2021 FDA TIDES (Peptides and Oligonucleotides) Harvest
Abstract
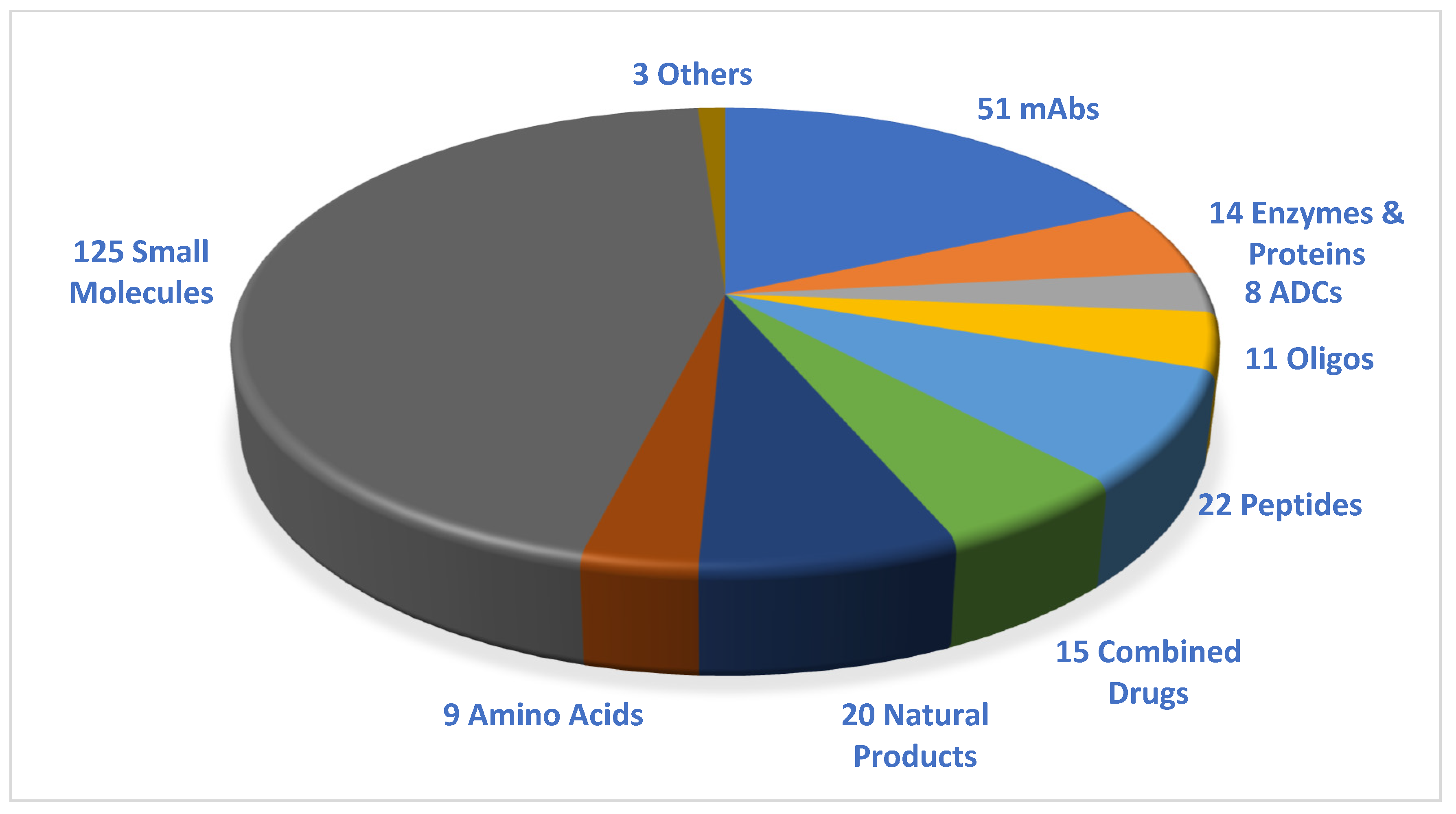
:1. Introduction
2. Oligonucleotides
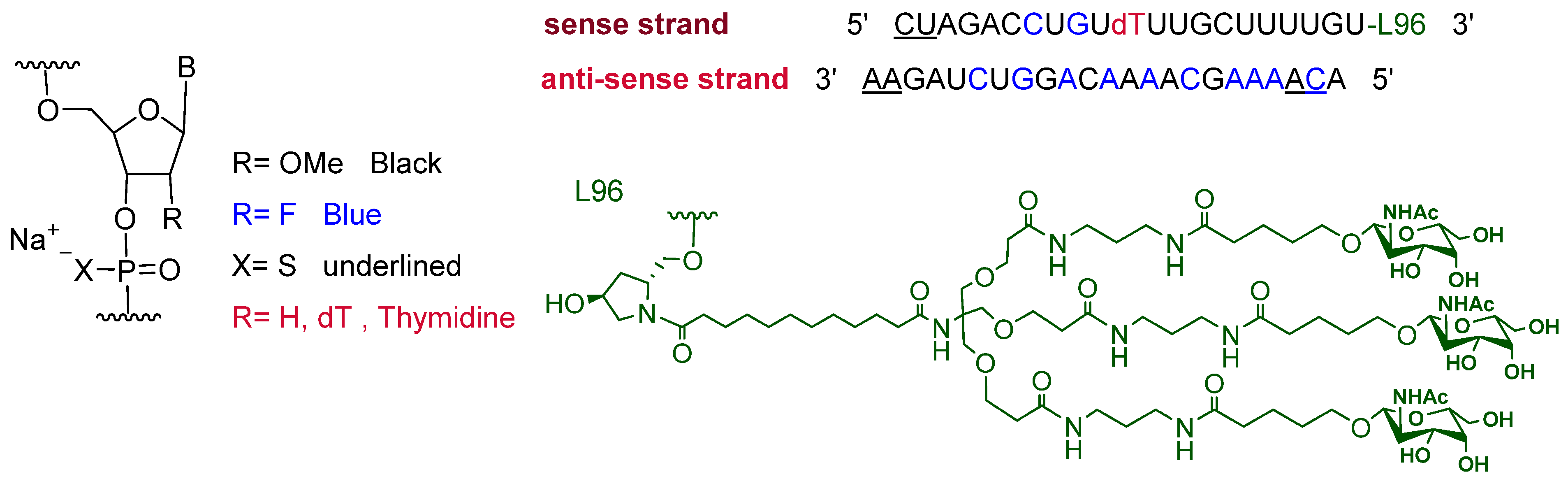
2.1. Inclisiran (LeqvioTM)
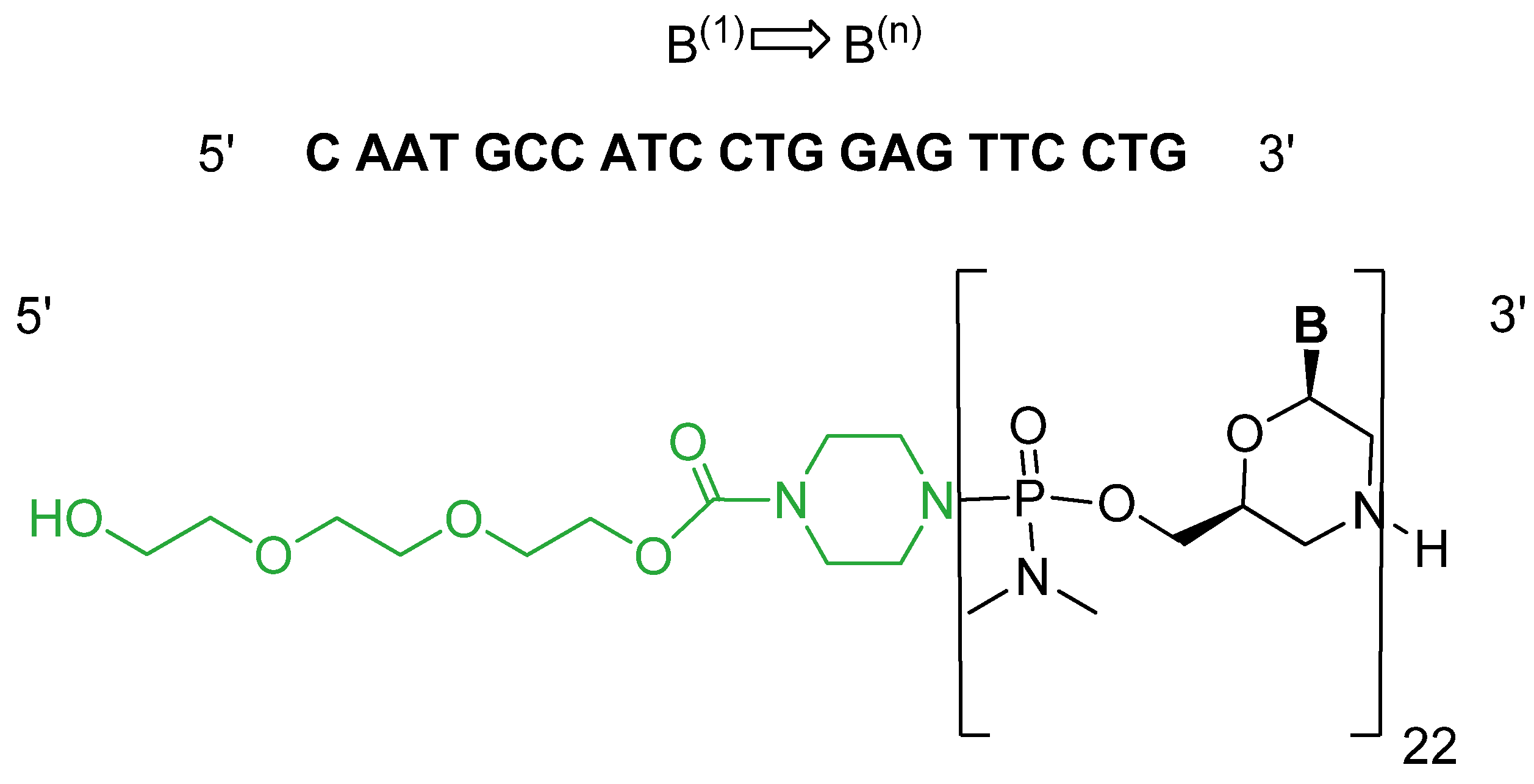
2.2. Casimersen (Amondys 45)
3. Peptides
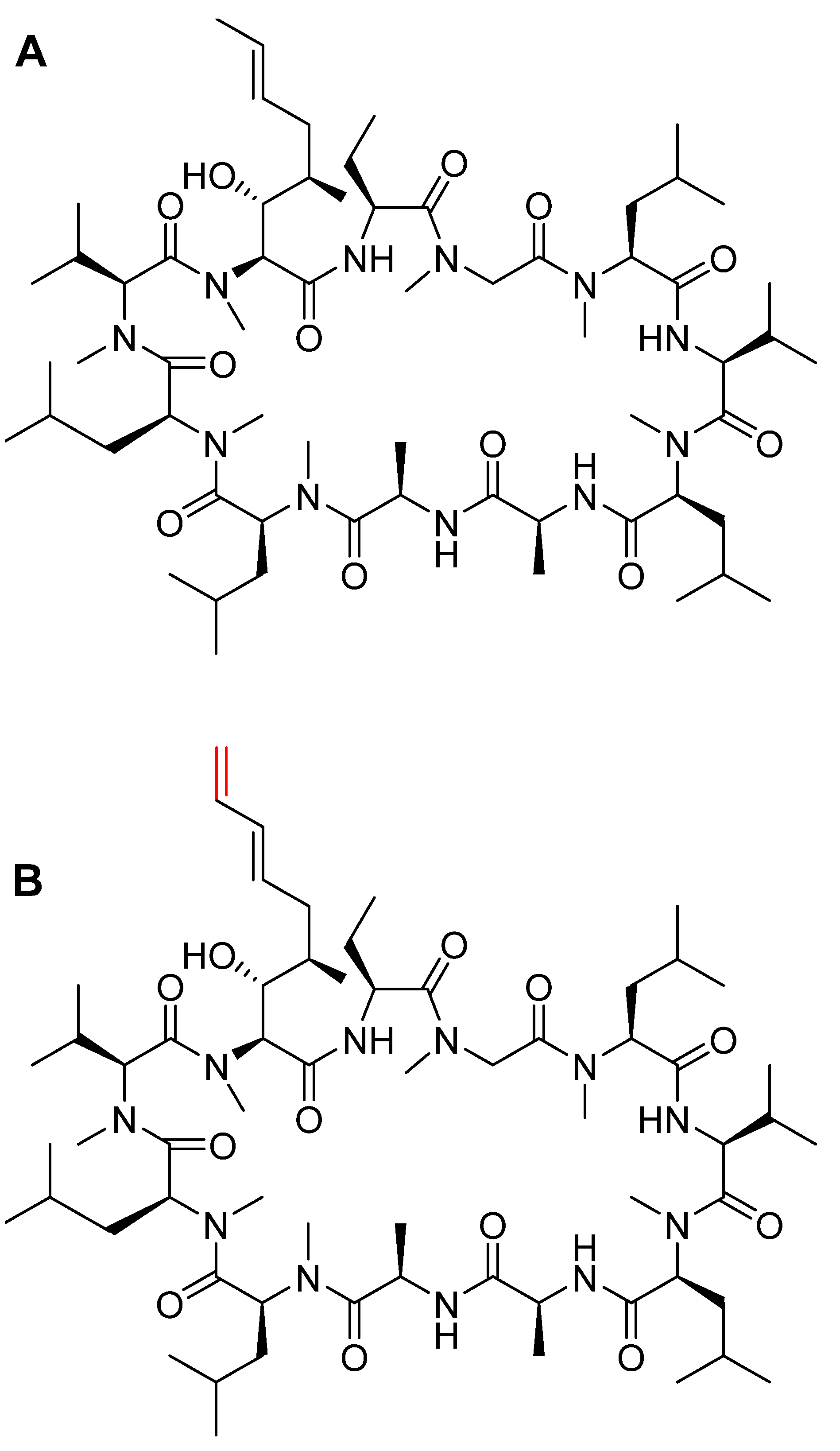
3.1. Vosoritide (VoxzogoTM)
3.2. Melphalan Flufenamide (Pepaxto®)
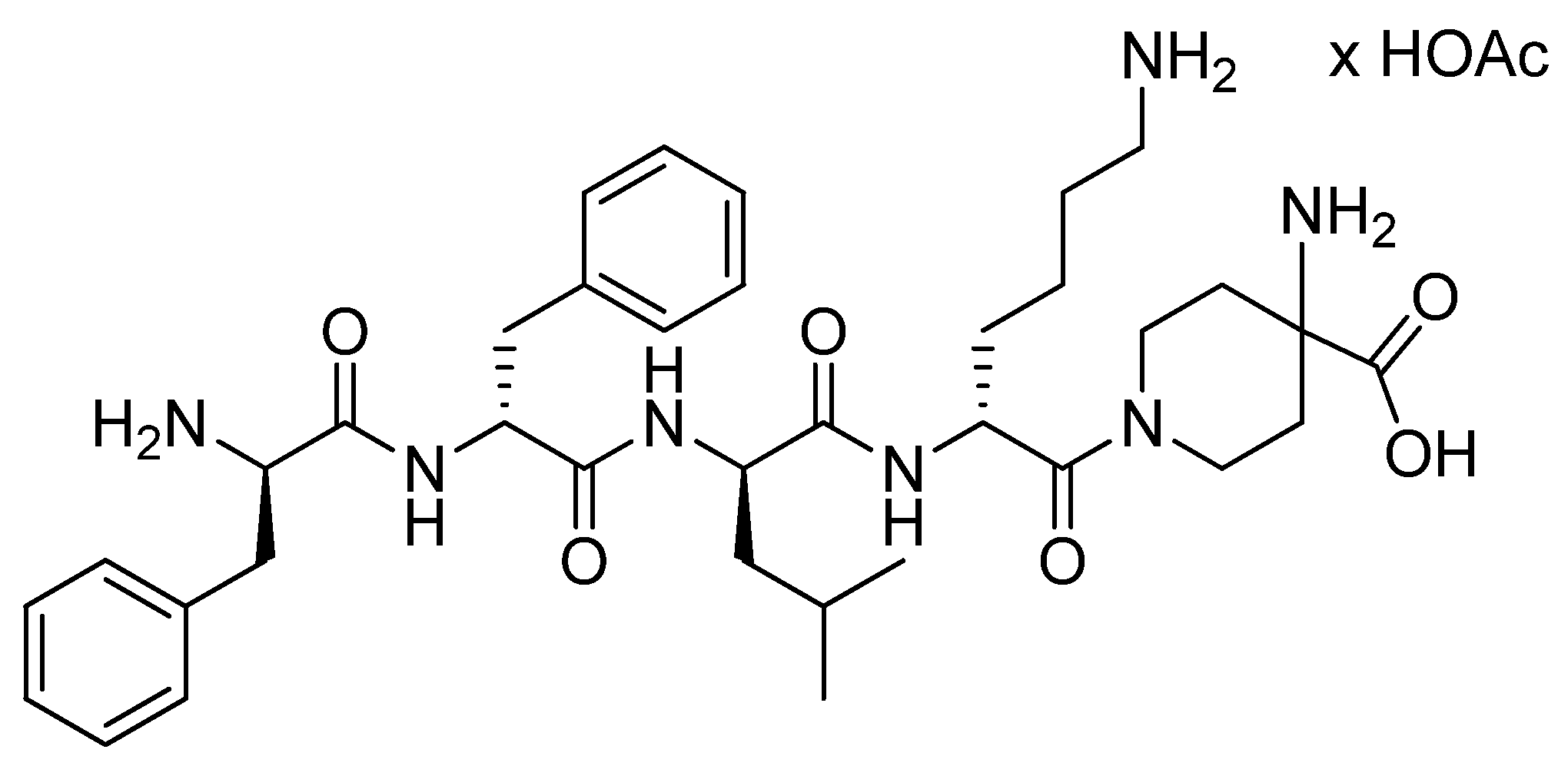
3.3. Voclosporin (LupkynisTM)
3.4. Pegcetacoplan (EmpaveliTM)
3.5. Dasiglucagon (ZegalogueTM)
3.6. Piflufolastat F 18 (PylarifyTM)
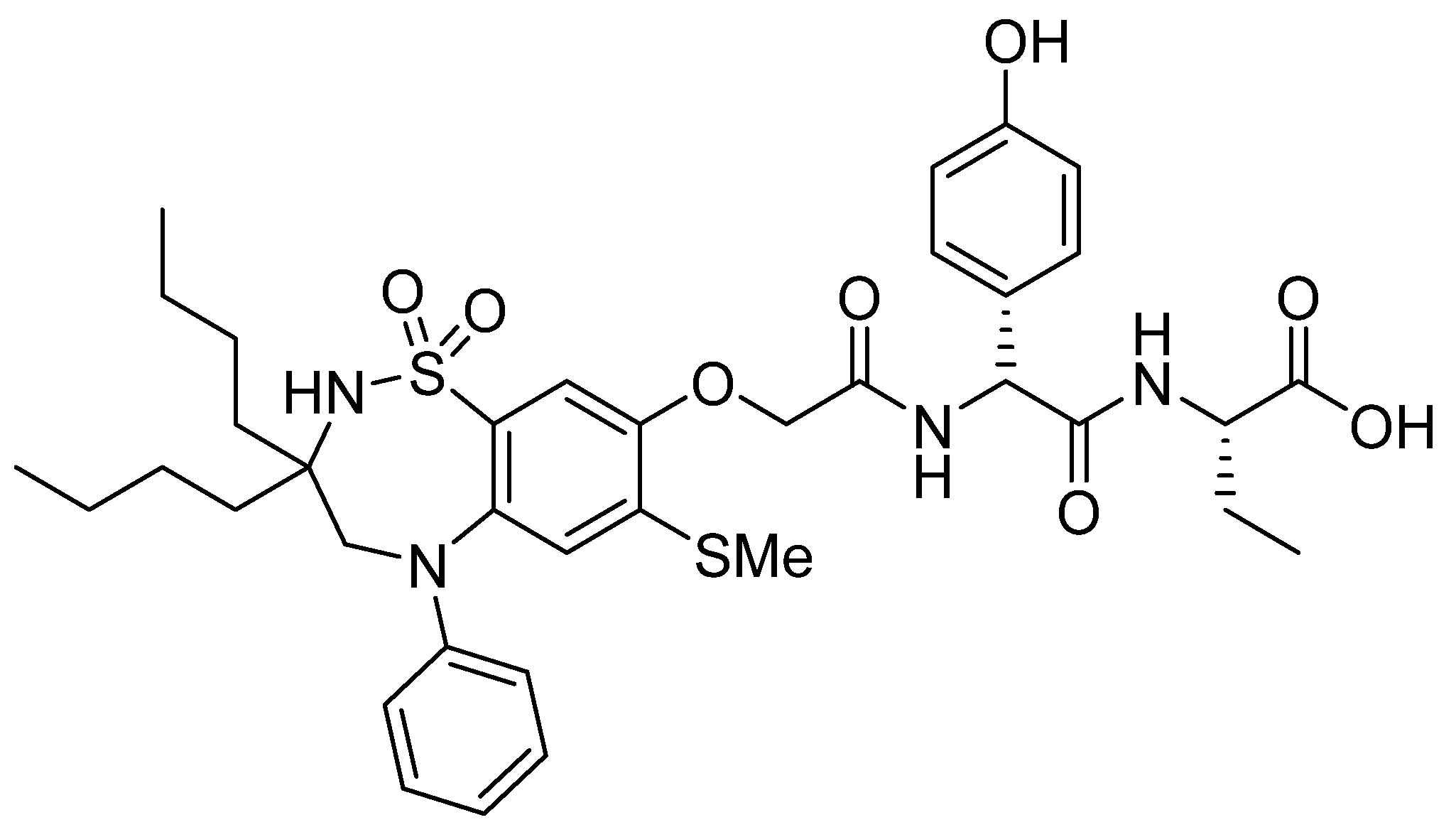
3.7. Difelikefalin (KorsuvaTM)
3.8. Odevixibat (BylvayTM)
4. Peptides in Antibody-Drug Conjugates (ADCs)
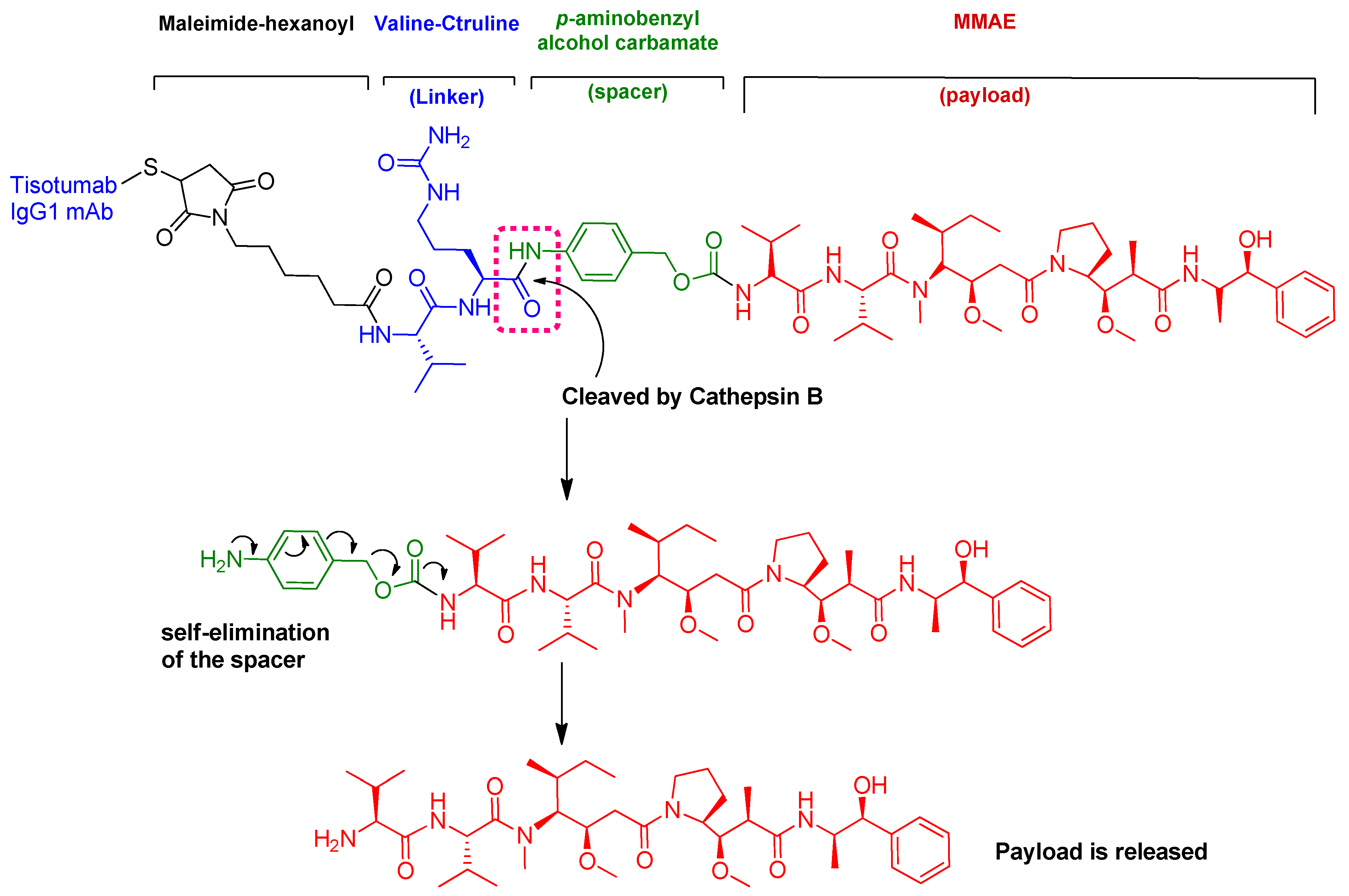
4.1. Tisotumab Vedotin-Tftv (TIVDAKTM)
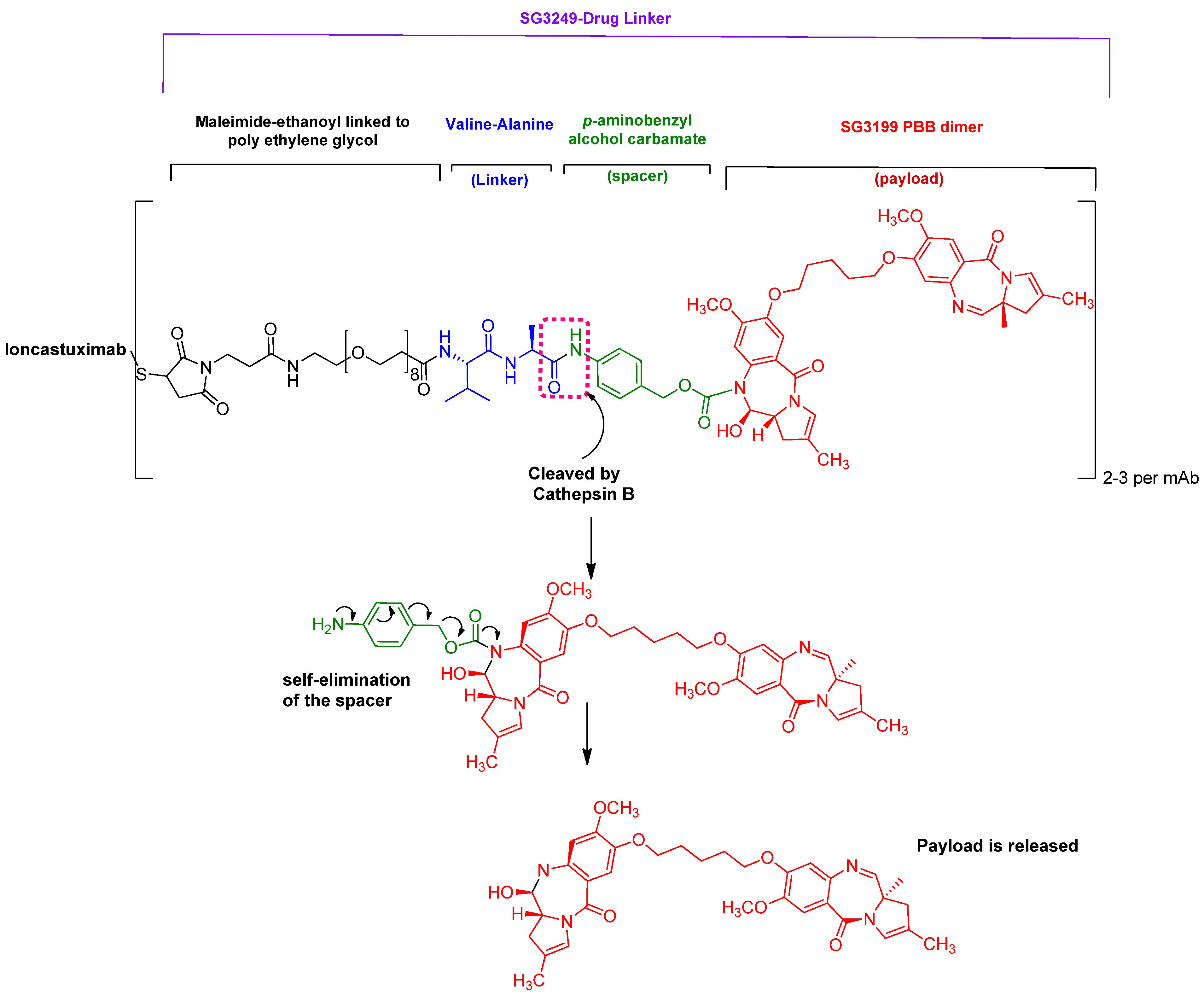
4.2. Loncastuximab Tesirine-Lpyl (ZynlontaTM)
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; Albericio, F.; de la Torre, B.G. 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2021, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; Al Shaer, D.; de la Torre, B.G.; Albericio, F. 2017 FDA Peptide Harvest. Pharmaceuticals 2018, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leqvio Assessment Report. 2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/leqvio-epar-public-assessment-report_en.pdf (accessed on 19 January 2022).
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021, 81, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.F.G.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Expert Opin. Drug Saf. 2021, 21, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Sirtori, C.R.; Ferri, N.; Corsini, A. New players in the treatment of hypercholesterolaemia: Focus on bempedoic acid and inclisiran. Eur. Heart J. Suppl. 2021, 23, E59–E62. [Google Scholar] [CrossRef]
- Inclisiran Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214012Orig1s000ltr.pdf (accessed on 19 January 2022).
- Amondys 45 Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf (accessed on 19 January 2022).
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2019 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2020, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Amondys 45 Approcal Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/213026Orig1s000ltr.pdf (accessed on 19 January 2022).
- Duggan, S. Vosoritide: First Approval. Drugs 2021, 81, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Voxzogo Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214938s000lbl.pdf (accessed on 19 January 2022).
- Savarirayan, R.; Tofts, L.; Irving, M.; Wilcox, W.; Bacino, C.A.; Hoover-Fong, J.; Font, R.U.; Harmatz, P.; Rutsch, F.; Bober, M.B.; et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: A randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 2020, 396, 684–692. [Google Scholar] [CrossRef]
- Chan, M.L.; Qi, Y.; Larimore, K.; Cherukuri, A.; Seid, L.; Jayaram, K.; Jeha, G.; Fisheleva, E.; Day, J.; Huntsman-Labed, A.; et al. Pharmacokinetics and Exposure-Response of Vosoritide in Children with Achondroplasia. Clin. Pharmacokinet. 2021, 61, 263–280. [Google Scholar] [CrossRef]
- Breinholt, V.M.; Rasmussen, C.E.; Mygind, P.H.; Kjelgaard-Hansen, M.; Faltinger, F.; Bernhard, A.; Zettler, J.; Hersel, U. TransCon CNP, a Sustained-Release C-Type Natriuretic Peptide Prodrug, a Potentially Safe and Efficacious New Therapeutic Modality for the Treatment of Comorbidities Associated with Fibroblast Growth Factor Receptor 3-Related Skeletal Dysplasias. J. Pharmacol. Exp. Ther. 2019, 370, 459–471. [Google Scholar] [CrossRef]
- Voxzogo Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214938Orig1s000ltr.pdf (accessed on 19 January 2022).
- Dhillon, S. Melphalan Flufenamide (Melflufen): First Approval. Drugs 2021, 81, 963–969. [Google Scholar] [CrossRef]
- Pepaxto Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214383s000lbl.pdf (accessed on 19 January 2022).
- Chauhan, D.; Ray, A.; Viktorsson, K.; Spira, J.; Paba-Prada, C.; Munshi, N.; Richardson, P.; Lewensohn, R.; Anderson, K.C. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin. Cancer Res. 2013, 19, 3019–3031. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Ravillah, D.; Das, D.S.; Song, Y.; Nordström, E.; Gullbo, J.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. A novel alkylating agent Melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br. J. Haematol. 2016, 174, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Byrgazov, K.; Anderson, C.; Salzer, B.; Bozsaky, E.; Larsson, R.; Gullbo, J.; Lehner, M.; Lehmann, F.; Slipicevic, A.; Kager, L.; et al. Targeting aggressive osteosarcoma with a peptidase-enhanced cytotoxic melphalan flufenamide. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937891. [Google Scholar] [CrossRef]
- Pepaxto Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214383Orig1s000ltr.pdf (accessed on 19 January 2022).
- Kuglstatter, A.; Mueller, F.; Kusznir, E.; Gsell, B.; Stihle, M.; Thoma, R.; Benz, J.; Aspeslet, L.; Freitag, D.; Hennig, M. Structural basis for the cyclophilin A binding affinity and immunosuppressive potency of E-ISA247 (voclosporin). Acta Crystallogr. Sect. D: Biol. Crystallogr. 2011, 67, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, F.E.; Isenberg, D. An evaluation of voclosporin for the treatment of lupus nephritis. Expert Opin. Pharmacother. 2018, 19, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Ling, S.Y.; Huizinga, R.B.; Mayo, P.R.; Larouche, R.; Freitag, D.G.; Aspeslet, L.J.; Foster, R.T. Cytochrome P450 3A and P-glycoprotein drug-drug interactions with voclosporin. Br. J. Clin. Pharmacol. 2014, 77, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupkynis Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213716s000lbl.pdf (accessed on 19 January 2022).
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Lupkynis Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/213716Orig1s000ltr.pdf (accessed on 19 January 2022).
- De Castro, C.; Grossi, F.; Weitz, I.C.; Maciejewski, J.; Sharma, V.; Roman, E.; Brodsky, R.A.; Tan, L.; Di Casoli, C.; El Mehdi, D.; et al. C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab. Am. J. Hematol. 2020, 95, 1334–1343. [Google Scholar] [CrossRef]
- Empaveli Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215014s000lbl.pdf (accessed on 19 January 2022).
- Bhak, R.H.; Mody-Patel, N.; Baver, S.B.; Kunzweiler, C.; Yee, C.W.; Sundaresan, S.; Swartz, N.; Duh, M.S.; Krishnan, S.; Sarda, S.P. Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: A matching-adjusted indirect comparison. Curr. Med. Res. Opin. 2021, 37, 1913–1923. [Google Scholar] [CrossRef]
- Empaveli Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/215014Orig1s000ltr.pdf (accessed on 19 January 2022).
- Baker, D.E. Dasiglucagon. Hosp. Pharm. 2021. Available online: https://journals.sagepub.com/doi/10.1177/00185787211046857 (accessed on 19 January 2022). [CrossRef]
- Zegalogue Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214231s000lbl.pdf (accessed on 19 January 2022).
- Blair, H.A. Dasiglucagon: First Approval. Drugs 2021, 81, 1115–1120. [Google Scholar] [CrossRef]
- Xu, B.; Tang, G.; Chen, Z. Dasiglucagon: An effective medicine for severe hypoglycemia. Eur. J. Clin. Pharmacol. 2021, 77, 1783–1790. [Google Scholar] [CrossRef]
- Zegalogue Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214231Orig1s000ltr.pdf (accessed on 19 January 2022).
- Pylarify Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf (accessed on 19 January 2022).
- Pylarify Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214793Orig1s000ltr.pdf (accessed on 19 January 2022).
- Gallium 68 PSMA-11 Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/212642Orig1s000ltr.pdf (accessed on 16 January 2021).
- Keam, S.J. Piflufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2021, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Korsuva Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214916s000lbl.pdf (accessed on 19 January 2022).
- Deeks, E.D. Difelikefalin: First Approval. Drugs 2021, 81, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Albert-Vartanian, A.; Boyd, M.R.; Hall, A.L.; Morgado, S.J.; Nguyen, E.; Nguyen, V.P.; Patel, S.P.; Russo, L.J.; Shao, A.J.; Raffa, R.B. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J. Clin. Pharm. Ther. 2016, 41, 371–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korsuva Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214916Orig1s000ltr.pdf (accessed on 19 January 2022).
- Bylvay Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215498s000lbl.pdf (accessed on 19 January 2022).
- Bylvay Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/215498Orig1s000ltr.pdf (accessed on 19 January 2022).
- Deeks, E.D. Odevixibat: First Approval. Drugs 2021, 81, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Davit-Spraul, A.; Gonzales, E.; Baussan, C.; Jacquemin, E. Progressive familial intrahepatic cholestasis. Orphanet J. Rare Dis. 2009, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bylvay Summary of Product Characteristics. 2021. Available online: https://www.albireopharma.com/wp-content/uploads/2021/10/ema-combined-h-4691_en.pdf (accessed on 19 January 2022).
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer. 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Markham, A. Tisotumab Vedotin: First Approval. Drugs 2021, 81, 2141–2147. [Google Scholar] [CrossRef]
- TIVDAK Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/761208Orig1s000_Corrected_ltr.pdf (accessed on 19 January 2022).
- TIVDAK Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761208Orig1s000lbledt.pdf (accessed on 19 January 2022).
- Zynlonta Drug Label. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761196s000lbl.pdf (accessed on 19 January 2022).
- Zynlonta Approval Letter. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/761196Orig1s000ltr.pdf (accessed on 19 January 2022).
- Wang, Y.; Fan, S.; Zhong, W.; Zhou, X.; Li, S. Development and Properties of Valine-Alanine based Antibody-Drug Conjugates with Monomethyl Auristatin E as the Potent Payload. Int J. Mol. Sci. 2017, 18, 1860. [Google Scholar] [CrossRef]



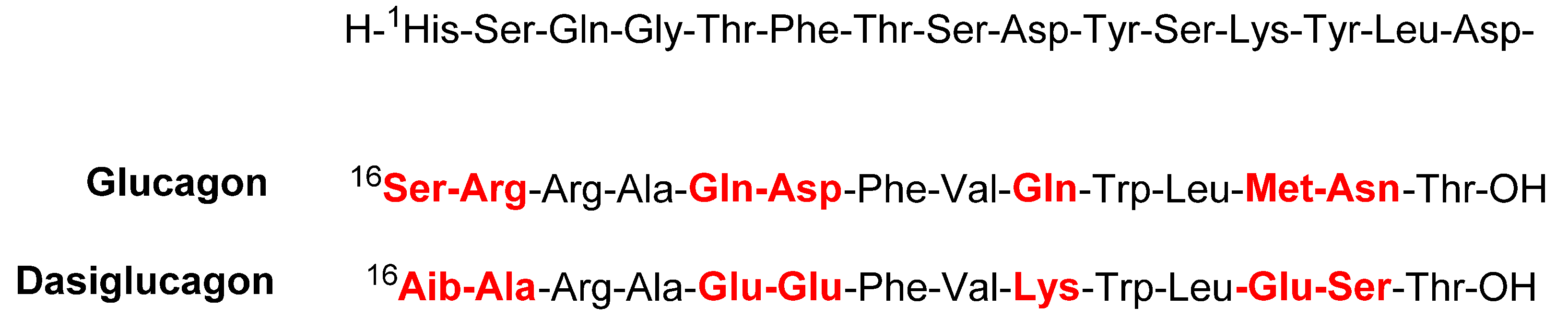

| # | Active Ingredient (Trade Name) | Indication | Therapeutic Target | Administration Route |
|---|---|---|---|---|
| Oligonucleotides | ||||
| 1 | Inclisiran (LeqvioTM) | Treatment of hypercholesterolemia | PCSK mRNA | Subcutaneously |
| 2 | Casimersen (Amondys 45) | Duchenne muscular dystrophy (DMD) amenable for exon 45 skipping | Exon 45 | Intravenously |
| Peptides | ||||
| 3 | Vosoritide (Voxzogo™) | Achondroplasia | Natriuretic peptide receptor B (NPR-B) | Subcutaneously |
| 4 | Melphalan flufenamide (PepaxtoTM) | Treatment of multiple myeloma (MM) and amyloid light-chain amyloidosis | Exerts anti-tumor activity through crosslinking of DNA | Intravenously |
| 5 | Voclosporin (Lupkynis™) | Treatment of lupus nephritis | T-cells | Orally |
| 6 | Pegcetacoplan (Empaveli™) | Treatment of paroxysmal nocturnal hemoglobinuria (PNH) in adult patients | Complement protein C3 and its activation C3b | Subcutaneously |
| 7 | Dasiglucagon (ZegalogueTM) | Hypoglycemia in diabetes patients aged over 6 years | Glucagon-receptor | Subcutaneously |
| 8 | Piflufolastat-F18 (PylarifyTM) | Positron emission tomography (PET) of prostate-specific membrane antigen (PSMA)-positive lesions in men with prostate cancer | PSMA | Intravenously |
| 9 | Difelikefalin (KorsuvaTM) | Pruritus associated with chronic kidney disease (CKD-aP) in adults undergoing hemodialysis (HD) | Kappa opioid receptor | Intravenously |
| 10 | Odevixibat (BylvayTM) | Pruritus in patients aged over 3 months with progressive familial intrahepatic cholestasis (PFIC) | Ileal bile acid transporter (IBAT) | Orally |
| Peptides in ADCs | ||||
| 11 | Tisotumab vedotin-tftv (TIVDAK™) (MMAE as cytotoxic and Valine-Citrulline as linker) | Treatment of recurrent or metastatic cervical cancer with disease progression during or after chemotherapy | Tissue factor (TF-011), | Intravenously |
| 12 | Loncastuximab tesirine-lpyl (ZynlontaTM) (Valine-Alanine as linker) | Treatment of adults with relapsed or refractory large B-cell lymphoma | CD19 | Intravenously |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2022, 15, 222. https://doi.org/10.3390/ph15020222
Al Shaer D, Al Musaimi O, Albericio F, de la Torre BG. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals. 2022; 15(2):222. https://doi.org/10.3390/ph15020222
Chicago/Turabian StyleAl Shaer, Danah, Othman Al Musaimi, Fernando Albericio, and Beatriz G. de la Torre. 2022. "2021 FDA TIDES (Peptides and Oligonucleotides) Harvest" Pharmaceuticals 15, no. 2: 222. https://doi.org/10.3390/ph15020222
APA StyleAl Shaer, D., Al Musaimi, O., Albericio, F., & de la Torre, B. G. (2022). 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals, 15(2), 222. https://doi.org/10.3390/ph15020222










