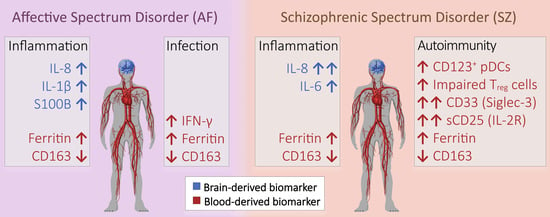
Old and New Biomarkers for Infection, Inflammation, and Autoimmunity in Treatment-Resistant Affective and Schizophrenic Spectrum Disorders
Abstract
:1. Introduction
2. Results
2.1. Plasma and CSF Cytokine Profiles
2.2. Immune Phenotype Stratification
2.3. Antigen Presenting Cells (APCs)

2.4. Pathogen Receptors (Toll-like Receptors, TLRs)

2.5. Lymphocyte Subpopulations Related to Virus Infections and Autoimmunity

2.6. Correlation Patterns of Plasma and CSF Cytokine Levels, and Immune Phenotypes
3. Discussion
3.1. Inflammation in AF and SZ Patients
3.2. Different Forms of Inflammation in AF and SZ
4. Materials and Methods
4.1. Patient Cohorts
4.2. Healthy Donors
4.3. Cytokine Measurements
4.4. Flow Cytometry
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copeland, W.E.; Shanahan, L.; Hinesley, J.; Chan, R.; Aberg, K.; Fairbank, J.A.; Oord, E.J.C.G.V.D.; Costello, E.J. Association of Childhood Trauma Exposure with Adult Psychiatric Disorders and Functional Outcomes. JAMA Netw. Open 2018, 1, e184493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.J.; Martin, J.; Lu, Y.; Brikell, I.; Lundström, S.; Larsson, H.; Lichtenstein, P. Association of Genetic Risk Factors for Psychiatric Disorders and Traits of These Disorders in a Swedish Population Twin Sample. JAMA Psychiatry 2019, 76, 280–289. [Google Scholar] [CrossRef]
- Beumer, W.; Gibney, S.M.; Drexhage, R.C.; Pont-Lezica, L.; Doorduin, J.; Klein, H.; Steiner, J.; Connor, T.J.; Harkin, A.; Versnel, M.A.; et al. The immune theory of psychiatric diseases: A key role for activated microglia and circulating monocytes. J. Leukoc. Biol. 2012, 92, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; McEwen, S.; Subotnik, K.L.; Hellemann, G.S.; Ghadiali, M.; Rahimdel, A.; Seo, M.J.; Irwin, M.R.; Nuechterlein, K.H. Changes in inflammation are related to depression and amount of aerobic exercise in first episode schizophrenia. Early Interv. Psychiatry 2020, 15, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Tripathi, A.; Pillai, A. Inflammatory Pathways in Psychiatric Disorders: The Case of Schizophrenia and Depression. Curr. Behav. Neurosci. Rep. 2020, 7, 128–138. [Google Scholar] [CrossRef]
- Maxeiner, H.-G.; Schneider, E.M.; Kurfiss, S.-T.; Brettschneider, J.; Tumani, H.; Bechter, K. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine 2014, 69, 62–67. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Ellul, P.; Mariotti-Ferrandiz, E.; Leboyer, M.; Klatzmann, D. Regulatory T Cells as Supporters of Psychoimmune Resilience: Toward Immunotherapy of Major Depressive Disorder. Front. Neurol. 2018, 9, 167. [Google Scholar] [CrossRef]
- Dik, W.A.; Heron, M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases. Neth J. Med. 2020, 78, 220–231. [Google Scholar]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, L.; Soukou, S.; Steglich, B.; Czarnewski, P.; Zhao, L.; Wende, S.; Bedke, T.; Ergen, C.; Manthey, C.; Agalioti, T.; et al. Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat. Commun. 2018, 9, 5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, G.; Zhang, W.; Dai, J.; Liu, J.; Li, F.; Wu, D.; Xiao, Y.; Shah, C.; Sweeney, J.A.; Wu, M.; et al. Increased Peripheral Interleukin 10 Relate to White Matter Integrity in Schizophrenia. Front. Neurosci. 2019, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Qusar, M.M.A.S.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, R. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916655. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.G.; Koppal, T.; Akama, K.T.; Guo, L.; Craft, J.M.; Samy, B.; Schavocky, J.P.; Watterson, D.; Van Eldik, L.J. Mechanism of glial activation by S100B: Involvement of the transcription factor NFκB. Neurobiol. Aging 2001, 22, 765–772. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lei, X.; Jiang, Z.; Fitzgerald, K.A. Cellular nucleic acid–binding protein is essential for type I interferon–mediated immunity to RNA virus infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2100383118. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Zorrilla, E.P.; Luborsky, L.; McKay, J.R.; Rosenthal, R.; Houldin, A.; Tax, A.; McCorkle, R.; Seligman, D.A.; Schmidt, K. The Relationship of Depression and Stressors to Immunological Assays: A Meta-Analytic Review. Brain Behav. Immun. 2001, 15, 199–226. [Google Scholar] [CrossRef] [Green Version]
- Benros, M.E.; Waltoft, B.L.; Nordentoft, M.; Østergaard, S.D.; Eaton, W.W.; Krogh, J.; Mortensen, P.B. Autoimmune Diseases and Severe Infections as Risk Factors for Mood Disorders. JAMA Psychiatry 2013, 70, 812–820. [Google Scholar] [CrossRef] [Green Version]
- Benros, M.E.; Eaton, W.W.; Mortensen, P.B. The Epidemiologic Evidence Linking Autoimmune Diseases and Psychosis. Biol. Psychiatry 2014, 75, 300–306. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Are medical comorbid conditions of bipolar disorder due to immune dysfunction? Acta Psychiatr. Scand. 2015, 132, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Kuzior, H.; Fiebich, B.L.; Yousif, N.M.; Saliba, S.W.; Ziegler, C.; Nickel, K.; Maier, S.J.; Süß, P.; Runge, K.; Matysik, M.; et al. Increased IL-8 concentrations in the cerebrospinal fluid of patients with unipolar depression. Compr. Psychiatry 2020, 102, 152196. [Google Scholar] [CrossRef] [PubMed]
- Hesse, R.; Wahler, A.; Gummert, P.; Kirschmer, S.; Otto, M.; Tumani, H.; Lewerenz, J.; Schnack, C.; von Arnim, C.A. Decreased IL-8 levels in CSF and serum of AD patients and negative correlation of MMSE and IL-1β. BMC Neurol. 2016, 16, 185. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, D.; Schoonenboom, N.; Scheltens, P.; Fenoglio, C.; Bouwman, F.; Venturelli, E.; Guidi, I.; Blankenstein, M.A.; Bresolin, N.; Scarpini, E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2006, 63, 538–543. [Google Scholar] [CrossRef]
- Espíndola, O.M.; Gomes, Y.C.P.; Brandão, C.O.; Torres, R.C.; Siqueira, M.; Soares, C.N.; Lima, M.A.S.D.; Leite, A.C.C.B.; Venturotti, C.O.; Carvalho, A.J.C.; et al. Inflammatory Cytokine Patterns Associated with Neurological Diseases in Coronavirus Disease 2019. Ann. Neurol. 2021, 89, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.T.; de Jongste, A.H.; Kraan, J.; Boonstra, J.G.; Sillevis Smitt, P.A.; Gratama, J.W. Flow cytometric characterization of cerebrospinal fluid cells. Cytom. Part B Clin. Cytom. 2011, 80, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wißfeld, J.; Nozaki, I.; Mathews, M.; Raschka, T.; Ebeling, C.; Hornung, V.; Brüstle, O.; Neumann, H. Deletion of Alzheimer’s disease associated CD33 results in an inflammatory human microglia phenotype. Glia 2021, 69, 1393–1412. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Miles, L.A.; Hermans, S.J.; Crespi, G.A.N.; Gooi, J.H.; Doughty, L.; Nero, T.L.; Markulić, J.; Ebneth, A.; Wroblowski, B.; Oehlrich, D.; et al. Small Molecule Binding to Alzheimer Risk Factor CD33 Promotes Aβ Phagocytosis. iScience 2019, 19, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Meniailo, M.E.; Malashchenko, V.V.; Shmarov, V.A.; Gazatova, N.D.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsova, G.V.; Seledtsov, V.I. Interleukin-8 favors pro-inflammatory activity of human monocytes/macrophages. Int. Immunopharmacol. 2018, 56, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, Y.J.; Kim, J.H.; Dinh, N.T.H.; Go, G.; Tae, S.; Park, K.S.; Park, H.T.; Lee, C.; Roh, T.Y.; et al. Outer Membrane Vesicles Derived From Escherichia coli Regulate Neutrophil Migration by Induction of Endothelial IL-8. Front. Microbiol. 2018, 9, 2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.F.; Narasipura, S.D.; Wallace, J.; Ritz, E.M.; Al-Harthi, L. Negative regulation of IL-8 in human astrocytes depends on β-catenin while positive regulation is mediated by TCFs/LEF/ATF2 interaction. Cytokine 2020, 136, 155252. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; Delpech, J.C. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hajduková, L.; Sobek, O.; Prchalová, D.; Bílková, Z.; Koudelková, M.; Lukášková, J.; Matuchová, I. Biomarkers of Brain Damage: S100B and NSE Concentrations in Cerebrospinal Fluid—A Normative Study. BioMed Res. Int. 2015, 2015, 379071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portela, L.V.; Tort, A.B.; Schaf, D.V.; Ribeiro, L.; Nora, D.B.; Walz, R.; Rotta, L.N.; Silva, C.T.; Busnello, J.V.; Kapczinski, F.; et al. The serum S100B concentration is age dependent. Clin. Chem. 2002, 48, 950–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peskind, E.R.; Griffin, W.S.; Akama, K.T.; Raskind, M.A.; Van Eldik, L.J. Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer’s disease. Neurochem. Int. 2001, 39, 409–413. [Google Scholar] [CrossRef]
- Uher, T.; Bob, P. Cerebrospinal fluid S100B levels reflect symptoms of depression in patients with non-inflammatory neurological disorders. Neurosci. Lett. 2012, 529, 139–143. [Google Scholar] [CrossRef]
- Heumann, D.; Gallay, P.; Barras, C.; Albrecht, H.; de Flaugergues, J.C.; Steck, A.; Glauser, M.P. Lipopolysaccharide binding protein (LBP) as a marker of protein leakage in cerebrospinal fluid. J. Endotoxin Res. 1995, 2, 1–7. [Google Scholar] [CrossRef]
- Pitts, A.F.; Carroll, B.T.; Gehris, T.L.; Kathol, R.G.; Samuelson, S.D. Elevated CSF protein in male patients with depression. Biol. Psychiatry 1990, 28, 629–637. [Google Scholar] [CrossRef]
- Mohamed, H.T.; El-Ghonaimy, E.A.; El-Shinawi, M.; Hosney, M.; Götte, M.; Woodward, W.A.; El-Mamlouk, T.; Mohamed, M.M. IL-8 and MCP-1/CCL2 regulate proteolytic activity in triple negative inflammatory breast cancer a mechanism that might be modulated by Src and Erk1/2. Toxicol. Appl. Pharmacol. 2020, 401, 115092. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, E.E.; Martin, A.S.; Soontornniyomkij, B.; Soontornniyomkij, V.; Achim, C.L.; Reuter, C.; Irwin, M.R.; Eyler, L.T.; Jeste, D.V. Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophr. Res. 2016, 181, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Proma, M.A.; Sohel, D.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Monocyte chemoattractant protein-1 levels are associated with major depressive disorder. J. Basic Clin. Physiol. Pharmacol. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Martínez, H.R.; Escamilla-Ocañas, C.E.; Camara-Lemarroy, C.R.; González-Garza, M.T.; Moreno-Cuevas, J.; García Sarreón, M.A. Increased cerebrospinal fluid levels of cytokines monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1β (MIP-1β) in patients with amyotrophic lateral sclerosis. Neurologia 2020, 35, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Cai, C.; Xu, Y.; Jiang, Y.; Chen, Z. Cerebrospinal fluid chemokine patterns in children with enterovirus 71-related encephalitis. Sci. Rep. 2018, 8, 1658. [Google Scholar] [CrossRef]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Brown, N.K.; Wu, W.; Khedri, Z.; Yu, H.; Chen, X.; van de Vlekkert, D.; D′Azzo, A.; Zheng, P.; Liu, Y. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. eLife 2014, 3, e04066. [Google Scholar] [CrossRef]
- Müller, N.; Wagner, J.K.; Krause, D.; Weidinger, E.; Wildenauer, A.; Obermeier, M.; Dehning, S.; Gruber, R.; Schwarz, M.J. Impaired monocyte activation in schizophrenia. Psychiatry Res. 2012, 198, 341–346. [Google Scholar] [CrossRef]
- Torrey, E.F.; Peterson, M.R. The viral hypothesis of schizophrenia. Schizophr. Bull. 1976, 2, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Kulaga, S.S.; Miller, C.W.T. Viral respiratory infections and psychosis: A review of the literature and the implications of COVID-19. Neurosci. Biobehav. Rev. 2021, 127, 520–530. [Google Scholar] [CrossRef]
- Özdin, S.; Böke, Ö. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in different stages of schizophrenia. Psychiatry Res. 2018, 271, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chithanathan, K.; Fan, F.; Xiu, M.; Fan, H.; Cui, Y.; Zhang, P.; Yu, T.; Yang, F.; Tian, B.; et al. Monocytic Subsets and Their Signature Genes Differentially Impact Cortex and Cognition in First-Episode Schizophrenia. medRxiv 2021, 1–57. [Google Scholar] [CrossRef]
- Krause, D.L.; Wagner, J.K.; Wildenauer, A.; Matz, J.; Weidinger, E.; Riedel, M.; Obermeier, M.; Gruber, R.; Schwarz, M.; Müller, N. Intracellular monocytic cytokine levels in schizophrenia show an alteration of IL-6. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Frommberger, U.H.; Bauer, J.; Haselbauer, P.; Fräulin, A.; Riemann, D.; Berger, M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. Eur. Arch. Psychiatry Clin. Neurosci. 1997, 247, 228–233. [Google Scholar] [CrossRef]
- Lin, A.; Kenis, G.; Bignotti, S.; Tura, G.J.; De Jong, R.; Bosmans, E.; Pioli, R.; Altamura, C.; Scharpé, S.; Maes, M. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophr. Res. 1998, 32, 9–15. [Google Scholar] [CrossRef]
- Bechter, K.; Reiber, H.; Herzog, S.; Fuchs, D.; Tumani, H.; Maxeiner, H.G. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: Identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 2010, 44, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.E.; Lawther, A.J.; Webster, M.J.; Asai, M.; Kondo, Y.; Matsumoto, M.; Walker, A.K.; Weickert, C.S. Nuclear factor kappa B activation appears weaker in schizophrenia patients with high brain cytokines than in non-schizophrenic controls with high brain cytokines. J. Neuroinflamm. 2020, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Corsi-Zuelli, F.; Deakin, B. Impaired regulatory T cell control of astroglial overdrive and microglial pruning in schizophrenia. Neurosci. Biobehav. Rev. 2021, 125, 637–653. [Google Scholar] [CrossRef]
- Murphy, C.E.; Weickert, C.S. A new suspect in the unsolved case of neuroinflammation in schizophrenia. Mol. Psychiatry 2021, 26, 7105–7106. [Google Scholar] [CrossRef]
- Moseman, E.A.; Liang, X.; Dawson, A.J.; Panoskaltsis-Mortari, A.; Krieg, A.M.; Liu, Y.J.; Blazar, B.R.; Chen, W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004, 173, 4433–4442. [Google Scholar] [CrossRef] [Green Version]
- Owaki, T.; Asakawa, M.; Fukai, F.; Mizuguchi, J.; Yoshimoto, T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol. 2006, 177, 7579–7587. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Steinwald, V.; Guralnik, A.; Brüstle, A.; Kleemann, P.; Rosenplänter, C.; Decker, T.; Lohoff, M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008, 20, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsi-Zuelli, F.; Deakin, B.; de Lima, M.H.F.; Qureshi, O.; Barnes, N.M.; Upthegrove, R.; Louzada-Junior, P.; Del-Ben, C.M. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav. Immun. Health 2021, 17, 100330. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J. The IL-2—IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clin. Immunol. 2020, 218, 108515. [Google Scholar] [CrossRef]
- Russell, S.E.; Moore, A.C.; Fallon, P.G.; Walsh, P.T. Soluble IL-2Rα (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLoS ONE 2012, 7, e47748. [Google Scholar] [CrossRef]
- Maxeiner, H.G.; Rojewski, M.T.; Schmitt, A.; Tumani, H.; Bechter, K.; Schmitt, M. Flow cytometric analysis of T cell subsets in paired samples of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Brain Behav Immun. 2009, 23, 134–142. [Google Scholar] [CrossRef]
- Kroken, R.A.; Sommer, I.E.; Steen, V.M.; Dieset, I.; Johnsen, E. Constructing the Immune Signature of Schizophrenia for Clinical Use and Research; An Integrative Review Translating Descriptives Into Diagnostics. Front. Psychiatry 2019, 9, 753. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Scaini, G.; Colpo, G.D.; Doifode, T.; Pinjari, O.F.; Teixeira, A.L.; Petronilho, F.; Macêdo, D.; Quevedo, J.; Barichello, T. Clozapine Prevents Poly (I:C) Induced Inflammation by Modulating NLRP3 Pathway in Microglial Cells. Cells 2020, 9, 577. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rish, E.Y.; Mansour, A.T.; Mansour, H.T.; Dahabiyeh, L.A.; Aleidi, S.M.; Bustanji, Y. Pregabalin inhibits in vivo and in vitro cytokine secretion and attenuates spleen inflammation in Lipopolysaccharide/Concanavalin A-induced murine models of inflammation. Sci. Rep. 2020, 10, 4007. [Google Scholar] [CrossRef] [Green Version]
- Westwell-Roper, C.; Stewart, S.E. Commentary: Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Front. Psychiatry 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Neutrophil–Lymphocyte Ratio (NLR) [Median] | Lymphocyte–Monocyte Ratio (LMR) [Median] | |
|---|---|---|
| AF | 2.06 (range 0.56–6.62) | 3.37 (range 1.71–6.80) |
| SZ | 2.34 (range 0.64–14.57) | 3.07 (range 1.19–9.01) (* vs. HDs, p = 0.02) |
| HDs | 2.44 (range 0.33–4.93) | 4.06 (range 1.44–10.23) |
| Group | ICD-10 Class | Total | Male | Female | Mean Age |
|---|---|---|---|---|---|
| Affective spectrum disorder (AF) | F30-F33 | n = 29 | n = 13 | n = 16 | 41 (range 28–69) |
| Schizophrenic spectrum disorder (SZ) | F20-F25 | n = 39 | n = 22 | n = 17 | 35 (range 20–65) |
| Subarachnoid hemorrhage (SAH) | I60 | n = 5 | n = 2 | n = 3 | 51 (range 40–68) |
| Healthy donors (HDs) 1 | - | n = 44 | n = 19 | n = 23 | 34 (range 18–68) |
| HLH/MAS | D76.1 | n = 7 | n = 3 | n = 4 | 30 (range 15–44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiber, C.; Schulz, T.; Schneider, J.M.; Bechter, K.; Schneider, E.M. Old and New Biomarkers for Infection, Inflammation, and Autoimmunity in Treatment-Resistant Affective and Schizophrenic Spectrum Disorders. Pharmaceuticals 2022, 15, 299. https://doi.org/10.3390/ph15030299
Scheiber C, Schulz T, Schneider JM, Bechter K, Schneider EM. Old and New Biomarkers for Infection, Inflammation, and Autoimmunity in Treatment-Resistant Affective and Schizophrenic Spectrum Disorders. Pharmaceuticals. 2022; 15(3):299. https://doi.org/10.3390/ph15030299
Chicago/Turabian StyleScheiber, Christian, Tanja Schulz, Julian M. Schneider, Karl Bechter, and E. Marion Schneider. 2022. "Old and New Biomarkers for Infection, Inflammation, and Autoimmunity in Treatment-Resistant Affective and Schizophrenic Spectrum Disorders" Pharmaceuticals 15, no. 3: 299. https://doi.org/10.3390/ph15030299
APA StyleScheiber, C., Schulz, T., Schneider, J. M., Bechter, K., & Schneider, E. M. (2022). Old and New Biomarkers for Infection, Inflammation, and Autoimmunity in Treatment-Resistant Affective and Schizophrenic Spectrum Disorders. Pharmaceuticals, 15(3), 299. https://doi.org/10.3390/ph15030299