Nuclear Molecular Imaging of Cardiac Remodeling after Myocardial Infarction
Abstract
:1. Introduction
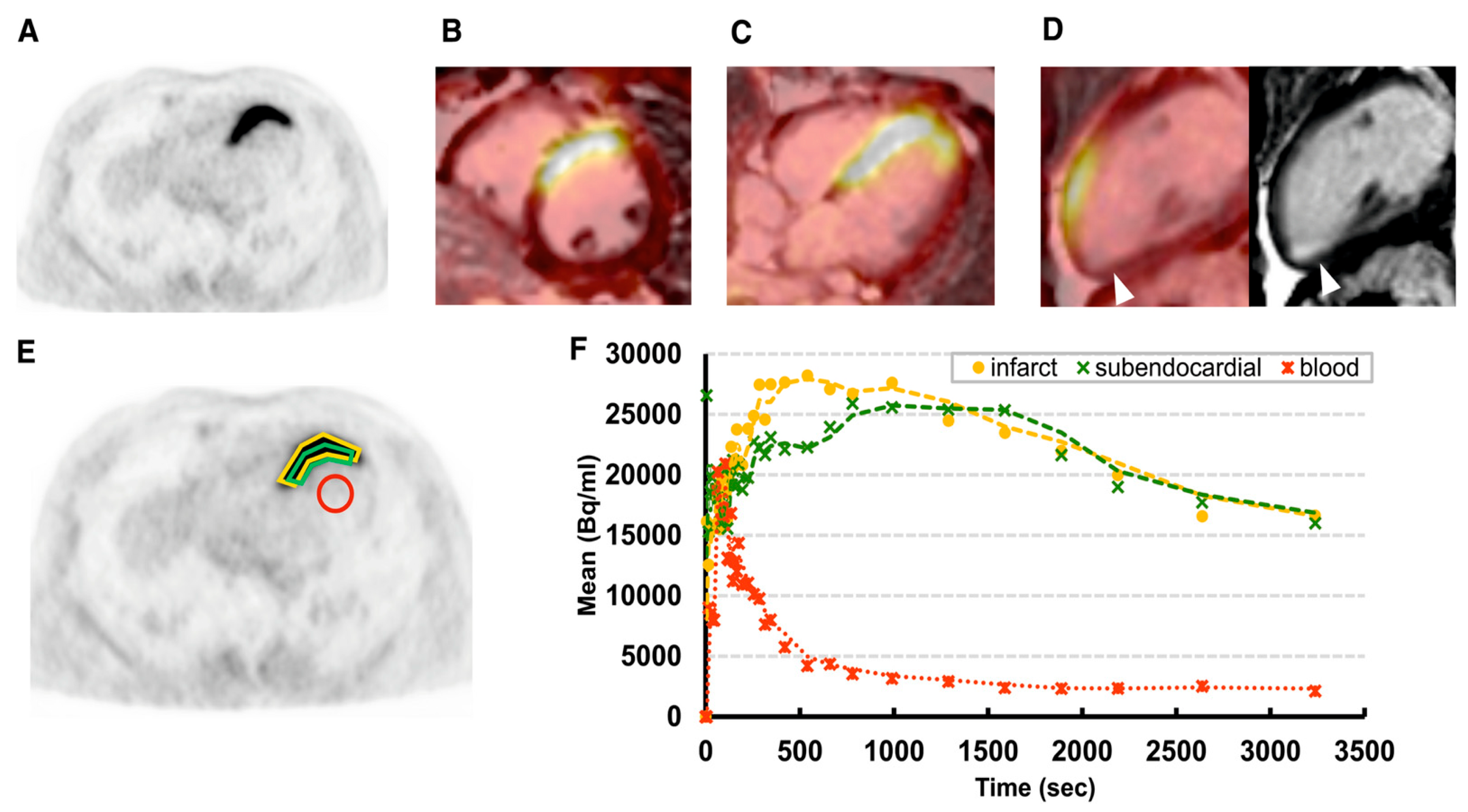
2. Inflammation
3. Angiogenesis
4. Fibrosis and Fibrosis Activity
5. Cardiac Molecular Imaging in Therapeutic Applications
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bankstahl, J.P.; Wang, Y.; Wollert, K.C.; Bengel, F.M. Clinically relevant strategies for lowering cardiomyocyte glucose uptake for 18F-FDG imaging of myocardial inflammation in mice. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Dirschinger, R.J.; Nekolla, S.G.; Kossmann, H.; Nicolosi, S.; Hanus, F.; van Marwick, S.; Kunze, K.P.; Meinicke, A.; Götze, K.; et al. Prospective Evaluation of 18 F-Fluorodeoxyglucose Uptake in Postischemic Myocardium by Simultaneous Positron Emission Tomography/Magnetic Resonance Imaging as a Prognostic Marker of Functional Outcome. Circ. Cardiovasc. Imaging 2016, 9, e004316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, J.; Viollet, C.; Doublier, S.; Videau, C.; Epelbaum, J.; Baud, L. Somatostatin binds to murine macrophages through two distinct subsets of receptors. J. Neuroimmunol. 2003, 138, 38–44. [Google Scholar] [CrossRef]
- Dalm, V.A.S.H.; Van Hagen, P.M.; Van Koetsveld, P.M.; Achilefu, S.; Houtsmuller, A.B.; Pols, D.; Van Der Lely, A.-J.; Lamberts, S.W.J.; Hofland, L.J. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am. J. Physiol. Metab. 2003, 285, E344–E353. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68 Ga-DOTATATE PET Compared to [18 F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Calcagno, C.; Dweck, M.R.; Evans, N.R.; Chowdhury, M.M.; Gopalan, D.; Newby, D.E.; Fayad, Z.A.; Bennett, M.R.; Rudd, J.H. 68Ga-DOTATATE PET Identifies Residual Myocardial Inflammation and Bone Marrow Activation After Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2489–2491. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Bankstahl, J.P.; Wang, Y.; Korf-Klingebiel, M.; Walte, A.; Wittneben, A.; Wollert, K.C.; Bengel, F.M. Targeting post-infarct inflammation by PET imaging: Comparison of 68Ga-citrate and 68Ga-DOTATATE with 18F-FDG in a mouse model. Eur. J. Nucl. Med. Mol. Imaging 2014, 42, 317–327. [Google Scholar] [CrossRef]
- Cotugno, G.; Aurilio, M.; Annunziata, P.; Capalbo, A.; Faella, A.; Rinaldi, V.; Strisciuglio, C.; Di Tommaso, M.; Aloj, L.; Auricchio, A. Noninvasive Repetitive Imaging of Somatostatin Receptor 2 Gene Transfer with Positron Emission Tomography. Hum. Gene Ther. 2011, 22, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DãRing, Y.; Epawig, L.; Eweber, C.; Enoels, H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front. Physiol. 2014, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Wester, H.J.; Keller, U.; Schottelius, M.; Beer, A.; Philipp-Abbrederis, K.; Hoffmann, F.; Šimeček, J.; Gerngross, C.; Lassmann, M.; Herrmann, K.; et al. Disclosing the CXCR4 Expression in Lymphoproliferative Diseases by Targeted Molecular Imaging. Theranostics 2015, 5, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Philipp-Abbrederis, K.; Herrmann, K.; Knop, S.; Schottelius, M.; Eiber, M.; Lueckerath, K.; Pietschmann, E.; Habringer, S.; Gerngroß, C.; Franke, K.; et al. In vivo molecular imaging of chemokine receptor CXCR 4 expression in patients with advanced multiple myeloma. EMBO Mol. Med. 2015, 7, 477–487. [Google Scholar] [CrossRef]
- Herrmann, K.; Lapa, C.; Wester, H.-J.; Schottelius, M.; Schiepers, C.; Eberlein, U.; Bluemel, C.; Keller, U.; Knop, S.; Kropf, S.; et al. Biodistribution and Radiation Dosimetry for the Chemokine Receptor CXCR4-Targeting Probe 68Ga-Pentixafor. J. Nucl. Med. 2015, 56, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Rischpler, C.; Nekolla, S.G.; Kossmann, H.; Dirschinger, R.J.; Schottelius, M.; Hyafil, F.; Wester, H.J.; Laugwitz, K.; Schwaiger, M. Upregulated myocardial CXCR4-expression after myocardial infarction assessed by simultaneous GA-68 pentixafor PET/MRI. J. Nucl. Cardiol. 2016, 23, 131–133. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Derlin, T.; Haghikia, A.; Napp, L.C.; Wang, Y.; Ross, T.L.; Schäfer, A.; Tillmannsv, J.; Wester, H.J.; Wollert, K.C.; et al. Molecular Imaging of the Chemokine Receptor CXCR4 After Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Lapa, C.; Reiter, T.; Werner, R.A.; Ertl, G.; Wester, H.-J.; Buck, A.K.; Bauer, W.R.; Herrmann, K. [68Ga]Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression After Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1466–1468. [Google Scholar] [CrossRef] [Green Version]
- Reiter, T.; Kircher, M.; Schirbel, A.; Werner, R.A.; Kropf, S.; Ertl, G.; Buck, A.K.; Wester, H.-J.; Bauer, W.R.; Lapa, C. Imaging of C-X-C Motif Chemokine Receptor CXCR4 Expression After Myocardial Infarction With [68Ga]Pentixafor-PET/CT in Correlation With Cardiac MRI. JACC Cardiovasc. Imaging 2018, 11, 1541–1543. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Guo, Z.; Shi, C.; Zhuang, R.; Hong, X.; Wang, X.; Xu, D.; Zhang, P.; Zhang, D.; et al. Radioiodinated Pentixather for SPECT Imaging of Expression of the Chemokine Receptor CXCR4 in Rat Myocardial-Infarction–Reperfusion Models. Anal. Chem. 2018, 90, 9614–9620. [Google Scholar] [CrossRef]
- Heo, G.S.; Kopecky, B.; Sultan, D.; Ou, M.; Feng, G.; Bajpai, G.; Zhang, X.; Luehmann, H.; Detering, L.; Su, Y.; et al. Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart. Circ. Res. 2019, 124, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bankstahl, J.P.; Wang, Y.; Wollert, K.C.; Bengel, F.M. Targeting Amino Acid Metabolism for Molecular Imaging of Inflammation Early After Myocardial Infarction. Theranostics 2016, 6, 1768–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef]
- Klein, R.; Celiker-Guler, E.; Rotstein, B.H.; Dekemp, R.A. PET and SPECT Tracers for Myocardial Perfusion Imaging. Semin. Nucl. Med. 2020, 50, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. New Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Rodriguez-Porcel, M.; Cai, W.; Gheysens, O.; Willmann, J.K.; Chen, K.; Wang, H.; Chen, I.Y.; He, L.; Wu, J.C.; Li, Z.-B.; et al. Imaging of VEGF Receptor in a Rat Myocardial Infarction Model Using PET. J. Nucl. Med. 2008, 49, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Horton, M.A. The αvβ3 integrin “vitronectin receptor”. Int. J. Biochem. Cell Biol. 1997, 29, 721–725. [Google Scholar] [CrossRef]
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of v 3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Mandic, L.; Traxler, D.; Gugerell, A.; Zlabinger, K.; Lukovic, D.; Pavo, N.; Goliasch, G.; Spannbauer, A.; Winkler, J.; Gyöngyösi, M. Molecular Imaging of Angiogenesis in Cardiac Regeneration. Curr. Cardiovasc. Imaging Rep. 2016, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Makowski, M.R.; Rischpler, C.; Ebersberger, U.; Keithahn, A.; Kasel, M.; Hoffmann, E.; Rassaf, T.; Kessler, H.; Wester, H.-J.; Nekolla, S.G.; et al. Multiparametric PET and MRI of myocardial damage after myocardial infarction: Correlation of integrin αvβ3 expression and myocardial blood flow. Eur. J. Pediatr. 2021, 48, 1070–1080. [Google Scholar] [CrossRef]
- Pfau, D.; Thorn, S.L.; Zhang, J.; Mikush, N.; Renaud, J.M.; Klein, R.; Dekemp, R.A.; Wu, X.; Hu, X.; Sinusas, A.J.; et al. Angiotensin Receptor Neprilysin Inhibitor Attenuates Myocardial Remodeling and Improves Infarct Perfusion in Experimental Heart Failure. Sci. Rep. 2019, 9, 5791. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Jones, P.; Haramis, H.; Battle, M.; Lear, R.; Barnett, D.J.; Edwards, C.; Crawford, H.; Black, A.; Godden, V. 99mTc-NC100692—A tracer for imaging vitronectin receptors associated with angiogenesis: A preclinical investigation. Nucl. Med. Biol. 2008, 35, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Bader, B.L.; Rayburn, H.; Crowley, D.; Hynes, R.O. Extensive Vasculogenesis, Angiogenesis, and Organogenesis Precede Lethality in Mice Lacking All αv Integrins. Cell 1998, 95, 507–519. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, L.E.; Wyder, L.; Lively, J.C.; Taverna, D.; Robinson, S.D.; Huang, X.; Sheppard, D.; Hynes, R.O.; Hodivala-Dilke, K.M. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat. Med. 2002, 8, 27–34. [Google Scholar] [CrossRef]
- Reynolds, A.R.; E Reynolds, L.; E Nagel, T.; Lively, J.C.; Robinson, S.D.; Hicklin, D.J.; Bodary, S.C.; Hodivala-Dilke, K.M. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004, 64, 8643–8650. [Google Scholar] [CrossRef] [Green Version]
- Antonov, A.S.; Kolodgie, F.D.; Munn, D.; Gerrity, R.G. Regulation of Macrophage Foam Cell Formation by αVβ3 Integrin. Am. J. Pathol. 2004, 165, 247–258. [Google Scholar] [CrossRef]
- Atkinson, S.J.; Ellison, T.S.; Steri, V.; Gould, E.; Robinson, S.D. Redefining the role(s) of endothelial αvβ3-integrin in angiogenesis. Biochem. Soc. Trans. 2014, 42, 1590–1595. [Google Scholar] [CrossRef]
- Notni, J.; Steiger, K.; Hoffmann, F.; Reich, D.; Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; Wester, H.-J. Complementary, Selective PET Imaging of Integrin Subtypes α5β1 and αvβ3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J. Nucl. Med. 2016, 57, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Fonsatti, E.; Sigalotti, L.; Arslan, P.; Altomonte, M.; Maio, M. Emerging Role of Endoglin (CD105) as a Marker of Angiogenesis with Clinical Potential in Human Malignancies. Curr. Cancer Drug Targets 2003, 3, 427–432. [Google Scholar] [CrossRef]
- Orbay, H.; Zhang, Y.; Valdovinos, H.F.; Song, G.; Hernandez, R.; Theuer, C.P.; A Hacker, T.; Nickles, R.J.; Cai, W. Positron emission tomography imaging of CD105 expression in a rat myocardial infarction model with (64)Cu-NOTA-TRC105. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 1–9. [Google Scholar]
- Corti, A.; Curnis, F.; Arap, W.; Pasqualini, R. The neovasculature homing motif NGR: More than meets the eye. Blood 2008, 112, 2628–2635. [Google Scholar] [CrossRef] [Green Version]
- Dijkgraaf, I.; Van de Vijver, P.; Dirksen, A.; Hackeng, T.M. Synthesis and application of cNGR-containing imaging agents for detection of angiogenesis. Bioorganic Med. Chem. 2013, 21, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, G.; De Saint-Hubert, M.; Dijkgraaf, I.; Bauwens, M.; Douma, K.; Wierts, R.; Pooters, I.; Akker, N.M.V.D.; Hackeng, T.M.; Post, M.J.; et al. Molecular imaging of angiogenesis after myocardial infarction by 111In-DTPA-cNGR and 99mTc-sestamibi dual-isotope myocardial SPECT. EJNMMI Res. 2015, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic Significance of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.H.; Helm, P.A.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.-C.; et al. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angew. Chem. Int. Ed. 2007, 46, 8171–8173. [Google Scholar] [CrossRef]
- Helm, P.A.; Caravan, P.; French, B.A.; Jacques, V.; Shen, L.; Xu, Y.; Beyers, R.J.; Roy, R.J.; Kramer, C.M.; Epstein, F.H. Postinfarction Myocardial Scarring in Mice: Molecular MR Imaging with Use of a Collagen-targeting Contrast Agent. Radiology 2008, 247, 788–796. [Google Scholar] [CrossRef]
- Muzard, J.; Sarda-Mantel, L.; Loyau, S.; Meulemans, A.; Louedec, L.; Bantsimba-Malanda, C.; Hervatin, F.; Marchal-Somme, J.; Michel, J.B.; Le Guludec, D.; et al. Non-Invasive Molecular Imaging of Fibrosis Using a Collagen-Targeted Peptidomimetic of the Platelet Collagen Receptor Glycoprotein VI. PLoS ONE 2009, 4, e5585. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, X.; Liu, K.; Feng, S.; Tang, B.; Li, Q.; Huang, D.; Yang, S. Molecular imaging of fibrosis using a novel collagen-binding peptide labelled with 99mTc on SPECT/CT. Amino Acids 2016, 49, 89–101. [Google Scholar] [CrossRef]
- De Haas, H.J.; Arbustini, E.; Fuster, V.; Kramer, C.M.; Narula, J. Molecular Imaging of the Cardiac Extracellular Matrix. Circ. Res. 2014, 114, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Verjans, J.W.; Lovhaug, D.; Narula, N.; Petrov, A.D.; Indrevoll, B.; Bjurgert, E.; Krasieva, T.B.; Petersen, L.B.; Kindberg, G.M.; Solbakken, M.; et al. Noninvasive Imaging of Angiotensin Receptors After Myocardial Infarction. JACC Cardiovasc. Imaging 2008, 1, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Bravo, P.E.; Higuchi, T.; Schuleri, K.H.; Lin, X.; Abraham, M.R.; Xia, J.; Mathews, W.B.; Dannals, R.F.; Lardo, A.C.; et al. Molecular Hybrid Positron Emission Tomography/Computed Tomography Imaging of Cardiac Angiotensin II Type 1 Receptors. J. Am. Coll. Cardiol. 2012, 60, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Borne, S.W.V.D.; Isobe, S.; Verjans, J.; Petrov, A.; Lovhaug, D.; Li, P.; Zandbergen, H.R.; Ni, Y.; Frederik, P.; Zhou, J.; et al. Molecular Imaging of Interstitial Alterations in Remodeling Myocardium After Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 2017–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verjans, J.; Wolters, S.; Laufer, W.; Schellings, M.; Lax, M.; Lovhaug, D.; Boersma, H.; Kemerink, G.; Schalla, S.; Gordon, P.; et al. Early molecular imaging of interstitial changes in patients after myocardial infarction: Comparison with delayed contrast-enhanced magnetic resonance imaging. J. Nucl. Cardiol. 2010, 17, 1065–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borne, S.W.V.D.; Isobe, S.; Zandbergen, H.R.; Li, P.; Petrov, A.; Wong, N.D.; Fujimoto, S.; Fujimoto, A.; Lovhaug, D.; Smits, J.F.; et al. Molecular Imaging for Efficacy of Pharmacologic Intervention in Myocardial Remodeling. JACC Cardiovasc. Imaging 2009, 2, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Meoli, D.F.; Sadeghi, M.M.; Krassilnikova, S.; Bourke, B.N.; Giordano, F.J.; Dione, D.P.; Su, H.; Edwards, D.S.; Liu, S.; Harris, T.D.; et al. Noninvasive imaging of myocardial angiogenesis following experimental myo-cardial infarction. J. Clin. Invest. 2004, 113, 1684–1691. [Google Scholar] [CrossRef]
- A Jenkins, W.S.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T.; et al. Cardiac αVβ3integrin expression following acute myocardial infarction in humans. Heart 2016, 103, 607–615. [Google Scholar] [CrossRef] [Green Version]
- De Haas, H.J.; Borne, S.W.V.D.; Boersma, H.H.; Slart, R.H.; Fuster, V.; Narula, J. Evolving role of molecular imaging for new understanding: Targeting myofibroblasts to predict remodeling. Ann. N. Y. Acad. Sci. 2012, 1254, 33–41. [Google Scholar] [CrossRef]
- Tillmanns, J.; Hoffmann, D.; Habbaba, Y.; Schmitto, J.; Sedding, D.; Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell. Cardiol. 2015, 87, 194–203. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity After Myocardial Infarction Using a 68Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Notohamiprodjo, S.; Nekolla, S.G.; Robu, S.; Asiares, A.V.; Kupatt, C.; Ibrahim, T.; Laugwitz, K.-L.; Makowski, M.R.; Schwaiger, M.; Weber, W.A.; et al. Imaging of cardiac fibroblast activation in a patient after acute myocardial infarction using 68Ga-FAPI-04. J. Nucl. Cardiol. 2021. [Google Scholar] [CrossRef]
- Kessler, L.; Kupusovic, J.; Ferdinandus, J.; Hirmas, N.; Umutlu, L.; Zarrad, F.; Nader, M.; Fendler, W.P.; Totzeck, M.; Wakili, R.; et al. Visualization of Fibroblast Activation After Myocardial Infarction Using 68Ga-FAPI PET. Clin. Nucl. Med. 2021, 46, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.B.; Reinhardt, F.; Finke, D.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010628. [Google Scholar] [CrossRef] [PubMed]
- Siebermair, J.; Köhler, M.I.; Kupusovic, J.; Nekolla, S.G.; Kessler, L.; Ferdinandus, J.; Guberina, N.; Stuschke, M.; Grafe, H.; Siveke, J.T.; et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J. Nucl. Cardiol. 2021, 28, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Guo, F.; Wang, Y.; Ding, H.; Huo, L. 68Ga-FAPI-04 Accumulation in Myocardial Infarction in a Patient with Neuroendocrine Carcinoma. Clin. Nucl. Med. 2020, 45, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Finke, D.; Heckmann, M.B.; Herpel, E.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Early Detection of Checkpoint Inhibitor-Associated Myocarditis Using 68Ga-FAPI PET/CT. Front. Cardiovasc. Med. 2021, 8, 614997. [Google Scholar] [CrossRef]
- Shi, X.; Lin, X.; Huo, L.; Li, X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J. Nucl. Cardiol. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Totzeck, M.; Siebermair, J.; Rassaf, T.; Rischpler, C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur. Heart J. 2019, 41. [Google Scholar] [CrossRef]
- Langer, L.B.; Hess, A.; Korkmaz, Z.; Tillmanns, J.; Reffert, L.M.; Bankstahl, J.P.; Bengel, F.M.; Thackeray, J.T.; Ross, T.L. Molecular imaging of fibroblast activation protein after myocardial infarction using the novel radiotracer [68Ga]MHLL. Theranostics 2021, 11, 7755–7766. [Google Scholar] [CrossRef]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zhu, W. Targeting angiogenesis in myocardial infarction: Novel therapeutics (Review). Exp. Ther. Med. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Scalise, R.; De Sarro, R.; Caracciolo, A.; Lauro, R.; Squadrito, F.; Carerj, S.; Bitto, A.; Micari, A.; Bella, G.; Costa, F.; et al. Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value. Med. Sci. 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Guan, J. Antifibrotic therapies to control cardiac fibrosis. Biomater. Res. 2016, 20, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, A.; Thackeray, J.T.; Wollert, K.C.; Bengel, F.M. Radionuclide Image-Guided Repair of the Heart. JACC Cardiovasc. Imaging 2020, 13, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.; Derlin, T.; Koenig, T.; Diekmann, J.; Wittneben, A.; Wang, Y.; Wester, H.-J.; Ross, T.L.; Wollert, K.C.; Bauersachs, J.; et al. Molecular imaging-guided repair after acute myocardial infarction by targeting the chemokine receptor CXCR. Eur. Heart J. 2020, 41, 3564–3575. [Google Scholar] [CrossRef]
- Sreenivasan, J.; Hooda, U.; Ranjan, P.; Jain, D. Nuclear Imaging for the Assessment of Cardiotoxicity from Chemotherapeutic Agents in Oncologic Disease. Curr. Cardiol. Rep. 2021, 23, 65. [Google Scholar] [CrossRef]
- Niu, N.; Huo, L.; Zhang, S.; Liu, Y.; Li, X. Immune checkpoint inhibitor-associated cardiotoxicity detected by 68Ga-DOTATATE PET/CT and 68Ga-FAPI PET/CT. Eur. Heart J. Cardiovasc. Imaging 2021. Epub ahead of print. [Google Scholar] [CrossRef]


| Marker | Probe | Imaging Modality | Application |
|---|---|---|---|
| Glucose transporters (GLUTs) | 18F-FDG | PET | Preclinical/clinical |
| SSRT-2 | 68Ga-DOTA-TOC | PET | Preclinical/clinical |
| 68Ga-DOTA-TATE | PET | Preclinical/clinical | |
| Chemokine receptors (CXCR4, CCR2) | 68Ga-pentixafor | PET | Preclinical/clinical |
| 131I-pentixather | SPECT | Preclinical | |
| 68Ga-DOTA-ECL1i | PET | Preclinical | |
| Mitochondrial membrane translocator protein (TSPO) | 18F-GE180 | PET | Preclinical |
| Amino acid metabolism and protein turnover | 11C-methionine | PET | Preclinical/clinical |
| Marker | Probe | Imaging Modality | Application |
|---|---|---|---|
| VEGF | 64Cu-DOTA-VEGF121 | PET | Preclinical (rat MI model) |
| αvβ3/αvβ5 integrins | 18F-galacto-RGD 68Ga-NODAGA-RGD 68Ga-TRAP-(RGD)3 68Ga-NOTA-RGD 18F-Alfatide II 68Ga-RGD 99mTc-RAFT-RGD 68Ga-PRGD2 18F-Fluciclatide 99mTc-NC100692 | PET PET PET PET PET PET SPECT PET PET SPECT | Preclinical (rat MI model) Preclinical (rat MI model) Preclinical (rat MI model) Preclinical (rat MI model) Preclinical (rat MI model) Preclinical (rat MI model) Preclinical (rat MI model) Clinical Clinical Preclinical (rat model of MI) |
| CD105 | 64Cu-NOTA-TRC105 | PET | Preclinical (rat model of MI) |
| CD13 | 111In-DTPA-cNGR | SPECT | Preclinical (Mouse MI model) |
| Marker | Probe | Imaging Modality | Application |
|---|---|---|---|
| Fibrosis collagen | 99mTc-streptavidin-B-collagelin | SPECT | Preclinical (rat MI model) |
| Fibroblast activity Angiotensin receptor | 99mTc-Losartan | SPECT | Preclinical (mouse MI model) |
| 11C-KR31173 | PET | Preclinical (pig MI model)/clinical | |
| Fibroblast activity αvβ3 integrin | 18F-galacto-RGD 99mTc-Cy5.5-RGD (CRIP) 99mTc-RGD (RIP) | PET SPECT SPECT | Preclinical (mouse and rat MI model)/clinical Preclinical (mouse MI model) Clinical |
| Fibroblast activity FAP | 68Ga-FAPI-04 | PET | Preclinical (rat MI model)/clinical |
| 68Ga-FAPI-46 | PET | Clinical | |
| 68Ga-MHLL1 | PET | Preclinical (Mouse MI model) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varasteh, Z.; Weber, W.A.; Rischpler, C. Nuclear Molecular Imaging of Cardiac Remodeling after Myocardial Infarction. Pharmaceuticals 2022, 15, 183. https://doi.org/10.3390/ph15020183
Varasteh Z, Weber WA, Rischpler C. Nuclear Molecular Imaging of Cardiac Remodeling after Myocardial Infarction. Pharmaceuticals. 2022; 15(2):183. https://doi.org/10.3390/ph15020183
Chicago/Turabian StyleVarasteh, Zohreh, Wolfgang A. Weber, and Christoph Rischpler. 2022. "Nuclear Molecular Imaging of Cardiac Remodeling after Myocardial Infarction" Pharmaceuticals 15, no. 2: 183. https://doi.org/10.3390/ph15020183






