2CH2CH2CH3)2 (2b)
3.3.8. Preparation of [Cu(NCCH3)2(Ph2PCH2N(CH2CH2CH2CH3)2)2]+BF4− (2c)
3.4. Electrochemical Measurements
3.5. DNA Strand Break Analysis
3.6. Partition Coefficient
3.7. Cell Cultures
3.8. Cytotoxic Activity
3.9. Intracellular Accumulation
3.10. Flow Cytometry
3.11. Three-Dimensional Culturing In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, C.; Kang, J.; Hwang, J.S.; Li, J.; Boese, A.C.; Wang, X.; Yang, L.; Boggon, T.J.; Chen, G.Z.; Saba, N.F.; et al. Cisplatin-mediated activation of glucocorticoid receptor induces platinum resistance via MAST1. Nat. Commun. 2021, 12, 4960. [Google Scholar] [CrossRef] [PubMed]
- Taber, A.; Christensen, E.; Lamy, P.; Nordentoft, I.; Prip, F.; Lindskrog, S.V.; Birkenkamp-Demtröder, K.; Okholm, T.L.H.; Knudsen, M.; Pedersen, J.S.; et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 2020, 11, 4858. [Google Scholar] [CrossRef] [PubMed]
- Jingwen, B.; Yaochen, L.; Guojun, Z. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef]
- Kozieł, S.A.; Lesiów, M.K.; Wojtala, D.; Dyguda-Kazimierowicz, E.; Bieńko, D.; Komarnicka, U. Interaction between DNA, Albumin and Apo-Transferrin and Iridium(III) Complexes with Phosphines Derived from Fluoroquinolones as a Potent Anticancer Drug. Pharmaceuticals 2021, 14, 685. [Google Scholar] [CrossRef]
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A critical review of palladium organometallic anticancer agents. Cell Rep. Phys. Sci. 2021, 2, 100446. [Google Scholar] [CrossRef]
- Teixeira, R.G.; Belisario, D.C.; Fontrodona, X.; Romero, I.; Tomaz, A.I.; Garcia, M.H.; Riganti, C.; Valente, A. Unprecedented collateral sensitivity for cisplatin-resistant lung cancer cells presented by new ruthenium organometallic compounds. Inorg. Chem. Front. 2021, 8, 1983–1996. [Google Scholar] [CrossRef]
- Máliková, K.; Masaryk, L.; Štarha, P. Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics 2021, 9, 26. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; Migoni, D.; Iaconisi, G.N.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. Synthesis and Evaluation of the Cytotoxic Activity of Water-Soluble Cationic Organometallic Complexes of the Type [Pt(η1-C2H4OMe)(L)(Phen)]+ (L = NH3, DMSO; Phen = 1,10-Phenanthroline). Pharmaceutics 2021, 13, 642. [Google Scholar] [CrossRef]
- Casini, A.; Vessières, A.; Meier-Menches, S.M. Metal-Based Anticancer Agents; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Bykowska, A.; Komarnicka, U.K.; Jeżowska-Bojczuk, M.; Kyzioł, A. CuI and CuII complexes with phosphine derivatives of fluoroquinolone antibiotics—A comparative study on the cytotoxic mode of action. J. Inorg. Biochem. 2018, 181, 1–10. [Google Scholar] [CrossRef]
- Galvez, L.; Rusz, M.; Schwaiger-Haber, M.; El Abiead, Y.; Hermann, G.; Jungwirth, U.; Berger, W.; Keppler, B.K.; Jakupec, M.A.; Koellensperger, G. Preclinical studies on metal based anticancer drugs as enabled by integrated metallomics and metabolomics. Metallomics 2019, 11, 1716–1728. [Google Scholar] [CrossRef] [Green Version]
- Ott, I.; Gust, R. Non-Platinum Metal Complexes as Anti-cancer Drugs. Arch. Pharm. Chem. Life Sci. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.-G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2020, ume 14, 5375–5392. [Google Scholar] [CrossRef]
- Sharma, S.A.; Sudhindra, P.; Roy, N.; Paira, P. Advances in novel iridium (III) based complexes for anticancer applications: A review. Inorg. Chim. Acta 2020, 513, 119925. [Google Scholar] [CrossRef]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque; Hun, L.T. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2015, 40, 1063–1090. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Starosta, R.; Płotek, M.; de Almeida, R.F.M.; Jeżowska-Bojczuk, M.; Kyzioł, A. Copper(i) complexes with phosphine derived from sparfloxacin. Part II: A first insight into the cytotoxic action mode. Dalton Trans. 2015, 45, 5052–5063. [Google Scholar] [CrossRef]
- Appleby, M.V.; Walker, P.G.; Pritchard, D.; van Meurs, S.; Booth, C.M.; Robertson, C. Cu(I) diimine complexes as immobilised antibacterial photosensitisers operating in water under visible light. Mater. Adv. 2020, 1, 3417. [Google Scholar] [CrossRef]
- Gizer, S.G.; Sahiner, N. The effect of sulphur on the antibacterial properties of succinic acid-Cu(II) and mercaptosuccinic acid-Cu(II) MOFs. Inorganica Chim. Acta 2021, 528, 120611. [Google Scholar] [CrossRef]
- Chen, F.; Moat, J.; Mcfeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Jiang, G.; Jiang, G.; Liu, J.; Lin, X.; Shen, J.; Xiong, Y.; Duan, X.; Wang, J.; Liao, X. Antibacterial activity of ruthenium polypyridyl complexes against Staphylococcus aureus and biofilms. JBIC J. Biol. Inorg. Chem. 2020, 25, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Feng, Y. Fluorination in enhancing photoactivated antibacterial activity of Ru(ii) complexes with photo-labile ligands. RSC Adv. 2020, 10, 25364. [Google Scholar] [CrossRef]
- Selvi, G.; Ozdemir, F.A.; Aykutoglu, G.; Özdemir, N.; Şerbetçi, Z.; Dinçer, M.; Dayan, O. Synthesis, catalytic, cytotoxic, and antibacterial properties of new Ru(II) and Pd(II) complexes bearing bidentate Schiff base ligand. Inorg. Nano-Metal Chem. 2020, 1697, 51. [Google Scholar] [CrossRef]
- de Paiva, R.E.F.; Neto, A.M.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.; DuChane, C.; Webb, E.; Rai, P.; Marano, J.; Bernier, C.; Merola, J.; Weger-Lucarelli, J. Noble Metal Organometallic Complexes Display Antiviral Activity against SARS-CoV-2. Viruses 2021, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Nareetsile, F.; Matshwele, J.T.; Ndlovu, S.; Ngaski, M. Transition Metal Complexes with HIV/AIDS Inhibitory Properties. Chem. Rev. Lett. 2020, 3, 140–160. [Google Scholar] [CrossRef]
- Almalki, S.A.; Bawazeer, T.M.; Asghar, B.; Alharbi, A.; Aljohani, M.M.; Khalifa, M.E.; El-Metwaly, N. Synthesis and characterization of new thiazole-based Co(II) and Cu(II) complexes; therapeutic function of thiazole towards COVID-19 in comparing to current antivirals in treatment protocol. J. Mol. Struct. 2021, 1244, 130961. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, D.S.; Yu, X.; Yang, Y.; Wang, D. Tunable Triazole-Phosphine-Copper Catalysts for the Synthesis of 2-Aryl-1H-benzo[d]imidazoles from Benzyl Alcohols and Diamines by Acceptorless Dehydrogenation and Borrowing Hydrogen Reactions. Adv. Synth. Catal. 2017, 19, 3332. [Google Scholar] [CrossRef]
- Păunescu, E.; McArthur, S.; Soudani, M.; Scopelliti, R.; Dyson, P.J. Nonsteroidal Anti-inflammatory—Organometallic Anticancer Compounds. Inorg. Chem. 2016, 55, 1788–1808. [Google Scholar] [CrossRef]
- Leung, C.-H.; Lin, S.; Zhong, H.-J.; Ma, D.-L. Metal complexes as potential modulators of inflammatory and autoimmune responses. Chem. Sci. 2015, 6, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.A.; Costa, V.A.; Franco, M.A.; de Oliveira, G.P.; Deflon, V.M.; Rocha, F.V. Palladium(II) complexes bearing thiosemicarbazone and phosphines as inhibitors of DNA-Topoisomerase II enzyme: Synthesis, characterizations and biological studies. Inorg. Chem. Commun. 2020, 112, 107708. [Google Scholar] [CrossRef]
- Jarrett, P.S.; Sadler, P.J. Nickel(II) bis(phosphine) complexes. Inorg. Chem. 1991, 30, 2098. [Google Scholar] [CrossRef]
- Mirzadeh, N.; Reddy, T.S.; Bhargava, S.K. Advances in diphosphine ligand-containing gold complexes as anticancer agents. Co-ord. Chem. Rev. 2019, 388, 343–359. [Google Scholar] [CrossRef]
- Marzano, C.; Tisato, F.; Porchia, M.; Pellei, M.; Gandin, V. Chapter 4—Phosphine Copper(I) Complexes as Anticancer Agents: Biological Characterization, Part II. In Copper(I) Chemistry of Phosphines, Functionalized Phosphines and Phosphorus Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; p. 83. [Google Scholar]
- Thomas, J.M.; Madarasi, P.K.; Sivasankar, C.; Samuelson, A.G. Chapter 7—Coordination chemistry of copper(I) complexes with bis(phosphine) ligands. In Copper(I) Chemistry of Phosphines, Functionalized Phosphines and Phosphorus Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; p. 165. [Google Scholar]
- Kuchar, J.; Rust, J.; Lehmann, C.W.; Mohr, F. Silver(I) Complexes with Camphorsulfonato and Phosphine Ligands: Structural Diversity and Antibacterial Activity. Inorg. Chem. 2020, 59, 10557–10568. [Google Scholar] [CrossRef]
- Kim, J.H.; Reeder, E.; Parkin, S.; Awuah, S.G. Gold(I/III)-Phosphine Complexes as Potent Antiproliferative Agents. Sci. Rep. 2019, 9, 12335. [Google Scholar] [CrossRef]
- Engelbrecht, Z.; Meijboom, R.; Cronjé, M.J. The ability of silver(I) thiocyanate 4-methoxyphenyl phosphine to induce apoptotic cell death in esophageal cancer cells is correlated to mitochondrial perturbations. BioMetals 2018, 31, 189–202. [Google Scholar] [CrossRef]
- Needham, R.J.; Prokes, I.; Habtemariam, A.; Romero-Canelón, I.; Clarkson, G.J.; Sadler, P.J. NMR studies of group 8 metallodrugs: 187Os-enriched organo-osmium half-sandwich anticancer complex. Dalton Trans. 2021, 50, 12970–12981. [Google Scholar] [CrossRef]
- Du, Q.; Yang, Y.; Guo, L.; Tian, M.; Ge, X.; Tian, Z.; Zhao, L.; Xu, Z.; Li, J.; Liu, Z. Fluorescent half-sandwich phosphine-sulfonate iridium(III) and ruthenium(II) complexes as potential lysosome-targeted anticancer agents. Dyes Pigments 2019, 162, 821. [Google Scholar] [CrossRef]
- Kantoury, M.; Moghadam, M.E.; Tarlani, A.A.; Divsalar, A. Structure Effect of Some New Anticancer Pt(II) Complexes of Amino Acid Derivatives with Small Branched or Linear Hydrocarbon Chains on Their DNA Interaction. Chem. Biol. Drug Des. 2016, 88, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Laha, P.; De, U.; Chandra, F.; Dehury, N.; Khullar, S.; Kim, H.S.; Patra, S. Alkyl chain-modified cyclometalated iridium complexes as tunable anticancer and imaging agents. Dalton Trans. 2018, 47, 15873–15881. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Haribabu, J.; Subarkhan, M.K.M.; Gayathri, D.; Balakrishnan, N.; Bhuvanesh, N.; Echeverria, C.; Karvembu, R. Impact of aliphatic acyl and aromatic thioamide substituents on the anticancer activity of Ru(ii)-p-cymene complexes with acylthiourea ligands—in vitro and in vivo studies. Dalton Trans. 2021, 50, 16311–16325. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; Wang, Y.; Guo, Z.; Wang, X. Hypotoxic copper complexes with potent anti-metastatic and anti-angiogenic activities against cancer cells. Dalton Trans. 2018, 47, 5049–5054. [Google Scholar] [CrossRef]
- Weninger, A.; Baecker, D.; Obermoser, V.; Egger, D.; Wurst, K.; Gust, R. Synthesis and Biological Evaluation of Zeise’s Salt Derivatives with Acetylsalicylic Acid Substructure. Int. J. Mol. Sci. 2018, 19, 1612. [Google Scholar] [CrossRef] [Green Version]
- Małecki, J.G.; Maron, A.M.; Palion, J.; Nycz, J.E.; Szala, M. A copper(I) phosphine complex with 5,7-dinitro-2-methylquinolin-8-ol as co-ligand. Transit. Met. Chem. 2014, 39, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Ralph, R.K.; Marshall, B.; Darkin, S. Anti-cancer drugs which intercalate into DNA: How do they act? Trends Biochem. Sci. 1983, 8, 212–214. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sasikala, W.D. Drug-DNA Intercalation: From Discovery to the Molecular Mechanism, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 92. [Google Scholar]
- Komarnicka, U.; Starosta, R.; Kyzioł, A.; Płotek, M.; Puchalska, M.; Jeżowska-Bojczuk, M. New copper(I) complexes bearing lomefloxacin motif: Spectroscopic properties, in vitro cytotoxicity and interactions with DNA and human serum albumin. J. Inorg. Biochem. 2016, 165, 25–35. [Google Scholar] [CrossRef]
- Gou, Y.; Huang, G.; Li, J.; Yang, F.; Liang, H. Versatile delivery systems for non-platinum metal-based anticancer therapeutic agents. Coord. Chem. Rev. 2021, 441, 213975. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiów, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper(I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly. Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926. [Google Scholar] [CrossRef]
- Szczepanik, W.; Czarny, A.; Zaczyńska, E.; Jezowska-Bojczuk, M. Preferences of kanamycin A towards copper(II). Effect of the resulting complexes on immunological mediators production by human leukocytes. J. Inorg. Biochem. 2004, 98, 245–253. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Tisato, F.; Santini, C. Copper Complexes as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2009, 9, 185–211. [Google Scholar] [CrossRef]
- Bancirova, M. Sodium azide as a specific quencher of singlet oxygen during chemiluminescent detection by luminol and Cypridina luciferin analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Pucelik, B.; Arnaut, L.G.; Dąbrowski, J.M. Lipophilicity of Bacteriochlorin-Based Photosensitizers as a Determinant for PDT Optimization through the Modulation of the Inflammatory Mediators. J. Clin. Med. 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Pucelik, B.; Sułek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.M.A.; Arnaut, L.G.; Dąbrowski, J.M. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 2786. [Google Scholar] [CrossRef] [Green Version]
- Sudhindra, P.; Sharma, S.A.; Roy, N.; Moharana, P.; Paira, P. Recent advances in cytotoxicity, cellular uptake and mechanism of action of ruthenium metallodrugs: A review. Polyhedron 2020, 192, 114827. [Google Scholar] [CrossRef]
- McKeage, M.J.; Berners-Price, S.J.; Galettis, P.; Bowen, R.J.; Brouwer, W.; Ding, L. Role of lipophilicity in determining cellular uptake and antitumour activity of gold phosphine complexes. Cancer Chemother. Pharmacol. 2000, 46, 343. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. A link between cancer genetics and chemotherapy. Cell 2002, 108, 153. [Google Scholar] [CrossRef] [Green Version]
- Kozieł, S.; Komarnicka, U.K.; Ziolkowska, A.; Skorska-Stania, A.; Pucelik, B.; Plotek, M.; Sebastian, V.; Bienko, A.; Stochel, G.; Kyzioł, A. Anticancer potency of novel organometallic Ir(III) complexes with phosphine derivatives of fluoroquinolones encapsulated in polymeric micelles. Inorg. Chem. Front. 2020, 7, 3386. [Google Scholar] [CrossRef]
- Jia, P.; Ouyang, R.; Cao, P.; Tong, X.; Zhou, X.; Lei, T.; Zhao, Y.; Guo, N.; Chang, H.; Miao, Y.; et al. Review: Recent advances and future development of metal complexes as anticancer agents. J. Coord. Chem. 2017, 70, 2175–2201. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Gu, Y.-Y.; Wang, Y.-J.; Bai, L.; Du, F.; Zhang, W.-Y.; He, M.; Liu, Y.-J.; Chen, Y.-Z. Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells. Molecules 2019, 24, 3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.-X.; Chen, M.-H.; Hu, X.-Y.; Ye, R.-R.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Ester-Modified Cyclometalated Iridium(III) Complexes as Mitochondria-Targeting Anticancer Agents. Sci. Rep. 2016, 6, 38954. [Google Scholar] [CrossRef] [PubMed]
- Komarnicka, U.K.; Pucelik, B.; Wojtala, D.; Lesiów, M.K.; Stochel, G.; Kyzioł, A. Evaluation of anticancer activity in vitro of a stable copper(I) complex with phosphine-peptide conjugate. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Lesiów, M.K.; Komarnicka, U.K.; Kyzioł, A.; Bieńko, A.; Pietrzyk, P. ROS-mediated lipid peroxidation as a result of Cu(II) interaction with FomA protein fragments of F. nucleatum: Relevance to colorectal carcinogenesis. Metallomics 2019, 11, 2066–2077. [Google Scholar] [CrossRef]









| MCF-7 | Du-145 | A549 | PANC-1 | HaCaT | Experimental LogP | |
|---|---|---|---|---|---|---|
| dibutylamine | >1000 | >1000 | >1000 | >1000 | >1000 | nd |
| diethylamine | >1000 | >1000 | >1000 | >1000 | >1000 | nd |
| 1 | 192 ± 20 | 86 ± 6 | 183 ± 12 | 185 ± 12 | 490 ± 12 | nd |
| 2 | 285 ± 11 | 57 ± 4 | 158 ± 11 | 142 ± 20 | 448 ± 25 | nd |
| 1a | 85 ± 8 | 19 ± 3 | 12 ± 3 | 91 ± 13 | 320 ± 19 | 3.03 |
| 2a | 56 ± 6 | 13 ± 2 | 16 ± 3 | 85 ± 16 | 302 ± 22 | 4.0 |
| 1b | 145 ± 15 | 23 ± 2 | 10 ± 1 | 128 ± 14 | 303 ± 20 | 3.1 |
| 2b | 121 ± 20 | 17 ± 3 | 14 ± 2 | 112 ± 18 | 347 ± 25 | 3.84 |
| Cisplatin | 64 ± 4 | 50 ± 2 | 11 ± 3 | 79 ± 5 | 102 ± 2 | nd. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarnicka, U.K.; Niorettini, A.; Kozieł, S.; Pucelik, B.; Barzowska, A.; Wojtala, D.; Ziółkowska, A.; Lesiów, M.; Kyzioł, A.; Caramori, S.; et al. Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes. Pharmaceuticals 2022, 15, 169. https://doi.org/10.3390/ph15020169
Komarnicka UK, Niorettini A, Kozieł S, Pucelik B, Barzowska A, Wojtala D, Ziółkowska A, Lesiów M, Kyzioł A, Caramori S, et al. Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes. Pharmaceuticals. 2022; 15(2):169. https://doi.org/10.3390/ph15020169
Chicago/Turabian StyleKomarnicka, Urszula K., Alessandro Niorettini, Sandra Kozieł, Barbara Pucelik, Agata Barzowska, Daria Wojtala, Aleksandra Ziółkowska, Monika Lesiów, Agnieszka Kyzioł, Stefano Caramori, and et al. 2022. "Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes" Pharmaceuticals 15, no. 2: 169. https://doi.org/10.3390/ph15020169
APA StyleKomarnicka, U. K., Niorettini, A., Kozieł, S., Pucelik, B., Barzowska, A., Wojtala, D., Ziółkowska, A., Lesiów, M., Kyzioł, A., Caramori, S., Porchia, M., & Bieńko, A. (2022). Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes. Pharmaceuticals, 15(2), 169. https://doi.org/10.3390/ph15020169







