Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation Considerations
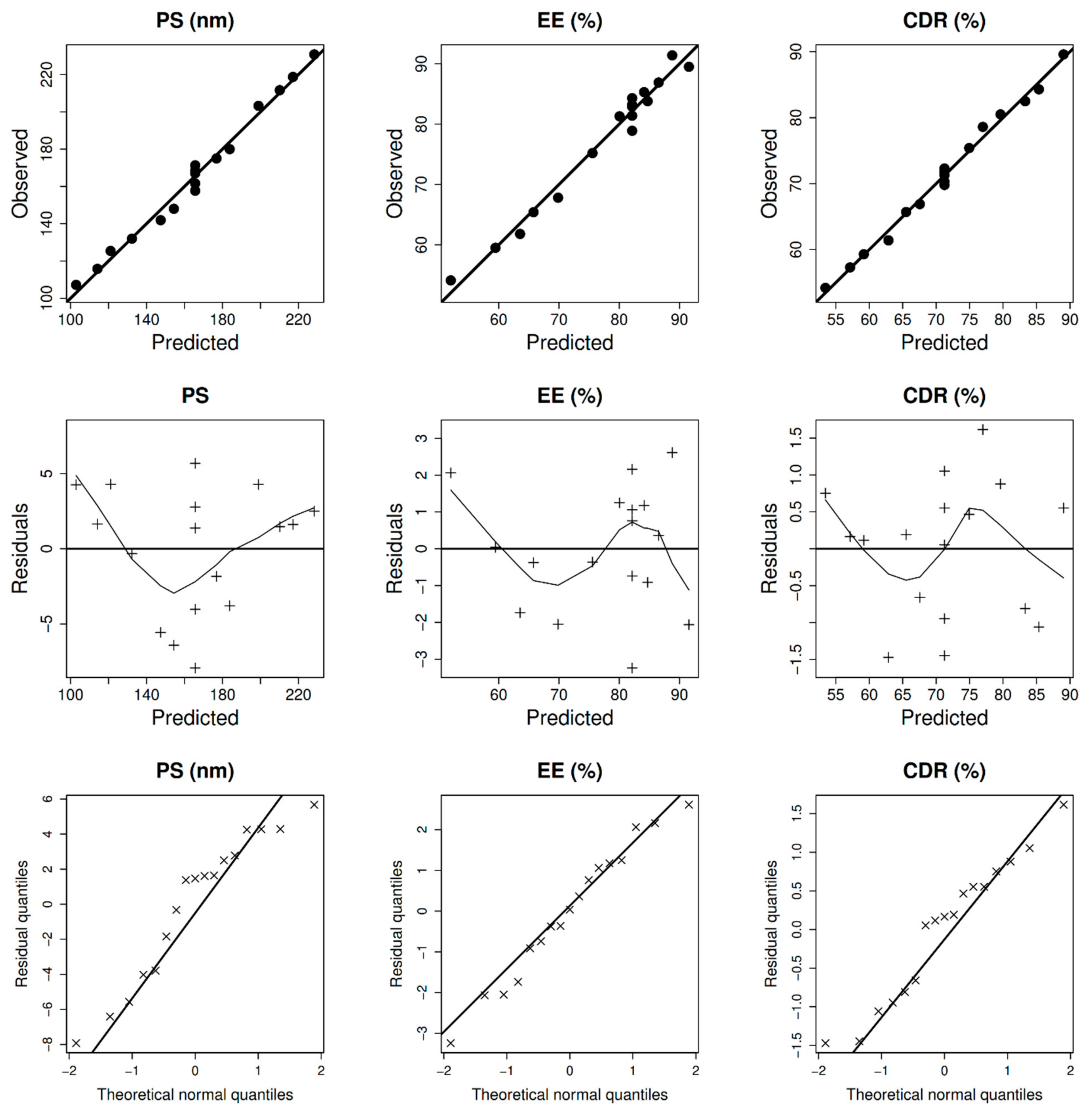
2.2. Design, Statistical Analysis, and Optimization
2.2.1. Particle Diameter, Entrapment, Surface Charge, and Cumulative Drug Release at 24 h
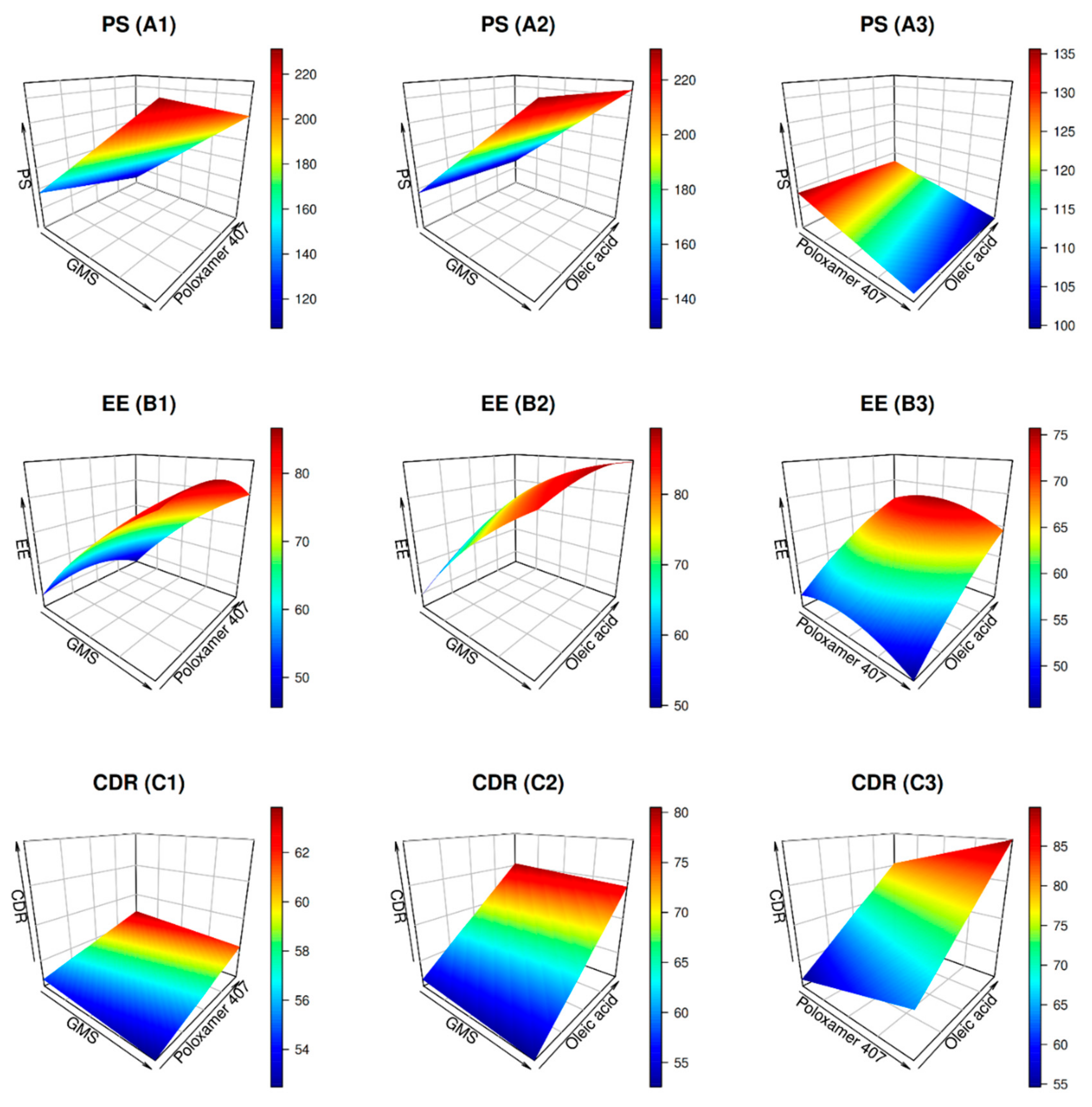
2.2.2. Optimization of Size
2.2.3. Optimization of Entrapment
2.2.4. Optimization of CDR
2.2.5. Composition of Optimal BER-NLCs
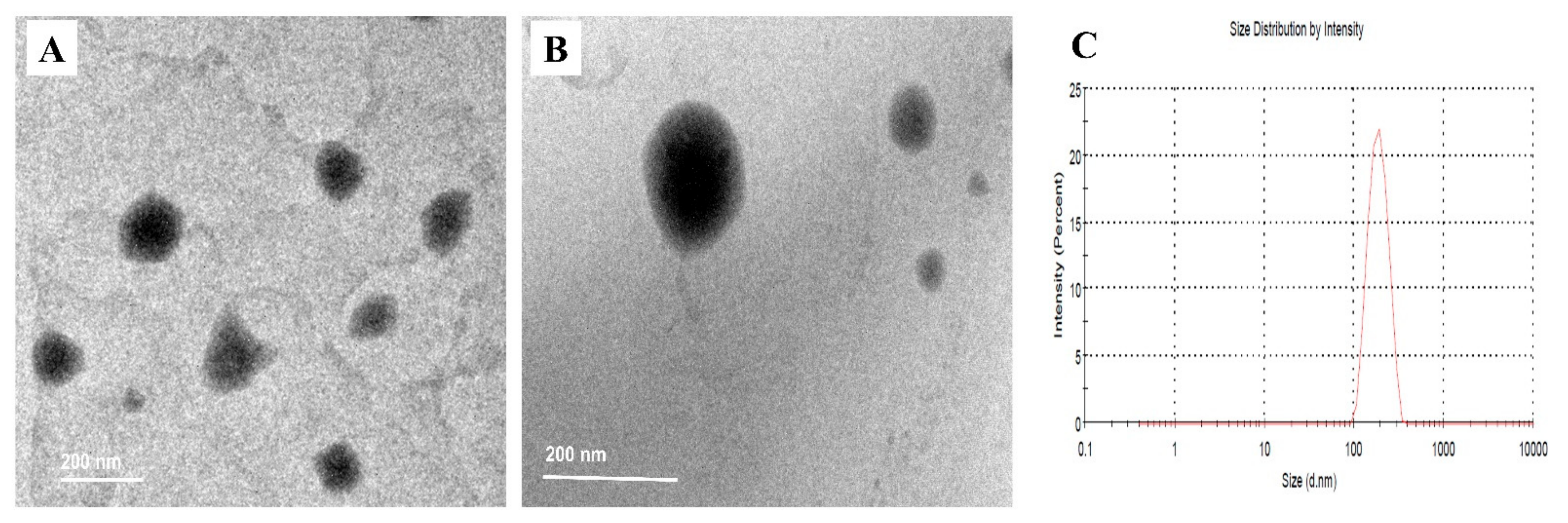
2.3. Characterization of BER-CTS-NLCs
2.3.1. BER-CTS-NLCs Size, Surface Charge, and Morphological Evaluation
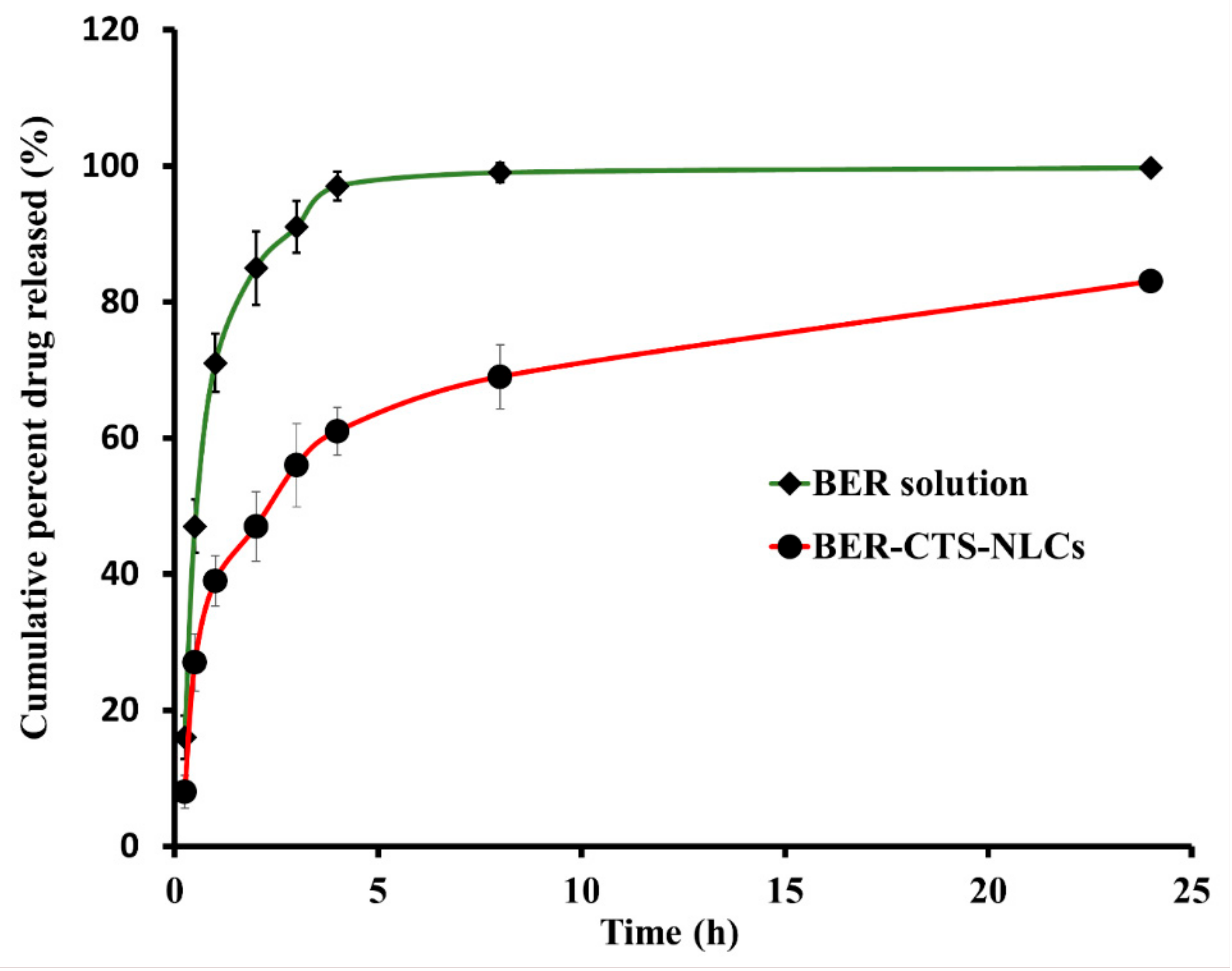
2.3.2. In Vitro Release Studies of BER
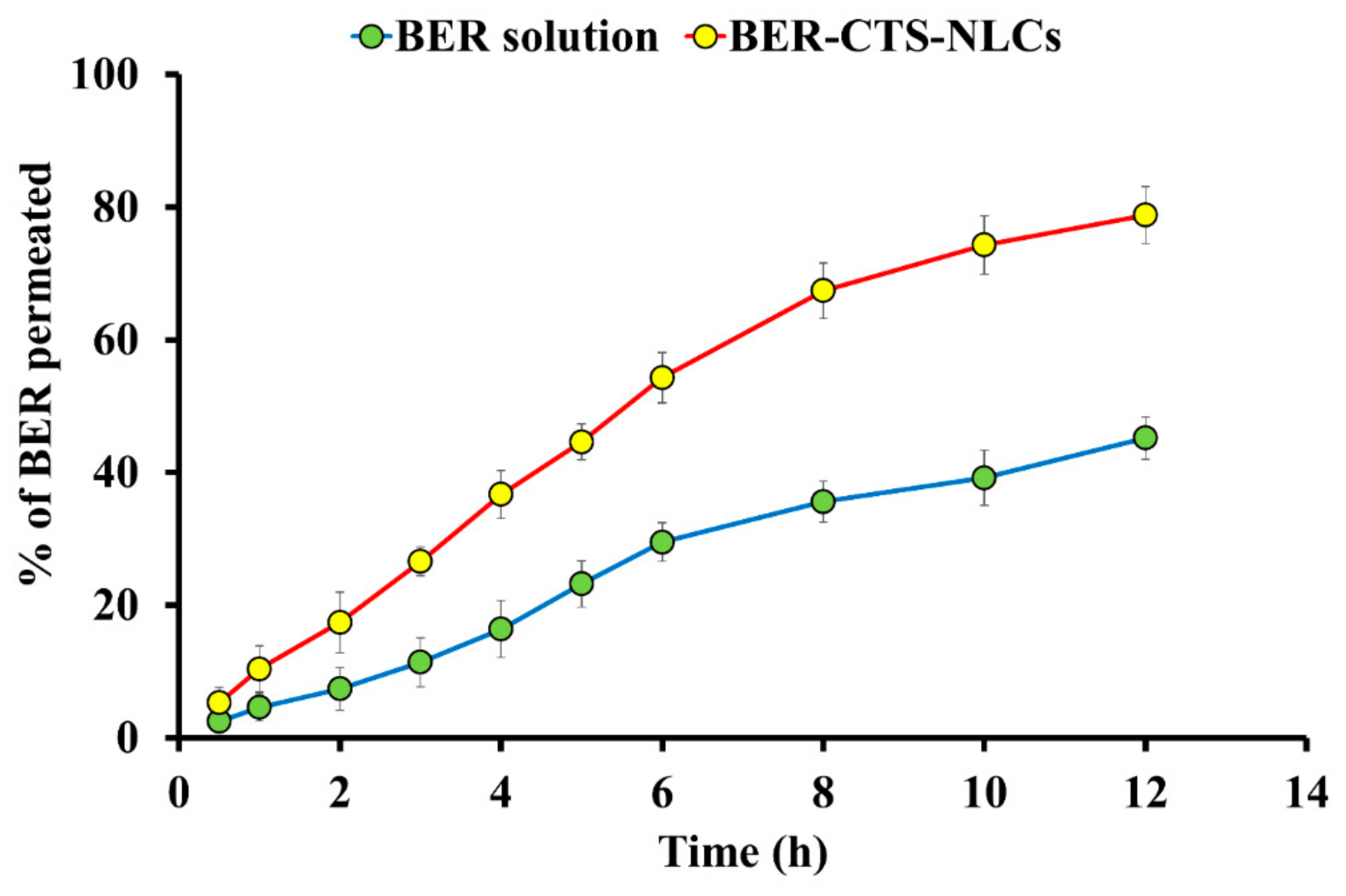
2.3.3. Ex Vivo BER Permeation Studies
2.3.4. pH Evaluation
2.4. In Vivo Experiments
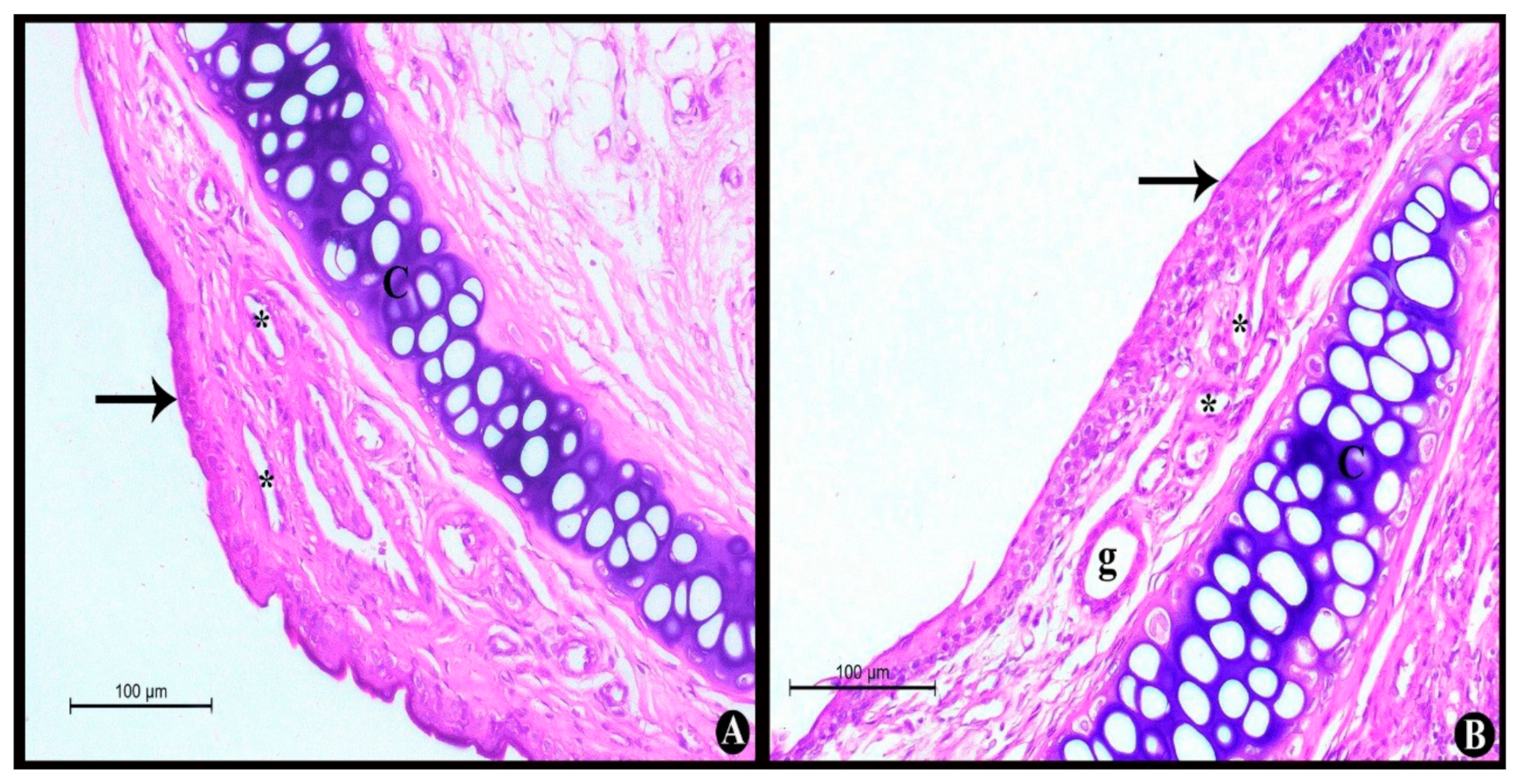
2.4.1. Nasal Histopathological Studies
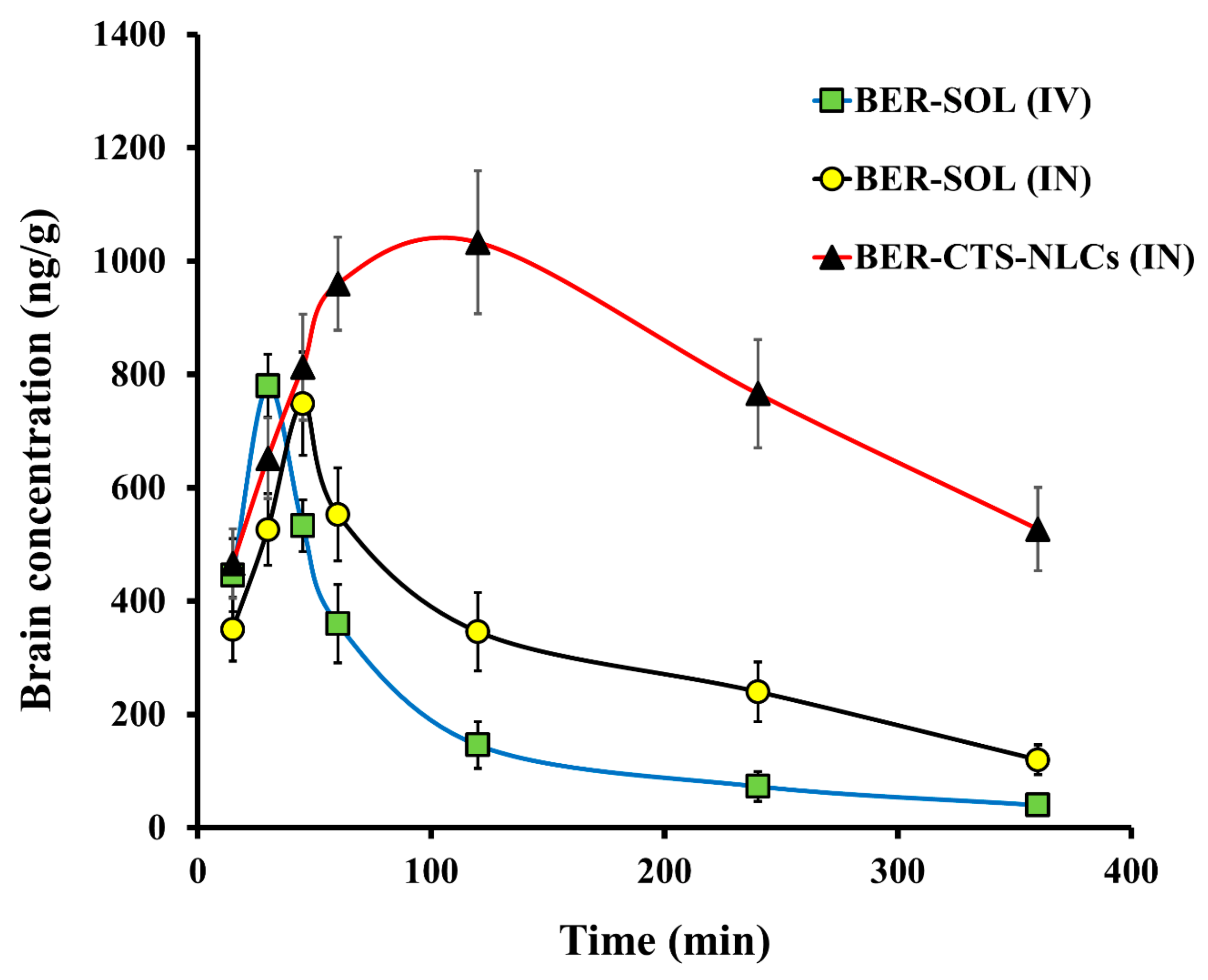
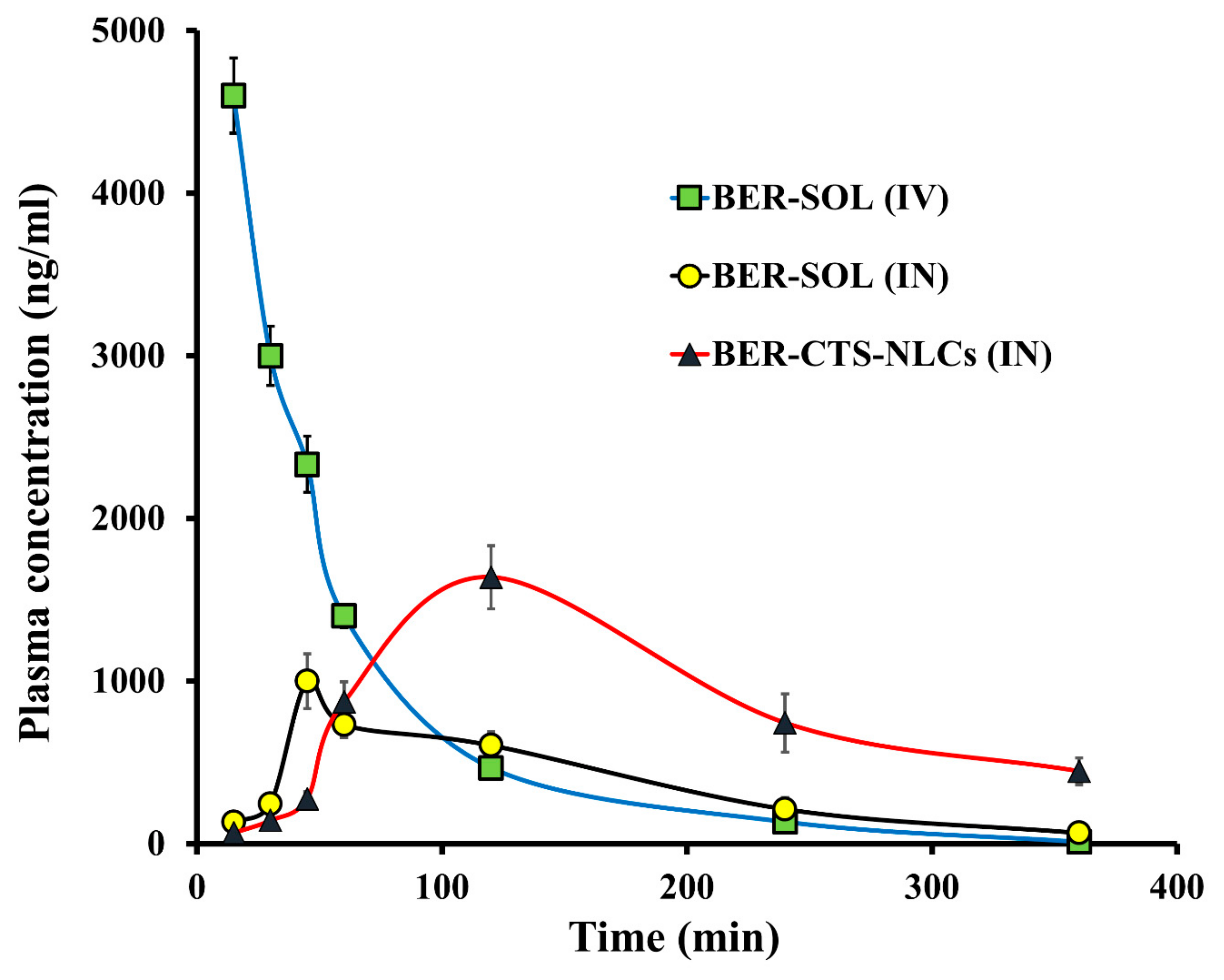
2.4.2. Pharmacokinetics Studies and Brain Distribution of BER
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Design and Optimization of Experiments
3.2.2. Preparation of Berberine Loaded NLCs (BER-NLCs)
3.2.3. Chromatographic Conditions
3.2.4. BER-NLCs Characterization and Optimization
Particle Diameter and Surface Charge
BER Entrapment
Cumulative Drug Release of BER after 24 h (CDR%)
3.2.5. Coating of Optimized BER-NLCs with CTS (BER-CTS-NLCs)
3.2.6. Characterization of BER-CTS-NLCs
BER-CTS-NLCs Size, Surface Charge, and Morphological Evaluation
In Vitro Release Studies of BER
Ex Vivo Drug Permeation Studies
Evaluation of pH
3.2.7. In Vivo Experiments
Nasal Histopathological Studies
Pharmacokinetic Study
Sample Preparation
Pharmacokinetic Analysis
3.2.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, K.-I.; Park, H.-S.; Kang, S.-R.; Nagappan, A.; Kim, J.-A.; Kim, E.-H.; Lee, W.-S.; Hah, Y.-S.; Chung, H.-J. Flavonoids isolated from Korea Citrus aurantium L. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 515901. [Google Scholar]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509. [Google Scholar] [CrossRef]
- Jin, Y.; Khadka, D.B.; Cho, W.-J. Pharmacological effects of berberine and its derivatives: A patent update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef]
- Ji, H.-F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Kulkarni, S.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 317–324. [Google Scholar] [CrossRef]
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M.J.B.R.I. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res. Int. 2014, 2014, 908915. [Google Scholar] [CrossRef]
- Lohmann, E.; Guerreiro, R.J.; Erginel-Unaltuna, N.; Gurunlian, N.; Bilgic, B.; Gurvit, H.; Hanagasi, H.A.; Luu, N.; Emre, M.; Singleton, A. Identification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patients. Neurobiol. Aging 2012, 33, 1850.e17–1850.e27. [Google Scholar] [CrossRef]
- Sullivan, S.E.; Young-Pearse, T.L. Induced pluripotent stem cells as a discovery tool for Alzheimer’s disease. Brain Res. 2017, 1656, 98–106. [Google Scholar] [CrossRef][Green Version]
- Whiteley, C.G. Arginine metabolising enzymes as targets against Alzheimers’ disease. Neurochem. Int. 2014, 67, 23–31. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Majdi, A.; Talebi, M.; Mahmoudi, J.; Babri, S. Regulation of nicotinic acetylcholine receptors in Alzheimer׳ s disease: A possible role of chaperones. Eur. J. Pharmacol. 2015, 755, 34–41. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Siemers, E. Drug Development in AD: Point of View from the Industry. J. Prev. Alzheimer’s Dis. 2015, 2, 216–218. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Piplani, P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol. Sci. 2016, 37, 1403–1435. [Google Scholar] [CrossRef]
- Shi, A.; Huang, L.; Lu, C.; He, F.; Li, X. Synthesis, biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and β-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011, 19, 2298–2305. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Wang, X.; Liu, X.; Liu, X.; Xie, L.; Wang, G. Modulation of glucagon-like peptide-1 release by berberine: In vivo and in vitro studies. Biochem. Pharmacol. 2010, 79, 1000–1006. [Google Scholar] [CrossRef]
- Asai, M.; Iwata, N.; Yoshikawa, A.; Aizaki, Y.; Ishiura, S.; Saido, T.C.; Maruyama, K. Berberine alters the processing of Alzheimer’s amyloid precursor protein to decrease Aβ secretion. Biochem. Biophys. Res. Commun. 2007, 352, 498–502. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid-and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef]
- Lohan, S.; Raza, K.; Mehta, S.; Bhatti, G.K.; Saini, S.; Singh, B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: A preclinical evidence. Int. J. Pharm. 2017, 530, 263–278. [Google Scholar] [CrossRef]
- Soudi, S.A.; Nounou, M.I.; Sheweita, S.A.; Ghareeb, D.A.; Younis, L.K.; El-Khordagui, L.K. Protective effect of surface-modified berberine nanoparticles against LPS-induced neurodegenerative changes: A preclinical study. Drug Deliv. Transl. Res. 2019, 9, 906–919. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; Carbone, C.; Russo, G.; Pappalardo, F.; Puglisi, G. Design and optimization of PEGylated nanoparticles intended for Berberine Chloride delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 521–530. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood–brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Graff, C.L.; Pollack, G.M. Nasal drug administration: Potential for targeted central nervous system delivery. J. Pharm. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef]
- Kaur, S.; Nautyal, U.; Singh, R.; Singh, S.; Devi, A. Nanostructure lipid carrier (NLC): The new generation of lipid nanoparticles. Asian. Pac. J. Health Sci. 2015, 2, 76–93. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J. Drug Deliv. Sci. Technol. 2016, 33, 37–50. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Du, W.; Li, H.; Tian, B.; Sai, S.; Gao, Y.; Lan, T.; Meng, Y.; Ding, C. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf. B Biointerfaces 2019, 183, 110446. [Google Scholar] [CrossRef]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to Brain Delivery of Rivastigmine by In Situ Gelling Cationic Nanostructured Lipid Carriers: Enhanced Brain Distribution and Pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef]
- Patel, H.P.; Gandhi, P.A.; Chaudhari, P.S.; Desai, B.V.; Desai, D.T.; Dedhiya, P.P.; Maulvi, F.A.; Vyas, B.A. Clozapine loaded nanostructured lipid carriers engineered for brain targeting via nose-to-brain delivery: Optimization and in vivo pharmacokinetic studies. J. Drug Deliv. Sci. Technol. 2021, 64, 102533. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Kunde, S.S.; Auti, S.T.; Kulkarni, Y.A.; Wairkar, S.J.L.S. Berberine loaded nanostructured lipid carrier for Alzheimer’s disease: Design, statistical optimization and enhanced in vivo performance. Life Sci. 2021, 285, 119990. [Google Scholar] [CrossRef] [PubMed]
- Ramtoola, Z.; Lyons, P.; Keohane, K.; Kerrigan, S.W.; Kirby, B.P.; Kelly, J.G. Investigation of the interaction of biodegradable micro-and nanoparticulate drug delivery systems with platelets. J. Pharm. Pharmacol. 2011, 63, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, L.; Battaglia, L.; Peira, E.; Chirio, D.; Muntoni, E.; Solazzi, I.; Gallarate, M.; Dosio, F. Solid lipid nanoparticles as vehicles of drugs to the brain: Current state of the art. Eur. J. Pharm. Biopharm. 2014, 87, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dadhania, P.; Vuddanda, P.R.; Jain, A.; Velaga, S.; Singh, S. Intranasal delivery of asenapine loaded nanostructured lipid carriers: Formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016, 6, 2032–2045. [Google Scholar] [CrossRef]
- Eid, H.M.; Naguib, I.A.; Alsantali, R.I.; Alsalahat, I.; Hegazy, A.M. Novel chitosan-coated niosomal formulation for improved management of bacterial conjunctivitis: A highly permeable and efficient ocular nanocarrier for azithromycin. J. Pharm. Sci. 2021, 110, 3027–3036. [Google Scholar] [CrossRef]
- Abou Youssef, N.A.H.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; Magda Abd Elsamea, E.-M.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: A detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J. Nanomater. 2012, 2012, 21. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharm. 2011, 415, 232–243. [Google Scholar] [CrossRef]
- Ferreira, M.; Chaves, L.L.; Lima, S.A.C.; Reis, S. Optimization of nanostructured lipid carriers loaded with methotrexate: A tool for inflammatory and cancer therapy. Int. J. Pharm. 2015, 492, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanoparticle Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Kokare, C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: Optimization and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, optimization, and in vitro/in vivo characterization of enhanced lipid nanoparticles for ocular delivery of ofloxacin: The influence of pegylation and chitosan coating. AAPS PharmSciTech 2019, 20, 183. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv. 2013, 20, 247–251. [Google Scholar] [CrossRef]
- Hussain, M.A.; Aungst, B.J. Nasal mucosal metabolism of an LH-RH fragment and inhibition with boroleucine. Int. J. Pharm. 1994, 105, 7–10. [Google Scholar] [CrossRef]
- Aungst, B.J.; Rogers, N.J. Site dependence of absorption-promoting actions of Laureth-9, Na salicylate, Na 2 EDTA, and aprotinin on rectal, nasal, and buccal insulin delivery. Pharm. Res. 1988, 5, 305–308. [Google Scholar] [CrossRef]
- Seju, U.; Kumar, A.; Sawant, K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, X.; Jiang, W.; Lu, W.; Su, L.; Shi, Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int. J. Pharm. 2004, 275, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Babbar, A.; Mishra, A.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, K. Plot3D: Plotting Multi-Dimensional Data. R Package Version 1.1.1. 2017. Available online: https://CRAN.R-project.org/package=plot3D, (accessed on 31 January 2022).
- Panda, D.S.; Eid, H.M.; Elkomy, M.H.; Khames, A.; Hassan, R.M.; Abo El-Ela, F.I.; Yassin, H.A. Berberine Encapsulated Lecithin–Chitosan Nanoparticles as Innovative Wound Healing Agent in Type II Diabetes. Pharmaceutics 2021, 13, 1197. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. Aaps Pharmscitech 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Chen, G.; Hussain, A.; Khan, H.; Abbas, A.; Zhou, Z.; Shafiq, M.; Ahmad, S.; Ali, U.; Usman, M.J.I.J.O.N. Cyclam-Modified Polyethyleneimine for Simultaneous TGFβ siRNA Delivery and CXCR4 Inhibition for the Treatment of CCl4-Induced Liver Fibrosis. Int. J. Nanomed. 2021, 16, 4451. [Google Scholar] [CrossRef]
- Elkomy, M.H.; El Menshawe, S.F.; Eid, H.M.; Ali, A.M. Development of a nanogel formulation for transdermal delivery of tenoxicam: A pharmacokinetic–pharmacodynamic modeling approach for quantitative prediction of skin absorption. Drug Dev. Ind. Pharm. 2017, 43, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Elmowafy, M.; Shalaby, K.; Azmy, A.F.; Ahmad, N.; Zafar, A.; Eid, H.M. Development and machine-learning optimization of mucoadhesive nanostructured lipid carriers loaded with fluconazole for treatment of oral candidiasis. Drug Dev. Ind. Pharm. 2021, 47, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2016, 23, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Callens, C.; Ceulemans, J.; Ludwig, A.; Foreman, P.; Remon, J.P. Rheological study on mucoadhesivity of some nasal powder formulations. Eur. J. Pharm. Biopharm. 2003, 55, 323–328. [Google Scholar] [CrossRef]
- Chen, G.; Ullah, A.; Xu, G.; Xu, Z.; Wang, F.; Liu, T.; Su, Y.; Zhang, T.; Wang, K. Topically applied liposome-in-hydrogels for systematically targeted tumor photothermal therapy. Drug Deliv. 2021, 28, 1923–1931. [Google Scholar] [CrossRef]
- Ullah, A.; Wang, K.; Wu, P.; Oupicky, D.; Sun, M.J.I.J.O.N. CXCR4-targeted liposomal mediated co-delivery of pirfenidone and AMD3100 for the treatment of TGFβ-induced HSC-T6 cells activation. Int. J. Nanomed. 2019, 14, 2927. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Elmenshawe, S.F.; Eid, H.M.; Ali, A.M. Topical ketoprofen nanogel: Artificial neural network optimization, clustered bootstrap validation, and in vivo activity evaluation based on longitudinal dose response modeling. Drug Deliv. 2016, 23, 3294–3306. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of an antiemetic drug, metoclopramide: In vitro/ex vivo studies. J. Pharm. Pharmacol. 2005, 57, 287–294. [Google Scholar] [CrossRef]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Transfersomal nanovesicles for nose-to-brain delivery of ofloxacin for better management of bacterial meningitis: Formulation, optimization by Box-Behnken design, characterization and in vivo pharmacokinetic study. J. Drug Deliv. Sci. Technol. 2019, 54, 101304. [Google Scholar] [CrossRef]
| Independent Variables | Levels | ||||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | |||||
| X1: GMS amount (mg) | 100 | 150 | 200 | ||||
| X2: Poloxamer 407 (% w/w) | 1 | 1.5 | 2 | ||||
| X3: Oleic acid amount (mg) | 15 | 22.5 | 30 | ||||
| F | X1 GMS (mg) | X2 Poloxamer 407 (w/w %) | X3 Oleic acid (mg) | Y1 PS (nm) | Y2 EE (%) | Y3 ZP (mV) € | Y4 CDR (%) |
| 1 | −1 | 0 | 1 | 115.8 ± 2.4 | 75.2 ± 3.5 | (−) 28.4 ± 2.1 | 84.3 ± 3.3 |
| 2 | 0 | −1 | −1 | 180.0 ± 3.6 | 67.8 ± 2.8 | (−) 28.3 ± 1.5 | 54.2 ± 4.2 |
| 3 | 0 | 0 | 0 | 157.7 ± 3.2 | 78.9 ± 3.1 | (−) 30.8 ± 2.4 | 69.8 ± 3.1 |
| 4 | 0 | 1 | 1 | 141.9 ± 2.5 | 81.3 ± 4.3 | (−) 34.9 ± 2.3 | 89.6 ± 4.5 |
| 5 | 0 | 0 | 0 | 161.6 ± 3.8 | 81.4 ± 3.4 | (−) 30.2 ± 1.6 | 70.3 ± 3.8 |
| 6 | −1 | 1 | 0 | 107.2 ± 2.7 | 59.5 ± 3.1 | (−) 29.6 ± 3.4 | 78.6 ± 2.1 |
| 7 | 1 | 1 | 0 | 203.2 ± 2.9 | 83.8 ± 3.3 | (−) 29.9 ± 2.5 | 75.4 ± 3.5 |
| 8 | 0 | 0 | 0 | 167.0 ± 3.5 | 83.2 ± 2.8 | (−) 34.8 ± 1.8 | 72.3 ± 2.4 |
| 9 | 0 | 0 | 0 | 168.4 ± 3.2 | 82.9 ± 4.6 | (−) 30.7 ± 2.4 | 71.3 ± 2.9 |
| 10 | −1 | −1 | 0 | 132.0 ± 1.5 | 61.8 ± 3.6 | (−) 31.5 ± 1.7 | 66.9 ± 3.1 |
| 11 | 0 | −1 | 1 | 175.0 ± 4.6 | 85.3 ± 4.8 | (−) 29.8 ± 2.4 | 80.5 ± 4.4 |
| 12 | 1 | −1 | 0 | 230.8 ± 3.8 | 91.4 ± 3.5 | (−) 31.3 ± 1.4 | 65.7 ± 3.2 |
| 13 | 1 | 0 | 1 | 211.6 ± 2.6 | 89.5 ± 2.7 | (−) 35.1 ± 3.6 | 82.5 ± 2.3 |
| 14 | −1 | 0 | −1 | 125.4 ± 2.3 | 54.1 ± 4.6 | (−) 33.1 ± 2.7 | 59.3 ± 2.8 |
| 15 | 1 | 0 | −1 | 218.7 ± 5.2 | 86.9 ± 2.4 | (−) 27.5 ± 1.9 | 57.3 ± 3.1 |
| 16 | 0 | 1 | −1 | 148.0 ± 3.1 | 65.4 ± 6.2 | (−) 32.1 ± 1.3 | 61.4 ± 4.2 |
| 17 | 0 | 0 | 0 | 171.3 ± 2.5 | 84.3 ± 4.9 | (−) 28.9 ± 2.3 | 71.8 ± 5.2 |
| Source | Size (nm) | EE% | CDR% | |||
|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | |||
| Model | 416.50 | <0.0001 | 162.22 | <0.0001 | 505.75 | <0.0001 |
| X1: GMS amount (mg) | 1134.32 | <0.0001 | 670.22 | <0.0001 | 8.19 | 0.0133 |
| X2: Poloxamer 407 (% w/w) | 108.84 | <0.0001 | 13.59 | 0.0050 | 173.20 | <0.0001 |
| X3: Oleic acid amount (mg) | 6.34 | 0.0257 | 241.61 | <0.0001 | 1335.85 | <0.0001 |
| X1×3 | 85.47 | <0.0001 | ||||
| X12 | 54.30 | <0.0001 | ||||
| X22 | 43.39 | 0.0001 | ||||
| X32 | 14.84 | 0.0039 | ||||
| Lack of Fit | 0.3386 | 0.9183 | 0.4527 | 0.7962 | 0.9338 | 0.5748 |
| Model | Linear | Reduced Quadratic | Linear | |||
| SD | 0.1573 | 0.0003 | 1.01 | |||
| R2 | 0.9897 | 0.9921 | 0.9915 | |||
| Adequate precision | 64.3041 | 42.7075 | 72.4640 | |||
| Predicted R2 | 0.9845 | 0.9687 | 0.9849 | |||
| Adjusted R2 | 0.9873 | 0.9860 | 0.9895 | |||
| %CV | 1.23 | 1.98 | 1.42 | |||
| Sqrt (PS) = 12.7979 + 1.87247 × X1 − 0.580015 × X2 − 0.140025 × X3. 1/(EE) = 0.0121807 − 0.0024 × X1 + 0.0003 × X2 − 0.0014 × X3 + 0.0012 × X1 .X3 + 0.0009 × X12 + 0.0008 × X22 + 0.0005 × X32. CDR% = 71.2471 − 1.025 × X1 + 4.7125 × X2 + 13.0875 × X3. | ||||||
| Independent Factors | Optimal Value | Response | ExperimentalValue | Design Expert® Predicted Value | Prediction Error (%) * |
|---|---|---|---|---|---|
| X1: GMS amount (mg) | 114.7 | PS (nm) | 142.1 ± 5.7 | 119.8 | 15.7 |
| X2: Poloxamer 407 (% w/w) | 1.8 | EE% | 80.3 ± 3.1 | 76.1 | 5.2 |
| X3: Oleic acid amount (mg) | 30 | CDR% | 85.6 ± 4.2 | 88.2 | 3 |
| Preparation | Flux Jss (µg cm−2 h−1) | Cumulative BER Permeated at 12 h (μg/cm2) | Permeability Coefficient (cm/h) |
|---|---|---|---|
| BER-CTS-NLCs | 10.63 ± 1.27 | 472.8 ± 25.87 | 0.01063 ± 0.00027 |
| BER-SOL | 5.79 ± 0.79 | 271.2 ± 19.25 | 0.00579 ± 0.00036 |
| Formulation | Tissue/Organ | Tmax (min) | Cmax (ng/mL) | Ke (min−1) | t1/2 (min) | AUC0–t (ng/mL × min) | Cbrain/Cblood at 30 min. | AUCbrain/AUCblood |
|---|---|---|---|---|---|---|---|---|
| BER-SOL (IV) | Brain | 30 | 780 | 0.005 | 128.5 | 74,944 | 0.26 | 0.24 |
| Blood | 15 | 4600 | 0.017 | 41.5 | 313,493 | |||
| BER-SOL (IN) | Brain | 45 | 748 | 0.005 | 144.6 | 137,275 | 2.14 | 0.99 |
| Blood | 45 | 1000 | 0.009 | 75.1 | 139,171 | |||
| BER-CTS-NLCs (IN) | Brain | 120 | 1033 | 0.003 | 247.2 | 469,403 | 4.56 | 1.22 |
| Blood | 120 | 1639 | 0.005 | 127.8 | 385,609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. https://doi.org/10.3390/ph15030281
Abo El-Enin HA, Elkomy MH, Naguib IA, Ahmed MF, Alsaidan OA, Alsalahat I, Ghoneim MM, Eid HM. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals. 2022; 15(3):281. https://doi.org/10.3390/ph15030281
Chicago/Turabian StyleAbo El-Enin, Hadel A., Mohammed H. Elkomy, Ibrahim A. Naguib, Marwa F. Ahmed, Omar A. Alsaidan, Izzeddin Alsalahat, Mohammed M. Ghoneim, and Hussein M. Eid. 2022. "Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route" Pharmaceuticals 15, no. 3: 281. https://doi.org/10.3390/ph15030281
APA StyleAbo El-Enin, H. A., Elkomy, M. H., Naguib, I. A., Ahmed, M. F., Alsaidan, O. A., Alsalahat, I., Ghoneim, M. M., & Eid, H. M. (2022). Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals, 15(3), 281. https://doi.org/10.3390/ph15030281








