Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery
Abstract
:1. Introduction
2. Summary Results of Literature Analysis
3. Clinical Trials for Various Cancer Types
3.1. Treatment of Breast Cancer
3.2. Treatment of Lung Cancer
3.3. Treatment of Colon Cancer
3.4. Treatment of Liver Cancer
3.5. Treatment of Leukemia or Blood Cancer
3.6. Treatment of Prostate Cancer
3.7. Treatment of Gynecological Cancer
3.8. Treatment of Miscellaneous Cancers and Meta-Analyses Study
4. Preclinical Evidence (Selected Important In Vitro vs. In Vivo Studies)
5. Toxicity Observations and Lack of Effect in Clinical Trials
6. Mushroom-Derived Active Components and Related Clinical Trials
7. Challenges for Mushroom Constituents as Anticancer Agents
8. Prospects for Development of Drugs from Mushrooms
9. Future Prospects
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | adverse event |
| AHCC®® | active hexose correlated compound |
| FFLZ | fucose-containing fraction of G. lucidum |
| HCC | hepatocellular carcinoma |
| HP | hematologic parameters |
| IC50 | half-maximal inhibitory concentration |
| LOA | loss of appetite |
| MDS | myelodys plastic syndromes |
| MM | medicinal mushrooms |
| OS | overall survival |
| QOL | quality of life |
| RCT | randomized, placebo-controlled, double-blind clinical trial |
| ROS | reactive oxygen species |
| SDL | superfine dispersed lentinan |
| SPG | polysaccharide schizophyllan |
References
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. The cultivation and environmental impact of mushrooms. In Oxford Research Encyclopedias Environmental Science; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gautam, N. Chemical, bioactive, and antioxidant potential of twenty wild culinary mushroom species. BioMed Res. Int. 2015, 2015, 346508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Fischer, M.; Koppold-Liebscher, D.A.; Murthy, V.; Kessler, C.S. Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, L.; Régis, W.C.B. Medicinal mushrooms in adjuvant cancer therapies: An approach to anticancer effects and presumed mechanisms of action. Nutrire 2017, 42, 28. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; Luyten, W. Medicinal mushrooms: Clinical perspective and challenges. Drug Discov. Today 2021, 27, 636–651. [Google Scholar] [CrossRef]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [Green Version]
- Grinde, B.; Hetland, G.; Johnson, E. Effects on gene expression and viral load of a medicinal extract from Agaricus blazei in patients with chronic hepatitis C infection. Int. Immunopharmacol. 2006, 6, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Tangen, J.-M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Trøseid, A.-M.S.; Wang, J.; Tjønnfjord, G.E.; Hetland, G. Clinical Study immunomodulatory effects of the Agaricus blazei Murrill-based mushroom extract AndoSan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. BioMed Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Fortes, R.C.; Recôva, V.L.; Melo, A.L.; Novaes, M.R.C.G. Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: A randomized, double-blind, placebo-controlled clinical study. Nutr. Hosp. 2008, 23, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Valadares, F.; Novaes, M.R.C.G.; Cañete, R. Effect of Agaricus sylvaticus supplementation on nutritional status and adverse events of chemotherapy of breast cancer: A randomized, placebo-controlled, double-blind clinical trial. Indian J. Pharmacol. 2013, 45, 217–222. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Hung, Y.C.; Chen, Y.H.; Chen, Y.H.; Huang, Y.C.; Kao, C.W.; Su, Y.L.; Chiu, H.H.E.; Rau, K.M. A preliminary randomised controlled study of short-term Antrodia cinnamomea treatment combined with chemotherapy for patients with advanced cancer. BMC Complement. Altern. Med. 2016, 16, 322. [Google Scholar] [CrossRef] [Green Version]
- Chay, W.Y.; Tham, C.K.; Toh, H.C.; Lim, H.Y.; Tan, C.K.; Lim, C.; Wang, W.W.; Choo, S.P. Coriolus versicolor (Yunzhi) use as therapy in advanced hepatocellular carcinoma patients with poor liver function or who are unfit for standard therapy. J. Altern. Complement. Med. 2017, 23, 648–652. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: A pilot clinical trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef] [Green Version]
- Griessmayr, P.C.; Gauthier, M.; Barber, L.G.; Cotter, S.M. Mushroom-derived Maitake PETfraction as single agent for the treatment of lymphoma in dogs. J. Vet. Intern. Med. 2007, 21, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Wesa, K.M.; Cunningham-Rundles, S.; Klimek, V.M.; Vertosick, E.; Coleton, M.I.; Yeung, K.S.; Lin, H.; Nimer, S.; Cassileth, B.R. Maitake mushroom extract in myelodysplastic syndromes (MDS): A phase II study. Cancer Immunol. Immunother. 2014, 64, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Urushima, H.; Sakaue, M.; Yukawa, S.; Honda, H.; Hirai, K.; Igura, T.; Hayashi, N.; Maeda, K.; Kitagawa, T.; et al. Reduction of adverse effects by a mushroom product, active hexose correlated compound (AHCC) in patients with advanced cancer during chemotherapy-the significance of the levels of HHV-6 DNA in saliva as a surrogate biomarker during chemotherapy. Nutr. Cancer 2014, 66, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, Y.; Hashine, K.; Kakehi, Y.; Yoshimura, K.; Satou, T.; Kuruma, H.; Namiki, S.; Shinohara, N. Dietary administration of mushroom mycelium extracts in patients with early stage prostate cancers managed expectantly: A phase II study. Jpn. J. Clin. Oncol. 2010, 40, 967–972. [Google Scholar] [CrossRef]
- DeVere White, R.W.; Hackman, R.M.; Soares, S.E.; Beckett, L.A.; Sun, B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology 2002, 60, 640–644. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef]
- Meera, C.R.; Janardhanan, K.K. Antitumor activity of a polysaccharide-protein complex isolated from a wood-rotting polypore macro fungus Phellinus rimosus (Berk) pilat. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 223–232. [Google Scholar] [CrossRef]
- Lee, H.; Cha, H.J. Poria cocos Wolf extracts represses pigmentation in vitro and in vivo. Cell. Mol. Biol. 2018, 64, 80–84. [Google Scholar] [CrossRef]
- Oka, S.; Tanaka, S.; Yoshida, S.; Hiyama, T.; Ueno, Y.; Ito, M.; Kitadai, Y.; Yoshihara, M.; Chayama, K. A water-soluble extract from culture medium of Ganoderma lucidum mycelia suppresses the development of colorectal adenomas. Hiroshima J. Med. Sci. 2010, 59, 1–6. [Google Scholar] [PubMed]
- Okamura, K.; Suzuki, M.; Yajima, A.; Chihara, T.; Fujiwara, A.; Fukuda, T.; Goto, S.; Ichinohe, K.; Jimi, S.; Kasamatsu, T.; et al. Clinical evaluation of Schizophyllan combined with irradiation in patients with cervical cancer: A randomized controlled study. Cancer 1986, 58, 865–872. [Google Scholar] [CrossRef]
- Fortes, R.C.; Novaes, M.R.C.G. The effects of Agaricus sylvaticus fungi dietary supplementation on the metabolism and blood pressure of patients with colorectal cancer during post surgical phase. Nutr. Hosp. 2011, 26, 176–186. [Google Scholar]
- Zhou, D.; Lin, L. Effect of Jinshuibao capsule on the immunological function of 36 patients with advanced cancer. Zhongguo Zhongxiyi jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 1995, 15, 476–478. [Google Scholar] [PubMed]
- Gao, Y.; Zhou, S.; Jiang, W.; Huang, M.; Dai, X. Effects of Ganopoly® (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol. Investig. 2003, 32, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dai, X.; Chen, G.; Ye, J.; Zhou, S. A randomized, placebo-controlled, multicenter study of Ganoderma lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromycetideae) polysaccharides (Ganopoly) in patients with advanced lung cancer. Int. J. Med. Mushrooms 2003, 5, 14. [Google Scholar] [CrossRef]
- Zuo, Z.; Zuo, Z.; Liying, Z. Clinical observation on alleviating chemotherapy’s side effect of psp in treating gastric careinoma. Liaoning J. Tradit. Chin. Med. 2001, 28, 668–669. [Google Scholar]
- Gao, Y.; Tang, W.; Dai, X.; Gao, H.; Chen, G.; Ye, J.; Chan, E.; Koh, H.L.; Li, X.; Zhou, S. Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J. Med. Food 2005, 8, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Fortes, R.C.; Lacorte Recôva, V.; Melo, A.L.; Carvalho, R.; Novaes, G.; De Vida, C. Life quality of postsurgical patients with colorectal cancer after supplemented diet with Agaricus sylvaticus fungus. Nutr. Hosp. 2010, 25, 586–596. [Google Scholar] [CrossRef]
- Nakano, H.; Namatame, K.; Nemoto, H.; Motohashi, H.; Nishiyama, K.; Kumada, K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: Effect on prolongation of survival and improvement of quality of life. Hepatogastroenterology 1999, 46, 2662–2668. [Google Scholar]
- Hazama, S.; Watanabe, S.; Ohashi, M.; Yagi, M.; SuzukI, M.; Matsuda, K.; Yamamoto, T.; Suga, Y.; Suga, T.; Nakazawa, S.; et al. Efficacy of orally administered superfine dispersed lentinan (β-1,3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res. 2009, 29, 2611–2617. [Google Scholar]
- Yang, P.; Liang, M.; Zhang, Y.; Shen, B. Clinical application of a combination therapy of lentinan, multi-electrode RFA and TACE in HCC. Adv. Ther. 2008, 25, 787–794. [Google Scholar] [CrossRef]
- Isoda, N.; Eguchi, Y.; Nukaya, H.; Hosho, K.; Suga, Y.; Sugae, T.; Nakazawa, S.; Sugano, K. Clinical efficacy of superfine dispersed lentinan (β-1,3-glucan) in patients with hepatocellular carcinoma. Hepatogastroenterology 2009, 56, 437–441. [Google Scholar] [PubMed]
- Mo, H.; Hong, M.; Zhang, J. Effect of Wuse-Lingzhi-Jiaonang on reducing side-effects of radiotherapy and improving immune function in patients with nasopharyngeal cancer—MD Anderson Cancer Center. Chin. J. Clin. Oncol. 1999, 26, 216–218. [Google Scholar]
- Oba, K.; KobayashI, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009, 29, 2739–2745. [Google Scholar] [PubMed]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, H.; Liu, W.; Wu, J.; Yue, X.; Wang, J.; Quan, L.; Liu, H.; Guo, L.; Wang, Z.; et al. Ganoderic acid a exerts antitumor activity against MDA-MB-231 human breast cancer cells by inhibiting the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 2018, 16, 6515–6521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliza, W.L.Y.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: Systematic review and meta-analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef]
- Chen, S.; Oh, S.R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Xie, Y.Z.; Yang, X.; Li, H.; Li, X.M.; Pan, H.H.; Cai, M.H.; Zhong, H.M.; Yang, B.B. Anticancer activity of Amauroderma rude. PLoS ONE 2013, 8, e66504. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Han, Y.; Huang, J.; Yu, X.; Jiao, C.; Yang, X.; Dhaliwal, P.; Xie, Y.; Yang, B.B. Purification and identification of a polysaccharide from medicinal mushroom Amauroderma rude with immunomodulatory activity and inhibitory effect on tumor growth. Oncotarget 2015, 6, 17777–17791. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.L.; Kuo, Y.H.; Tsai, C.T.; Huang, Y.T.; Chen, S.C.; Chang, H.W.; Lin, E.; Lin, W.H.; Hseu, Y.C. Anti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem. Toxicol. 2011, 49, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Yang, H.L.; Der Way, T.; Kumar, K.J.S.; Juan, Y.C.; Cho, H.J.; Lin, K.Y.; Hsu, L.S.; Chen, S.C.; Hseu, Y.C. Inhibition of cell growth and induction of apoptosis by Antrodia camphorata in HER-2/neu-overexpressing breast cancer cells through the induction of ROS, depletion of HER-2/neu, and disruption of the PI3K/Akt signaling pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 702857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.K.; Wu, A.T.H.; Geethangili, M.; Te Huang, M.; Chao, W.J.; Wu, C.H.; Deng, W.P.; Yeh, C.T.; Tzeng, Y.M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lin, Y.Y.; Yang, Y.H.; Lin, C.L.; Kuan, F.C.; Lu, C.N.; Chang, G.H.; Tsai, M.S.; Hsu, C.M.; Yeh, R.A.; et al. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement. Altern. Med. 2018, 18, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-L.; Liu, H.-W.; Shrestha, S.; Thiyagarajan, V.; Huang, H.-C.; Hseu, Y.-C. Antrodia salmonea induces apoptosis and enhances cytoprotective autophagy in colon cancer cells. Aging 2021, 13, 15964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Hsieh, H.W.; Lin, C.C.; Wang, S.Y. Antcin-A modulates epithelial-to-mesenchymal transition and inhibits migratory and invasive potentials of human breast cancer cells via p53-mediated mir-200c activation. Planta Med. 2019, 85, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, Q.; Ji, S.; Huang, Y.; Liu, K.-D.; Zhang, Z.-X.; Bo, T.; Tzeng, Y.-M.; Guo, D.-A.; Ye, M. Metabolites identification and multi-component pharmacokinetics of ergostane and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J. Pharm. Biomed. Anal. 2015, 111, 266–276. [Google Scholar] [CrossRef]
- Chang, C.T.; Hseu, Y.C.; Thiyagarajan, V.; Huang, H.C.; Hsu, L.S.; Huang, P.J.; Liu, J.Y.; Liao, J.W.; Yang, H.L. Antrodia salmonea induces G2 cell-cycle arrest in human triple-negative breast cancer (MDA-MB-231) cells and suppresses tumor growth in athymic nude mice. J. Ethnopharmacol. 2017, 196, 9–19. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lin, Y.C.; Rajendran, P.; Thigarajan, V.; Mathew, D.C.; Lin, K.Y.; Der Way, T.; Liao, J.W.; Yang, H.L. Antrodia salmonea suppresses invasion and metastasis in triple-negative breast cancer cells by reversing EMT through the NF-κB and Wnt/β-catenin signaling pathway. Food Chem. Toxicol. 2019, 124, 219–230. [Google Scholar] [CrossRef]
- Jiang, J.; Sliva, D. Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. Int. J. Oncol. 2010, 37, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, X.; Liang, Y.N.; Wang, L.; Song, Z.X.; Liu, J.L.; Tang, Z.S. Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR. Molecules 2016, 21, 1267. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.; Lau, C.B.S. Differential anti-tumor activity of Coriolus versicolor (Yunzhi) extract through p53- and/or Bcl-2-dependent apoptotic pathway in human breast cancer cells. Cancer Biol. Ther. 2005, 4, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Yue, G.G.L.; Ko, C.H.; Lee, J.K.M.; Gao, S.; Li, L.F.; Li, G.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. In vivo and in vitro anti-tumor and anti-metastasis effects of Coriolus versicolor aqueous extract on mouse mammary 4T1 carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chou, P.Y.; Chien, Y.C.; Wu, C.H.; Wu, T.S.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomedicine 2012, 19, 768–778. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kunitomo, M.; Kagota, S.; Shinozuka, K.; Nakamura, K. Inhibitory effect of cordycepin on hematogenic metastasis of B16-F1 mouse melanoma cells accelerated by adenosine-5′-diphosphate-PubMed. Anticancer Res. 2009, 29, 3857–3860. [Google Scholar]
- Wei, C.; Yao, X.; Jiang, Z.; Wang, Y.; Zhang, D.; Chen, X.; Fan, X.; Xie, C.; Cheng, J.; Fu, J.; et al. Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol. Res. 2019, 144, 79–89. [Google Scholar] [CrossRef]
- Tsao, S.M.; Hsu, H.Y. Fucose-containing fraction of Ling-Zhi enhances lipid rafts-dependent ubiquitination of TGFβ receptor degradation and attenuates breast cancer tumorigenesis. Sci. Rep. 2016, 6, 36563. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.S.; Lu, J.J.; Guo, J.J.; Li, Y.B.; Tan, W.; Dang, Y.Y.; Zhong, Z.F.; Xu, Z.T.; Chen, X.P.; Wang, Y.T. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 2012, 83, 408–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Meng, Q.; Guo, W.; Chen, Q.; Zhang, Y. Grifola frondosa polysaccharides induce breast cancer cell apoptosis via the mitochondrial-dependent apoptotic pathway. Int. J. Mol. Med. 2017, 40, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-fraction on the activation of NK cells in cancer patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef]
- Alonso, E.N.; Orozco, M.; Nieto, A.E.; Balogh, G.A. Genes related to suppression of malignant phenotype induced by maitake D-fraction in breast cancer cells. J. Med. Food 2013, 16, 602–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and antimetastatic activity of Maitake D-fraction in triple-negative breast cancer cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldan-Deamicis, A.; Alonso, E.; Brie, B.; Braico, D.A.; Balogh, G.A. Maitake Pro4X has anti-cancer activity and prevents oncogenesis in BALBc mice. Cancer Med. 2016, 5, 2427–2441. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2010, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chiu, L.; Cheung, P.; Ooi, V. Growth-inhibitory effects of a beta-glucan from the mycelium of Poria cocos on human breast carcinoma MCF-7 cells: Cell-cycle arrest and apoptosis induction-PubMed. Oncol. Rep. 2006, 15, 637–643. [Google Scholar] [PubMed]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Suzuki, M.; Kanayama, N.; et al. Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J. Cancer Res. Clin. Oncol. 2005, 131, 527–538. [Google Scholar] [CrossRef]
- Masuda, Y.; Nawa, D.; Nakayama, Y.; Konishi, M.; Nanba, H. Soluble β-glucan from Grifola frondosa induces tumor regression in synergy with TLR9 agonist via dendritic cell-mediated immunity. J. Leukoc. Biol. 2015, 98, 1015–1025. [Google Scholar] [CrossRef]
- Chan, G.C.F.; Chan, W.K.; Sze, D.M.Y. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Togo, T.; Mizuno, S.; Konishi, M.; Nanba, H. Soluble -glucan from Grifola frondosa induces proliferation and Dectin-1/Syk signaling in resident macrophages via the GM-CSF autocrine pathway. J. Leukoc. Biol. 2012, 91, 547–556. [Google Scholar] [CrossRef]
- Fujimiya, Y.; Suzuki, Y.; Katakura, R.; Ebina, T. Tumor-specific cytocidal and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycete, Agaricus blazei Murill. Anticancer Res. 1999, 19, 113–118. [Google Scholar] [PubMed]
- Wu, J.Y.; Zhang, Q.X.; Leung, P.H. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine 2007, 14, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Takaku, T.; Kimura, Y.; Okuda, H. Isolation of an antitumor compound from Agaricus blazei Murill and its mechanism of action. J. Nutr. 2001, 131, 1409–1413. [Google Scholar] [CrossRef]
- Mansour, A.; Daba, A.; Baddour, N.; El-Saadani, M.; Aleem, E. Schizophyllan inhibits the development of mammary and hepatic carcinomas induced by 7,12 dimethylbenz(α)anthracene and decreases cell proliferation: Comparison with tamoxifen. J. Cancer Res. Clin. Oncol. 2012, 138, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Sun, J.; Wang, H.X.; Ng, T.B. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Li, D.F.; Feng, L.; Xiang, Y.; Liu, W.; Sun, H.; Wang, D.C. Structural basis for the tumor cell apoptosis-inducing activity of an antitumor lectin from the edible mushroom Agrocybe aegerita. J. Mol. Biol. 2009, 387, 694–705. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, W.K.; Ng, T.B.; Ooi, V.E.C.; Chang, S.T. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 1996, 31, 205–211. [Google Scholar] [CrossRef]
- Yap, H.Y.Y.; Fung, S.Y.; Ng, S.T.; Tan, C.S.; Tan, N.H. Shotgun proteomic analysis of tiger milk mushroom (Lignosus rhinocerotis) and the isolation of a cytotoxic fungal serine protease from its sclerotium. J. Ethnopharmacol. 2015, 174, 437–451. [Google Scholar] [CrossRef]
- Cheung, Y.H.; Sheridan, C.M.; Lo, A.C.Y.; Lai, W.W. Lectin from Agaricus bisporus inhibited S phase cell population and Akt phosphorylation in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7469–7475. [Google Scholar] [CrossRef] [Green Version]
- Koh, G.Y.; Chou, G.; Liu, Z. Purification of a water extract of chinese sweet tea plant (Rubus suavissimus S. Lee) by alcohol precipitation. J. Agric. Food Chem. 2009, 57, 5000–5006. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of biologically active Ganoderma lucidum compounds and synthesis of improved derivatives that confer anti-cancer activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Qin, C.; Tao, L.; Liu, X.; Shi, Z.; Ma, X.; Jia, J.; Tan, Y.; Cui, C.; Lin, J.; et al. Clustered patterns of species origins of nature-derived drugs and clues for future bioprospecting. Proc. Natl. Acad. Sci. USA 2011, 108, 12943–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Wang, Y.; Wang, X.; Li, J.; Cui, H.; Niu, M. Ganoderic acids suppress growth and angiogenesis by modulating the NF-κB signaling pathway in breast cancer cells. Int. J. Clin. Pharmacol. Ther. 2012, 50, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Y.; Yin, X.; Li, Z.H.; Li, Y.; Liu, J.K.; Feng, T.; Zhao, B.H. Mycophenolic acid derivatives from cultures of the mushroom Laetiporus sulphureu. Chin. J. Nat. Med. 2014, 12, 685–688. [Google Scholar] [CrossRef]
- He, J.B.; Tao, J.; Miao, X.S.; Bu, W.; Zhang, S.; Dong, Z.J.; Li, Z.H.; Feng, T.; Liu, J.K. Seven new drimane-type sesquiterpenoids from cultures of fungus Laetiporus sulphureus. Fitoterapia 2015, 102, 1–6. [Google Scholar] [CrossRef]
- Erkel, G.; Anke, T.; Sterner, O. Inhibition of NF-κB activation by panepoxydone. Biochem. Biophys. Res. Commun. 1996, 226, 214–221. [Google Scholar] [CrossRef]
- Arora, R.; Yates, C.; Gary, B.D.; McClellan, S.; Tan, M.; Xi, Y.; Reed, E.; Piazza, G.A.; Owen, L.B.; Dean-Colomb, W. Panepoxydone targets NF-kB and FOXM1 to inhibit proliferation, induce apoptosis and reverse epithelial to mesenchymal transition in breast cancer. PLoS ONE 2014, 9, e98370. [Google Scholar] [CrossRef] [Green Version]
- Quang, D.N.; Lam, D.M.; Hanh, N.T.H.; Queb, D.D. Cytotoxic constituents from the fungus Daldinia concentrica (Xylariaceae). Nat. Prod. Res. 2013, 27, 486–490. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Wang, H.; Ng, T. Purification and characterization of a laccase from the edible wild mushroom Tricholoma mongolicum. J. Microbiol. Biotechnol. 2010, 20, 1069–1076. [Google Scholar] [CrossRef]
- Jiang, J.; Thyagarajan-Sahu, A.; Loganathan, J.; Eliaz, I.; Terry, C.; Sandusky, G.E.; Sliva, D. BreastDefendTM prevents breast-to-lung cancer metastases in an orthotopic animal model of triple-negative human breast cancer. Oncol. Rep. 2012, 28, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.J.; Chung, C.-K.; Jeong, Y.; Ham, S.-S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, W.S.; Go, S.I.; Nagappan, A.; Baek, J.Y.; Lee, J.D.; Lee, S.J.; Park, C.; Kim, G.Y.; Kim, H.J.; et al. Pachymic acid induces apoptosis of ej bladder cancer cells by DR5 up-regulation, ROS generation, modulation of Bcl-2 and IAP family members. Phytother. Res. 2015, 29, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, J.; Lu, C.; Cai, D. Pachymic acid induces apoptosis via activating ROS-dependent JNK and ER stress pathways in lung cancer cells. Cancer Cell Int. 2015, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Fan, L. Evaluation of anticancer activities of Poria cocos ethanol extract in breast cancer: In vivo and in vitro, identification and mechanism. J. Ethnopharmacol. 2020, 257, 112851. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Leonard, J. In vitro effects on proliferation, apoptosis and colony inhibition in ER-dependent and ER-independent human breast cancer cells by selected mushroom species. Oncol. Rep. 2006, 15, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Andrej, J.; Silva Daniel, S. Pleurotus ostreatus inhibits proliferation of human breast and colon cancer cells through p53-dependent as well as p53-independent pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar]
- Chen, J.; Seviour, R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [Green Version]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an Edible Mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar] [PubMed]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.G. Immune responses to commensal and environmental microbes. Nat. Immunol. 2007, 8, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Kunze, R. Water-Soluble Low Molecular Weight β-Glucans for Modulating Immunological Responses in Mammalian System. U.S. Patent 6,143,883, 7 November 2000. [Google Scholar]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, T.; Ohno, N. Contribution of dectin-1 and granulocyte macrophage–colony stimulating factor (GM-CSF) to immunomodulating actions of β-glucan. Int. Immunopharmacol. 2008, 8, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, Y.; Liu, F. Purification and characterization of a∼ 43 kDa antioxidant protein with antitumor activity from Pholiota nameko. J. Sci. Food Agric. 2016, 96, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.R.; Guo, C.L.; Lou, D.J.; Li, R.T.; Xiang, Q.; Zou, Y.L.; Cui, X.M.; Yang, X.Y. One new sterpurane sesquiterpene from cultures of the basidiomycete Pholiota nameko. Nat. Prod. Res. 2020, 34, 2754–2759. [Google Scholar] [CrossRef]
- Yang, X.Y.; Niu, W.R.; Li, R.T.; Cui, X.M.; Liu, J.K. Two new sesquiterpenes from cultures of the higher fungus Pholiota nameko. Nat. Prod. Res. 2018, 33, 1992–1996. [Google Scholar] [CrossRef]
- Lee, J.; Maarisit, W.; Abdjul, D.; Yamazaki, H.; Takahashi, H.; Kirikoshi, R.; Kanno, S.; Namikoshi, M. Structures and biological activities of triterpenes and sesquiterpenes obtained from Russula lepida. Phytochemistry 2016, 127, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Maarisit, W.; Yamazaki, H.; Kanno, S.; Tomizawa, A.; Lee, J.; Namikoshi, M. Protein tyrosine phosphatase 1B inhibitory properties of seco-cucurbitane triterpenes obtained from fruiting bodies of Russula lepida. J. Nat. Med. 2017, 71, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Song, Y.L.; Yin, Z.Q.; Guo, J.J.; Wang, S.P.; Zhao, W.W.; Chen, X.P.; Zhang, Q.W.; Lu, J.J.; Wang, Y.T. Ganoderiol A-enriched extract suppresses migration and adhesion of MDA-MB-231 cells by inhibiting FAK-SRC-paxillin cascade pathway. PLoS ONE 2013, 8, e76620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Aouidate, A.; Wang, S.; Yu, Q.; Li, Y.; Yuan, S. Discovering anti-cancer drugs via computational methods. Front. Pharmacol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Darsey, J.A.; Ghosh, A.; Li, H.-Y.; Yang, M.Q.; Wang, S. Artificial intelligence and cancer drug development. Recent Pat. Anti-Cancer Drug Discov. 2021, 16. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer screening and early detection in the 21st century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, L.G.; Ferreira, L.L.G.; Andricopulo, A.D. Recent advances and perspectives in cancer drug design. An. Acad. Bras. Ciênc. 2018, 90, 1233–1250. [Google Scholar] [CrossRef] [Green Version]
- Markham, M.J.; Wachter, K.; Agarwal, N.; Bertagnolli, M.M.; Chang, S.M.; Dale, W.; Diefenbach, C.S.M.; Rodriguez-Galindo, C.; George, D.J.; Gilligan, T.D.; et al. Clinical cancer advances 2020: Annual report on progress against cancer from the American Society of Clinical oncology. J. Clin. Oncol. 2020, 38, 1081–1101. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663. [Google Scholar] [CrossRef]
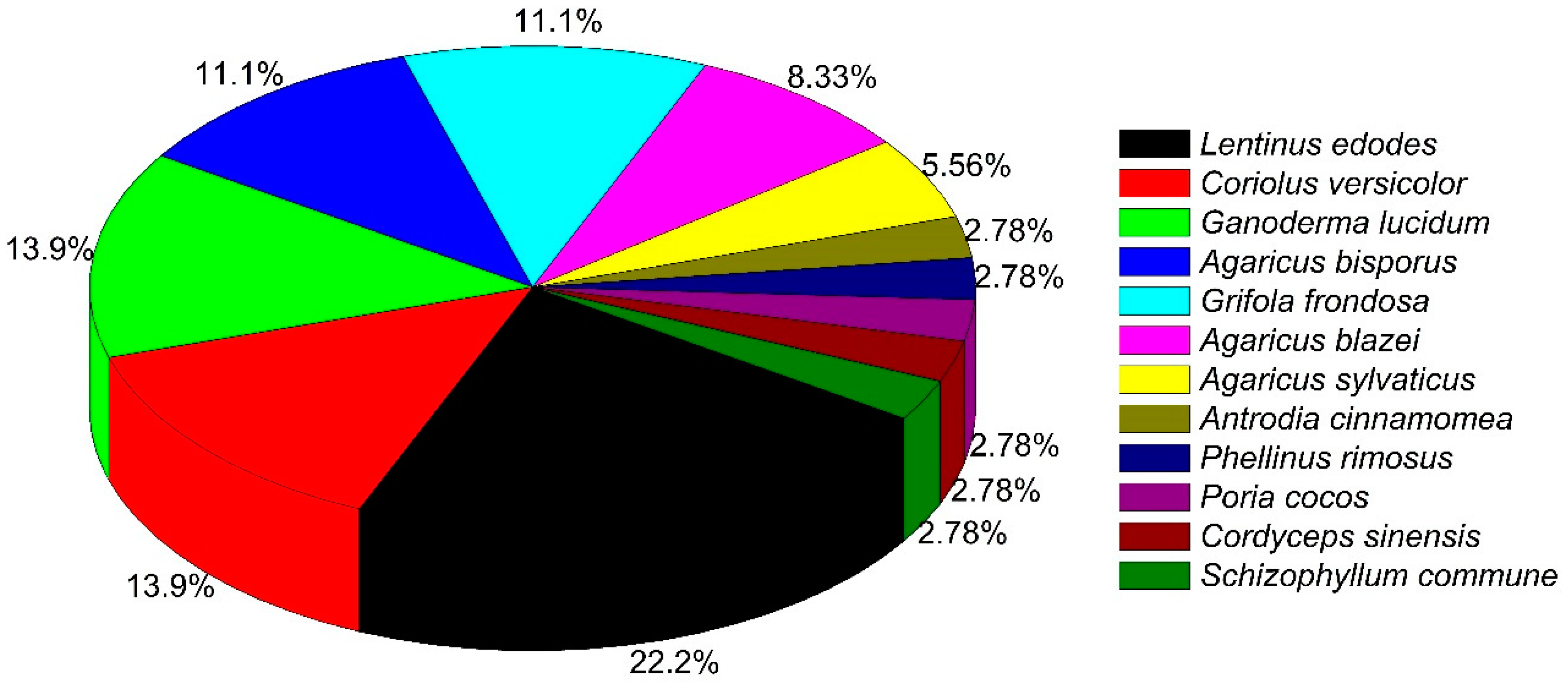

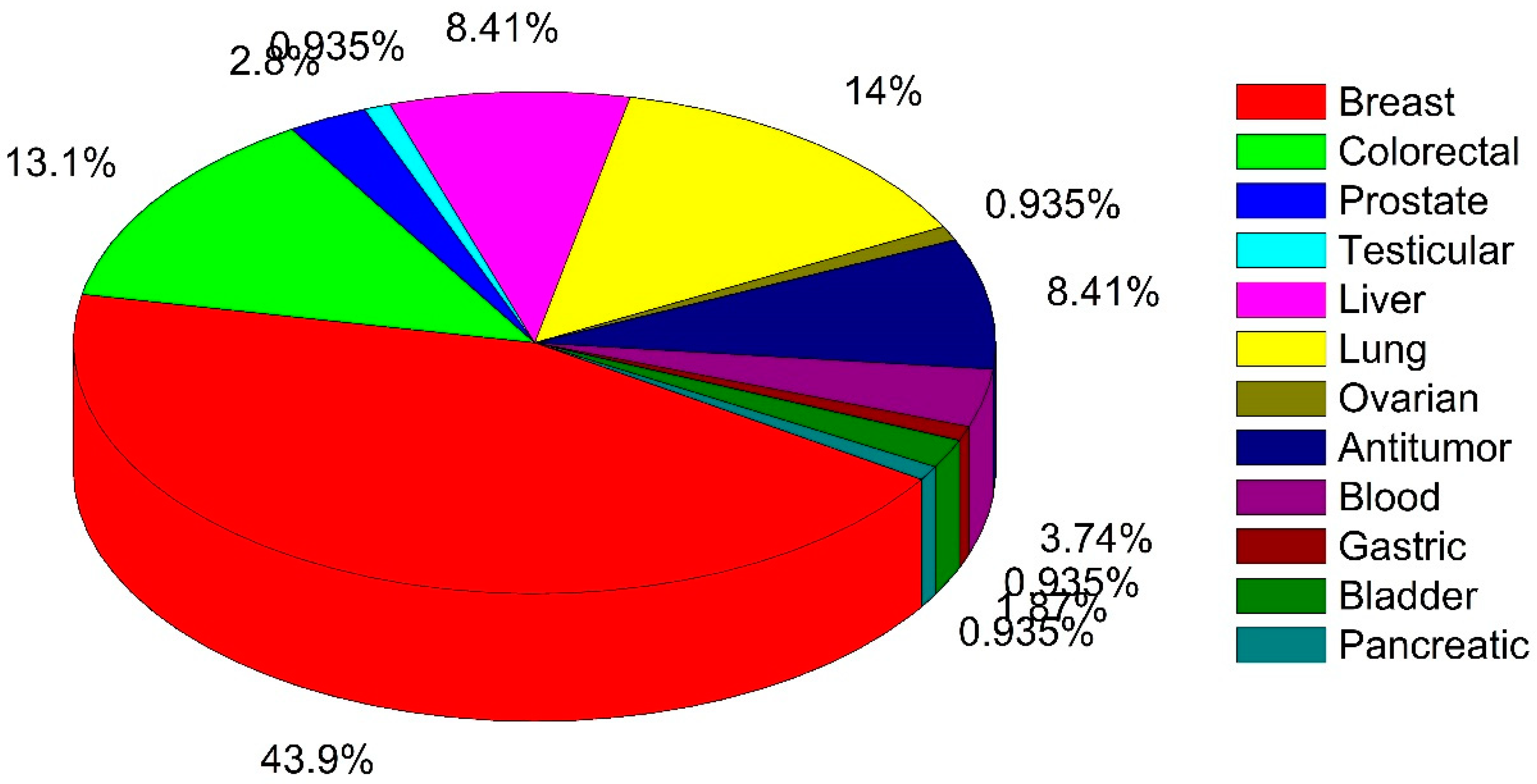

| Scientific Name | Type of Study | Major Outcomes | Reference |
|---|---|---|---|
| Agaricus bisporus | Phase I trial, n = 32 | Appeared to reduce prostate cancer by decreasing immunosuppressive factors. | [10] * |
| Agaricus blazei | Randomized, placebo-controlled, double-blind clinical trial (RCT), n = 40 | AndoSanTM as adjuvant therapy to high dose of melphalan improved a few immune-modulating effects. In addition, increase in serum levels (IL-1, IL-5, and IL- 7) and expression of antibodies and killer immunoglobulin receptor (KIR) genes were observed. | [12] * |
| Agaricus blazei | RCT, n = 100 | Between treated and non-treated groups, there was no significant difference w.r.t. lymphokine-activated killer and monocyte activities among cervical, ovarian, and endometrial cancer patients undergoing chemotherapy. Additionally, several side effects were improved by verum only when treated with mushroom extract | [13] * |
| Agaricus sylvaticus | RCT, n = 56 | Significant reduction in fasting plasma glucose, total cholesterol, creatinine, aspartate aminotransferase, alanine aminotransferase, IgA, IgM, and systolic and diastolic blood pressure. | [31] |
| Agaricus sylvaticus | RCT, n = 46 | Improved nutritional status with reduced adverse effects (nausea, vomiting, and anorexia), in patients with breast cancer, stage II and III. | [15] |
| Cordyceps sinensis | Clinical study, n = 36 | Jinshuibao capsule (containing constituents similar to Cordyceps sinensis) restored cellular immunological function, improved quality of life (QOL), but had no substantial effect on humoral immune function. | [32] |
| Ganoderma lucidum | Pilot clinical trial, n = 48 | Treated breast cancer patients showed significant enhancements in physical well-being and fatigue with a reduced amount of anxiety and depression. | [19] |
| Ganoderma lucidum | Open label, n = 36 | Ganopoly®® significant increase in mean plasma concentrations of IL-2, IL-6, and IFN-γ, whereas the levels of IL-1 and TNF-α were significantly decreased. The mean absolute number of CD56+ cells was significantly increased, whereas the numbers of CD3+-, CD4+-, and CD8+-expressing cells were just marginally increased compared with baseline levels, with the CD4:CD8 T cell ratios unchanged. PHA responses were enhanced in most patients; and mean NK activity was increased compared with baselines. | [33] |
| Ganoderma lucidum | RCT, n = 68 | A significant increase in Karnofsky scores compared with placebo among the advanced-stage lung cancer patients. Less disease progression. In addition, several cancer-related symptoms and immune parameters were significantly improved in verum. | [34] |
| Ganoderma lucidum | Controlled clinical Trial, n = 198 | Decrease in both number and size of colorectal adenomas for the verum group. | [29] |
| Grifola frondosa | Phase I/II, dose escalation trial, n = 34 | Maitake extracts affects both immunological stimulatory and inhibitory parameters in peripheral blood with treated post-menopausal breast cancer patients. | [20] * |
| Lentinula edodes | Phase II clinical trial, n = 74 | Mushroom extract failed to reduce by >50% prostate- specific antigen in early stage prostrate cancer patients. | [24] * |
| Lentinus edodes | Clinical trial, n = 62 | Administration of L. edodes extract in prostate cancer patients failed to stabilize or halt progression of disease. | [25] * |
| Schizophyllum commune | Clinical trial, n = 220 | Tumor-reducing effect in cervical cancer patients with stage II or III. Time to recurrence was longer in in stage II but not stage III cancer, compared with control group; 48-month survival time of patients with stage II but not stage III cancer in the SPG group was significantly longer than in the control group. | [30] |
| Trametes versicolor | Controlled trial, n = 60 | Significantly improved symptoms of Qi and Yin deficiency in gastric cancer patients after chemotherapy. | [35] |
| Cancer Type | In Vitro Study | In Vivo Study | Clinical Trial |
|---|---|---|---|
| Miscellaneous tumors | Agaricus bisporus, Agaricus blazei, Antrodia camphorata, Grifola frondosa, Phellinus linteus, Phellinus rimosus, Ramaria flava | Agaricus blazei, Agaricus sylvaticus, Antrodia camphorata, Amauroderma rude, Cordyceps sinensis, Flammulina velutipes, Ganoderma lucidum, Grifola frondosa, Lentinus edodes, Lepista inversa, Pleurotus nebrodensis, Tricholoma mongolicum | Phellinus rimosus |
| Bladder | Phellinus linteus, Poria cocos | - | - |
| Blood | Agaricus blazei, Cordyceps sinensis, Grifola frondosa, Pleurotus ostreatus | - | Grifola frondosa |
| Breast | Agaricus bisporus, Agaricus blazei, Amauroderma rude, Antrodia cinnamomea, Antrodia camphorata, Antrodia salmonea, Amauroderma rude, Cordyceps sinensis, Coriolus versicolor, Cortinarius xiphidipus, Fuscoporia torulosa, Ganoderma lucidum, Grifola frondosa, Inonotus obliquus, Laetiporus sulphureus, Lentinus crinitus, Lentinus polychrous, Lignosus rhinocerotis, Lignosus tigris, Marasmius oreades, Phellinus linteus, Phellinus rimosus, Pholiota adiposa, Pholiota nameko, Pleurotus abalones, Pleurotus djamor, Pleurotus highking, Pleurotus nebrodensis, Pleurotus ostreatus, Poria cocos, Tricholoma mongolicum, Xylaria schweinitzii | Agaricus bisporus, Agaricus blazei, Amauroderma rude, Antrodia salmonea, Ganoderma lucidum, Lignosus tigris, Phellinus rimosus, Poria cocos, Schizophyllum commune | Agaricus bisporus, Agaricus sylvaticus, Coriolus versicolor, Ganoderma lucidum, Grifola frondosa |
| Cancer cachexia | - | Antrodia cinnamomea | |
| Cervical | - | - | Agaricus blazei, Schizophyllum commune |
| Chronic hepatitis C infection | - | - | Agaricus blazei |
| Colorectal | Agaricus bisporus, Agaricus blazei, Antrodia salmonea, Cerrena unicolor, Ganoderma lucidum, Grifola frondosa, Inonotus obliquus, Lentinan, Marasmius oreades, Phellinus linteus, Pleurotus sajor-caju, Pleurotus ostreatus, Pycnoporus sanguineus, Sarcodon aspratus, Taiwanofungus salmoneus | Agaricus blazei | Agaricus sylvaticus, Ganoderma lucidum, Lentinan |
| Endometrial | - | - | Agaricus blazei |
| Gastric | Agaricus blazei | - | Trametes versicolor, Lentinan |
| Liver | Agaricus blazei, Auricularia auricula-judae, Cordyceps sinensis, Coriolus versicolo, Lentinan, Russula alatoreticula, Thelephora aurantiotincta, Tricholoma mongolicum, Xylaria schweinitzii | Agaricus blazei, Auricularia auricula-judae, Ganoderma lucidum, Phellinus linteus, Schizophyllum commune | Coriolus versicolo, Lentinan |
| Lung | Agaricus blazei, Antrodia cinnamomea, Cordyceps sinensis, Flammulina velutipes, Ganoderma lucidum, Grifola frondosa, Inonotus obliquus, Lentinula edodes, Phellinus linteus, Lentinus squarrosulus, Pleurotus nebrodensis, Pleurotus nebrodensis | Poria cocos | Ganoderma lucidum, Grifola frondosa |
| Lymphoma in dogs | - | - | Grifola frondosa |
| Myeloma | - | - | Agaricus blazei |
| Nasopharyngeal | - | - | Ganoderma lucidum |
| Ovarian | Antrodia salmonea | - | Agaricus blazei, Agaricus bisporus, Volvariella volvacea |
| Pancreatic | Agaricus blazei | - | - |
| Prostate | Fuscoporia torulosa, Ganoderma lucidum, Lentinula edodes, Phellinus linteus | - | Agaricus bisporus, Lentinula edodes |
| Testicular | Cordyceps sinensis | - | - |
| Other advanced cancers | - | - | Antrodia cinnamomea, Cordyceps sinensis, Ganoderma lucidum, Lentinula edodes |
| Name of the Mushroom | Type of Cancer | Type of Studies (References) | Overall Strength of Recommendation | ||||
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | In Silico | Clinical Study | Active Constituents | |||
| Agaricus bisporus | Breast, colon, prostate cancer | *** | ** | *** | ** | ** | ** |
| Agaricus blazei | Several types of cancer: myeloma, leukemia, chronic hepatitis C infection, breast, cervical, ovarian, lung, pancreatic, and endometrial | *** | *** | - | *** | *** | *** |
| Agaricus sylvaticus | Colorectal and breast cancer | *** | ** | - | *** | * | ** |
| Amauroderma rude | Breast cancer | *** | * | - | - | ** | * |
| Antrodia cinnamomea | Breast and lung cancer | *** | *** | - | * | ** | ** |
| Antrodia camphorata | Miscellaneous tumor | ** | * | - | - | * | * |
| Antrodia salmonea | Breast, colon, and ovarian cancer | *** | ** | - | - | * | * |
| Auricularia auricula-judae | Hepatoma | * | - | * | - | * | * |
| Cerrena unicolor | Colon cancer, miscellaneous tumors | *** | * | - | - | - | * |
| Cordyceps sinensis | Lung and testicular cancer | *** | * | ** | * | ** | ** |
| Coriolus versicolor | Breast, gastric, and liver cancer | *** | *** | *** | *** | ** | *** |
| Cortinarius xiphidipus | Several types | * | - | - | - | - | - |
| Flammulina velutipes | Lung cancer and miscellaneous tumor | ** | - | * | - | *** | * |
| Fuscoporia torulosa | Brest and prostate cancer | * | - | - | - | - | - |
| Ganoderma lucidum | Breast, lung, colorectal, and Nasopharyngeal cancer | *** | *** | *** | *** | *** | *** |
| Grifola frondosa | Blood, breast, and lung cancer | *** | ** | * | *** | *** | *** |
| Inonotus obliquus | Breast cancer | *** | ** | * | - | *** | ** |
| Lentinus edodes | Breast, lung, colorectal, gastric, and liver cancer | *** | ** | ** | *** | *** | *** |
| Lentinus squarrosulus | Lung cancer | * | - | - | - | - | - |
| Lepista inversa | Several cancer cell lines | * | - | - | - | - | - |
| Lignosus rhinocerotis | Breast cancer | ** | * | * | - | ** | * |
| Lignosus tigris | Breast cancer | ** | * | - | - | * | * |
| Marasmius oreades | Colon and breast cancer | ** | * | - | - | * | * |
| Phellinus linteus | Colon, liver, lungs, and prostate cancer | *** | ** | * | - | *** | ** |
| Phellinus rimosus | Colon and liver cancer | *** | * | - | - | ** | * |
| Pholiota nameko | Breast cancer | ** | * | - | - | * | * |
| Pleurotus abalones | Breast cancer | ** | * | - | - | * | * |
| Pleurotus highking | Breast cancer | ** | * | - | - | * | * |
| Pleurotus nebrodensis | Liver, lungs, and breast cancer | *** | * | - | - | ** | * |
| Pleorotus ostreatus | Blood, lungs, and breast cancer | *** | ** | * | - | * | * |
| Poria cocos | Breast and pancreatic cancer | *** | ** | ** | * | *** | ** |
| Pycnoporus sanguineus | Colon cancer | * | - | - | - | - | - |
| Ramaria flava | Liver cancer | ** | - | - | - | - | - |
| Russula alatoreticula | Liver cancer | * | - | - | - | - | - |
| Schizophyllum commune | Breast, liver, and cervical cancer | *** | * | - | * | ** | ** |
| Thelephora aurantiotincta | Liver cancer | * | - | - | - | - | - |
| Taiwanofungus salmoneus | Colon and liver cancer | *** | * | - | - | ** | * |
| Tricholoma mongolicum | Breast and liver cancer | *** | - | - | - | * | * |
| Xylaria schweinitzii | Breast, liver, and lung cancer | * | - | - | - | * | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals 2022, 15, 176. https://doi.org/10.3390/ph15020176
Panda SK, Sahoo G, Swain SS, Luyten W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals. 2022; 15(2):176. https://doi.org/10.3390/ph15020176
Chicago/Turabian StylePanda, Sujogya Kumar, Gunanidhi Sahoo, Shasank S. Swain, and Walter Luyten. 2022. "Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery" Pharmaceuticals 15, no. 2: 176. https://doi.org/10.3390/ph15020176







