Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-Time Imaging in Photoimmunotherapy
Abstract
:1. Introduction
2. Results
2.1. Quality Control of Cetuximab-IR700
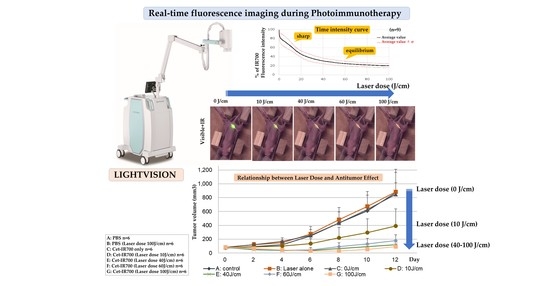
2.2. In Vivo Real-Time Fluorescence Imaging during PIT
2.3. Real-Time Fluorescence Imaging Analysis of IR700
2.4. Relationship between Laser Dose and Antitumor Effect
2.5. Histological Analysis of PIT
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. Synthesis of IR700-Conjugated Antibodies
4.3. Flow Cytometry
4.4. In Vitro Fluorescence Microscopic Images and PIT
4.5. Animal Model
4.6. In Vivo Real-Time Fluorescence Imaging
4.7. In Vivo PIT with Cet-IR700
4.8. Histological Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, K.; Takakura, H.; Shimizu, Y.; Ogawa, M. Changes in plasma membrane damage inducing cell death after treatment with near-infrared photoimmunotherapy. Cancer Sci. 2018, 109, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, K.; Kagawa, S.; Ishida, M.; Watanabe, S.; Noma, K.; Takehara, K.; Tazawa, H.; Hashimoto, Y.; Tanabe, S.; Matsuoka, J.; et al. Viral transduction of the HER2-extracellular domain expands trastuzumab-based photoimmunotherapy for HER2-negative breast cancer cells. Breast Cancer Res. Treat. 2015, 149, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, R.; Hanaoka, H.; Sato, K.; Nagaya, T.; Harada, T.; Mitsunaga, M.; Kim, I.; Paik, C.H.; Wu, A.M.; Choyke, P.L.; et al. Photoimmunotherapy targeting prostate-specific membrane antigen: Are antibody fragments as effective as antibodies? J. Nucl. Med. 2015, 56, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Sato, N.; Xu, B.; Nakamura, Y.; Nagaya, T.; Choyke, P.L.; Hasegawa, Y.; Kobayashi, H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 2016, 8, 352ra110. [Google Scholar] [CrossRef] [Green Version]
- Shirasu, N.; Yamada, H.; Shibaguchi, H.; Kuroki, M.; Kuroki, M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer 2014, 135, 2697–2710. [Google Scholar] [CrossRef]
- Hanaoka, H.; Nakajima, T.; Sato, K.; Watanabe, R.; Phung, Y.; Gao, W.; Harada, T.; Kim, I.; Paik, C.H.; Choyke, P.L.; et al. Photoimmunotherapy of hepatocellular carcinoma-targeting Glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine 2015, 10, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of B-cell lymphoma. Mol. Oncol. 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 2017, 8, 8807–8817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Choyke, P.L.; Kobayashi, H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE 2014, 9, e113276. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Thankarajan, E.; Jadhav, S.; Luboshits, G.; Gellerman, G.; Patsenker, L. Quantification of Drug Release Degree In Vivo Using Antibody-Guided, Dual-NIR-Dye Ratiometric System. Anal Chem. 2021, 93, 8265–8272. [Google Scholar] [CrossRef]
- Sato, K.; Watanabe, R.; Hanaoka, H.; Harada, T.; Nakajima, T.; Kim, I.; Paik, C.; Choyke, P.; Kobayashi, H. Photoimmunotherapy: Comparative effectiveness of two monoclonal antibodies targeting the epidermal growth factor receptor. Mol. Oncol. 2014, 8, 620–632. [Google Scholar] [CrossRef]
- Nagaya, T.; Sato, K.; Harada, T.; Nakamura, Y.; Choyke, P.; Kobayashi, H. Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen. PLoS ONE 2015, 10, e0136829. [Google Scholar] [CrossRef]
- Rosenberg, A.; Fujimura, D.; Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Real-Time Fluorescence Imaging Using Indocyanine Green to Assess Therapeutic Effects of Near-Infrared Photoimmunotherapy in Tumor Model Mice. Mol. Imaging 2020, 19, 1536012120934965. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Fujimura, D.; Furusawa, A.; Okada, R.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Diagnostic imaging in near-infrared photoimmunotherapy using a commercially available camera for indocyanine green. Cancer Sci. 2021, 112, 1326–1330. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Fujimura, D.; Furusawa, A.; Okada, R.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Fluorescence Imaging of Tumor-Accumulating Antibody-IR700 Conjugates Prior to Near-Infrared Photoimmunotherapy (NIR-PIT) Using a Commercially Available Camera Designed for Indocyanine Green. Mol. Pharm. 2021, 18, 1238–1246. [Google Scholar] [CrossRef]
- Okuyama, S.; Fujimura, D.; Inagaki, F.; Okada, R.; Maruoka, Y.; Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Real-time IR700 Fluorescence Imaging During Near-infrared Photoimmunotherapy Using a Clinically approved Camera for Indocyanine Green. Cancer Diagn. Progn. 2021, 1, 29–34. [Google Scholar] [CrossRef]
- Panzardi, G.; Donati, M.C.; Longobardi, G.; Pogi, P. Choroidal angiography with indocyanine green dye: Absorption and fluorescence techniques. Eur. J. Ophthalmol. 1992, 2, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano 2013, 7, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Nagaya, T.; Liu, Y.; Horng, H.; Lin, J.; Sato, K.; Kobayashi, H.; Chen, Y. 3D mesoscopic fluorescence tomography for imaging micro-distribution of antibody-photon absorber conjugates during near infrared photoimmunotherapy in vivo. J. Control Release 2018, 279, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nakajima, T.; Mizoi, K.; Tsushima, Y.; Ogihara, T. Imaging modalities for monitoring acute therapeutic effects after near-infrared photoimmunotherapy in vivo. J. Biophotonics 2021, 15, e202100266. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, S.; Muguruma, N.; Okamoto, K.; Kurihara, T.; Sato, Y.; Miyamoto, Y.; Kitamura, S.; Miyamoto, H.; Taguchi, T.; Tsuneyama, K.; et al. A Novel Theranostic Combination of Near-infrared Fluorescence Imaging and Laser Irradiation Targeting c-KIT for Gastrointestinal Stromal Tumors. Theranostics 2018, 8, 2313–2328. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Mitsunaga, M.; Ito, K.; Kobayashi, H.; Saruta, M. Cancer neovasculature-targeted near-infrared photoimmunotherapy (NIR-PIT) for gastric cancer: Different mechanisms of phototoxicity compared to cell membrane-targeted NIR-PIT. Gastric Cancer 2020, 23, 82–94. [Google Scholar] [CrossRef]
- Koganemaru, S.; Kuboki, Y.; Koga, Y.; Kojima, T.; Yamauchi, M.; Maeda, N.; Kagari, T.; Hirotani, K.; Yasunaga, M.; Matsumura, Y.; et al. U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer. Mol. Cancer Ther. 2019, 18, 2043–2050. [Google Scholar] [CrossRef] [Green Version]
- Yasunaga, M.; Saijou, S.; Hanaoka, S.; Anzai, T.; Tsumura, R.; Matsumura, Y. Significant antitumor effect of an antibody against TMEM180, a new colorectal cancer-specific molecule. Cancer Sci. 2019, 110, 761–770. [Google Scholar] [CrossRef]
- Takashima, H.; Koga, Y.; Tsumura, R.; Yasunaga, M.; Tsuchiya, M.; Inoue, T.; Negishi, E.; Harada, M.; Yoshida, S.; Matsumura, Y. Reinforcement of antitumor effect of micelles containing anticancer drugs by binding of an anti-tissue factor antibody without direct cytocidal effects. J. Control Release 2020, 323, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Fiji, Image J. Available online: https://imagej.net/Fiji (accessed on 13 February 2022).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takashima, K.; Koga, Y.; Anzai, T.; Migita, K.; Yamaguchi, T.; Ishikawa, A.; Sakashita, S.; Yasunaga, M.; Yano, T. Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-Time Imaging in Photoimmunotherapy. Pharmaceuticals 2022, 15, 223. https://doi.org/10.3390/ph15020223
Takashima K, Koga Y, Anzai T, Migita K, Yamaguchi T, Ishikawa A, Sakashita S, Yasunaga M, Yano T. Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-Time Imaging in Photoimmunotherapy. Pharmaceuticals. 2022; 15(2):223. https://doi.org/10.3390/ph15020223
Chicago/Turabian StyleTakashima, Kenji, Yoshikatsu Koga, Takahiro Anzai, Kayo Migita, Toru Yamaguchi, Akihiro Ishikawa, Shingo Sakashita, Masahiro Yasunaga, and Tomonori Yano. 2022. "Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-Time Imaging in Photoimmunotherapy" Pharmaceuticals 15, no. 2: 223. https://doi.org/10.3390/ph15020223
APA StyleTakashima, K., Koga, Y., Anzai, T., Migita, K., Yamaguchi, T., Ishikawa, A., Sakashita, S., Yasunaga, M., & Yano, T. (2022). Evaluation of Fluorescence Intensity and Antitumor Effect Using Real-Time Imaging in Photoimmunotherapy. Pharmaceuticals, 15(2), 223. https://doi.org/10.3390/ph15020223