From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19
Abstract
:1. Introduction
2. Alginate: Origin and Properties
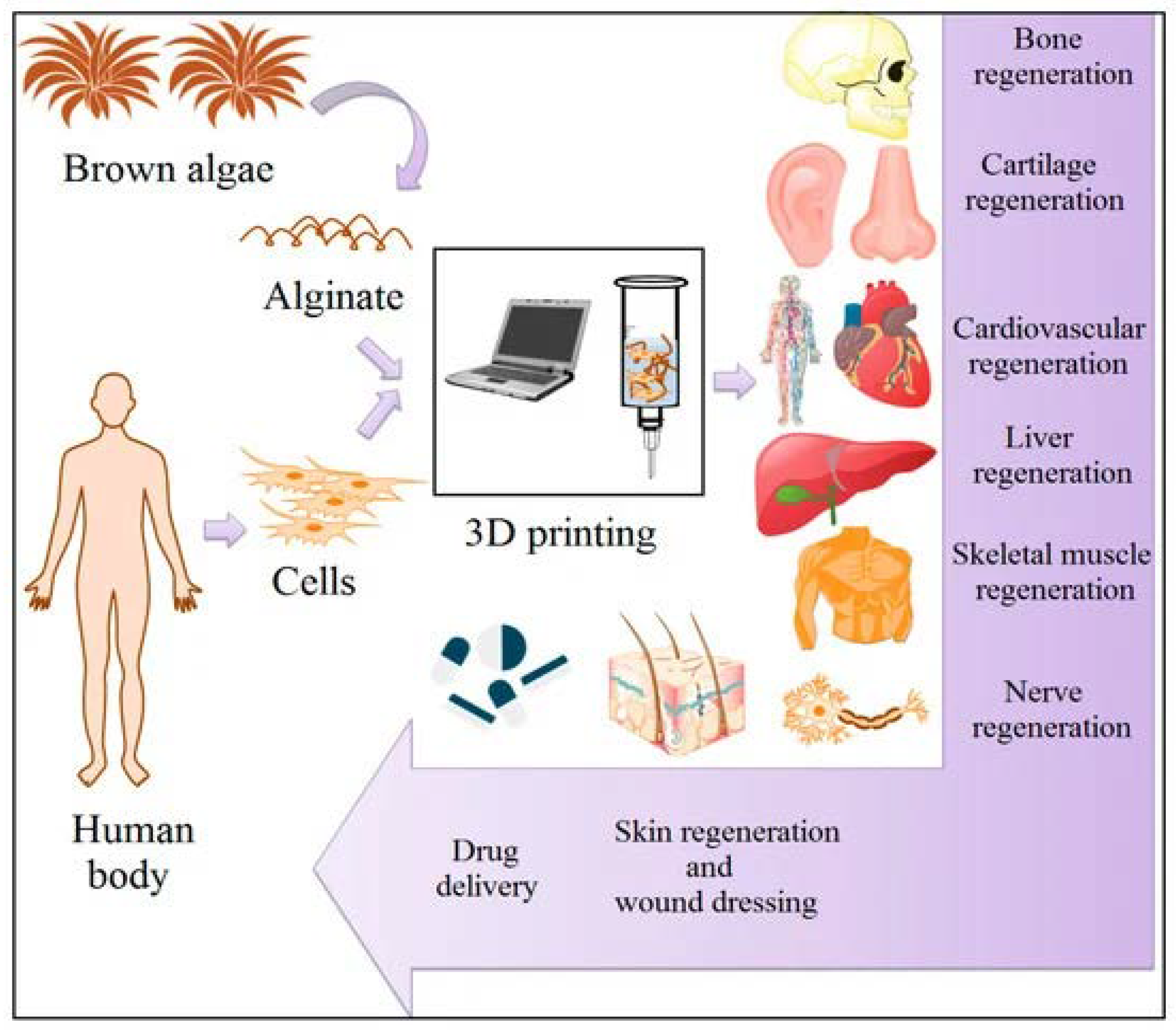
3. Biomedical Applications of Alginate
4. Drug Delivery Applications of Alginate
5. Applications of Alginate in CVDs
6. COVID-19 Consequences in CVD Patients
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Cardiovascular Diseases (CVDs). Available online: www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Elflein, J. Coronavirus (COVID-19) Disease Pandemic—Statistics Facts. Available online: https://www.statista.com/topics/5994/the-coronavirus-disease-covid-19-outbreak/ (accessed on 11 January 2022).
- Shehata, A.A.; Parvin, R.; Nagy, A.; Wang, Y.; Azhar, T.M.; Attia, Y.A.; Azhar, E.I.; Paul, A.K.; Rahmatullah, M. An overview of the ongoing challenges in SARS-CoV-2 global control. Ger. J. Microbiol. 2021, 1, 1–18. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, M.; Zhang, J.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; Liu, J.; Wan, J. Advances in the relationship between coronavirus infection and cardiovascular diseases. Biomed. Pharmacother. 2020, 127, 110230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; Chapter 7. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [Green Version]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Negar, K.H.; Nafisef, B.; Nasim, N.; Mojdeh, S. Reduced graphene oxide facilitates biocompatibility of alginate for cardiac repair. J. Bioact. Compat. Polym. 2020, 35, 363–377. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Yahav, M.; Ahmadi, Y. Alginates: Source, chemistry, and properties. In Alginates—Versatile Polymers in Biomedical Applications and Therapeutics; Hasnain, S., Kumar, A., Eds.; Taylor Francis Group (Apple Academic Press Inc.): Burlington, ON, Canada, 2019; Chapter 1; pp. 1–24. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial biopolymer formation from sodium alginate and algae extract using aminoglycosides. PLoS ONE 2019, 14, e0214411. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ko, S.C.; Oh, G.W.; Heo, S.Y.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Choi, S.W.; Jung, W.K. Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. Int. J. Biol. Macromol. 2016, 93, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Smidsrod, O.; Skjak-Bræk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Linker, A.; Jones, R.S. A New Polysaccharide Resembling Alginic Acid Isolated from Pseudomonads. J. Biol. Chem. 1966, 241, 3845–3851. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Nivens, D.E.; Ohman, D.E.; Williams, J.; Franklin, M.J. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 2001, 183, 1047–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, I.D.; Gatland, K.; Campisano, A.; Jordens, J.Z.; Rehm, B.H. Impact of alginate overproduction on attachment and biofilm architecture of a supermucoid Pseudomonas aeruginosa strain. Appl. Environ. Microbiol. 2009, 75, 6022–6025. [Google Scholar] [CrossRef] [Green Version]
- Sabra, W.; Zeng, A.P. Microbial Production of Alginates: Physiology and Process Aspects. In Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–173. [Google Scholar] [CrossRef]
- Cote, G.L.; Krull, L.H. Characterization of the Exocellular Polysaccharides from Azotobacter chroococcum. Carbohydr. Res. 1988, 181, 143–152. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from algae. In Polysaccharides and Polyamides in the Food Industry: Properties, Production, and Patents; Steinbüchel, A., Rhee, S.K., Eds.; Wiley: Weinheim, Germany, 2005; pp. 1–30. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; p. 441. [Google Scholar]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B. Recent advances in alginates as material for biomedical applications. In Alginates—Versatile Polymers in Biomedical Applications and Therapeutics; Yahav, M., Ahmadi, Y., Eds.; Taylor Francis Group (Apple Academic Press Inc.): Burlington, ON, Canada, 2019; Chapter 2; pp. 25–87. [Google Scholar]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Milivojevic, M.; Pajić-Lijaković, I.; Levic, S.; Nedovic, V.; Bugarski, B. Alginic Acid: Sources, Modifications and Main Applications. In Alginic Acid: Chemical Structure, Uses and Health Benefits; Moore, A., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 45–88. [Google Scholar]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.; Leong, K.W. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jay, S.M.; Saltzman, W.M. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. J. Control. Release 2009, 134, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.J.; Min, G.H. Oral Controlled Release of Melatonin Using Polymer-Reinforced and Coated Alginate Beads. Int. J. Pharm. 1996, 144, 37–46. [Google Scholar] [CrossRef]
- Edelman, E.R.; Nathan, A.; Katada, M.; Gates, J.; Karnovsky, M.J. Perivascular Graft heparin delivery using biodegradable polymer wraps. Biomaterials 2000, 21, 2279–2286. [Google Scholar] [CrossRef]
- Chen, A.Z.; Chen, M.Y.; Wang, S.B.; Huang, X.N.; Liu, Y.G.; Chen, Z.X. Poly(L-histidine)-chitosan/alginate complex microcapsule as a novel drug delivery agent. J. Appl. Polym. Sci. 2012, 124, 3728–3736. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.B.; Kim, S.J.; Lee, Y.M. Rapid Temperature pH Response of Porous Alginate-g-poly(N-isopropylacrylamide) Hydrogels. Polymer 2002, 43, 7549–7558. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Zorzin, L.; Cocchietto, M.; Voinovich, D.; Marcuzzi, A.; Fnipovic-Grcic, J.; Mulloni, C.; Crembiale, G.; Casarsa, C.; Bulla, R.; Sava, G. Lysozyme-containing chitosan-coated alginate microspheres for oral immunisation. J. Drug Deliv. Sci. Technol. 2006, 16, 413–420. [Google Scholar] [CrossRef]
- Park, H.; Kim, P.H.; Hwang, T.; Kwon, O.J.; Park, T.J.; Choi, S.W.; Yun, C.O.; Kim, J.H. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. Int. J. Pharm. 2012, 427, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.; Ribeiro, A.J.; Ferreira, D.; Veiga, F. Insulin encapsulation in reinforced alginate microspheres prepared by internal gelation. Eur. J. Pharm. Sci. 2006, 29, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orive, G.; Hernandez, R.M.; Rodriguez Gascon, A.; Calafiore, R.; Chang, T.M.; de Vos, P.; Hortelano, G.; Hunkeler, D.; Lacik, I.; Pedraz, J.L. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004, 22, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Review paper: Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef]
- Leor, J.; Amsalem, Y.; Cohen, S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol. Ther. 2005, 105, 151–163. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering Part 1. Structure, Gelation Rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [Green Version]
- Boland, T.; Tao, X.; Damon, B.J.; Manley, B.; Kesari, P.; Jalota, S.; Bhaduri, S. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater. Sci. Eng. C 2007, 27, 372–376. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Lu, W.W.; Chan, D.; Cheung, K.M.; Liu, W.G.; Zhao, F.; Li, Z.Y.; Leong, J.C.; Luk, K.D. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem. Biophys. Res. Commun. 2006, 347, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chung, H.Y.; Shin, H.I.; Park, D.J.; Choi, J.H. Osteogenic activity of chitosan-based hybrid scaffold prepared by polyelectrolyte complex formation with alginate. Tissue Eng. Regen. Med. 2014, 11, 106–112. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Ivanov, R.V.; Petrenko, A.Y.; Lozinsky, V.I. Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2011, 22, 1529–1540. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.H.; Kim, J.H.; Lee, J.H.; Park, J.H.; Knowles, J.C.; Kim, H.W. Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part A 2014, 20, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Schroder, H.C.; Grebenjuk, V.; Diehl-Seifert, B.; Mailander, V.; Steffen, R.; Schlossmacher, U.; Muller, W.E. The marine sponge-derived inorganic polymers, biosilica and polyphosphate, as morphogenetically active matrices/scaffolds for the differentiation of human multipotent stromal cells: Potential application in 3D printing and distraction osteogenesis. Mar. Drugs 2014, 12, 1131–1147. [Google Scholar] [CrossRef] [Green Version]
- Mouriño, V.; Newby, P.; Boccaccini, A.R. Preparation and Characterization of Gallium Releasing 3-D Alginate Coated 45S5 Bioglass® Based Scaffolds for Bone Tissue Engineering. Adv. Eng. Mater. 2010, 12, B283–B291. [Google Scholar] [CrossRef]
- Nakaoka, R.; Hirano, Y.; Mooney, D.J.; Tsuchiya, T.; Matsuoka, A. Study on the potential of RGD- and PHSRN-modified alginates as artificial extracellular matrices for engineering bone. J. Artif. Organs 2013, 16, 284–293. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martinez Avila, H.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bierwolf, J.; Lutgehetmann, M.; Deichmann, S.; Erbes, J.; Volz, T.; Dandri, M.; Cohen, S.; Nashan, B.; Pollok, J.M. Primary human hepatocytes from metabolic-disordered children recreate highly differentiated liver-tissue-like spheroids on alginate scaffolds. Tissue Eng. Part A 2012, 18, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Mashayekhan, S.; Fadaoddini, S.; Haghirsharifzamini, Y. Design, fabrication and characterization of oxidized alginate-gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016, 31, 152–161. [Google Scholar] [CrossRef]
- Solovieva, E.V.; Fedotov, A.Y.; Mamonov, V.E.; Komlev, V.S.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2018, 13, 025007. [Google Scholar] [CrossRef] [Green Version]
- Galateanu, B.; Dimonie, D.; Vasile, E.; Nae, S.; Cimpean, A.; Costache, M. Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnol. 2012, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Ruvinov, E.; Sapir, Y.; Cohen, S. Cardiac Tissue Engineering: Principles, Materials, and Applications; Morgan Claypool Publishers: Davis, CA, USA, 2012; Volume 4. [Google Scholar]
- Zia, K.M.; Zia, F.; Zuber, M.; Rehman, S.; Ahmad, M.N. Alginate based polyurethanes: A review of recent advances and perspective. Int. J. Biol. Macromol. 2015, 79, 377–387. [Google Scholar] [CrossRef]
- Huang, Q.; Cai, Y.; Zhang, X.; Liu, J.; Liu, Z.; Li, B.; Wong, H.; Xu, F.; Sheng, L.; Sun, D.; et al. Aligned Graphene Mesh-Supported Double Network Natural Hydrogel Conduit Loaded with Netrin-1 for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 112–122. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Niu, C.; Zhang, L.; Li, G.; Yang, Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J. Biomed. Mater. Res. A 2018, 106, 1951–1964. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Voicu, G.; Ficai, A.; Hoteteu, M. Montmorillonite-alginate nanocomposite as a drug delivery system incorporation and in vitro release of irinotecan. Int. J. Pharm. 2014, 463, 184–192. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Thirawong, N.; Korkerd, K. Swelling, erosion and release behavior of alginate-based matrix tablets. Eur. J. Pharm. Biopharm. 2007, 66, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Liu, T.; Wu, Y.; Guo, H.; Wang, P.; Tian, Q.; Wang, Y.; Yuan, Z. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials 2012, 33, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.D.; Bresson, R.; Charpentier, P.; Coliche, V.; Erlher, S.; Ha Van, G.; Magalon, G.; Martini, J.; Moreau, Y.; Pradines, S.; et al. Efficacy and tolerance of calcium alginate versus vaseline gauze dressings in the treatment of diabetic foot lesions. Diabetes Metab. 2002, 28, 223–229. [Google Scholar] [PubMed]
- Lansdown, A.B. Calcium: A Potential Central Regulator in Wound Healing in the Skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef]
- Iliescu, R.I.; Andronescu, E.; Ghitulica, C.D.; Berger, D.; Ficai, A. Montmorillonite-alginate nanocomposite beads as drug carrier for oral administration of carboplatin—Preparation and characterization. UPB Bull. 2011, 73, 2–16. [Google Scholar]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Gaumann, A.; Laudes, M.; Jacob, B.; Pommersheim, R.; Laue, C.; Vogt, W.; Schrezenmeir, J. Effect of Media Composition on Long-Term in Vitro Stability of Barium Alginate and Polyacrylic Acid Multilayer Microcapsules. Biomaterials 2000, 21, 1911–1917. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Preparation and characterization of a novel pH-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr. Polym. 2009, 78, 731–737. [Google Scholar] [CrossRef]
- Chatfieid, S. A Comparison of the Efficacy of the Alginate Preparation, Gaviscon Advance, with Placebo in the Treatment of Gastro-Oesophageal Reflux Disease. Curr. Med. Res. Opin. 1999, 15, 152–159. [Google Scholar] [CrossRef]
- Sutton, M.G.S.J.; Sharpe, N. Left Ventricular Remodeling After Myocardial Infarction: Pathophysiology and Therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, H.; Wang, H.; Wei, Y.; Hu, S. Artificial Matrix Helps Neonatal Cardiomyocytes Restore Injured Myocardium in Rats. Artif. Organs 2006, 30, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Christman, K.L.; Lee, R.J. Biomaterials for the treatment of myocardial infarction. J. Am. Coll. Cardiol. 2006, 48, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, V.F.; Lee, R.T. Biomaterials to enhance stem cell function in the heart. Circ. Res. 2011, 109, 910–922. [Google Scholar] [CrossRef]
- Levit, R.D.; Landazuri, N.; Phelps, E.A.; Brown, M.E.; Garcia, A.J.; Davis, M.E.; Joseph, G.; Long, R.; Safley, S.A.; Suever, J.D.; et al. Cellular encapsulation enhances cardiac repair. J. Am. Heart Assoc. 2013, 2, e000367. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 283–590. [Google Scholar] [CrossRef]
- Zmora, S.; Glicklis, R.; Cohen, S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials 2002, 23, 4087–4094. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.N.H.; Mehdinavaz Aghdam, R.; Ebrahimi, S.A.S.; Chehrehsaz, Y. Tri-layered alginate/PCL electrospun scaffold for cardiac tissue engineering. Polym. Int. 2022. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res. Ther. 2016, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majka, M.; Sułkowski, M.; Badyra, B.; Musiałek, P. Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl. Med. 2017, 6, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Kim, S.W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.W.; Williams, D.R.; Lee, J.Y. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: Mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials 2019, 225, 119513. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.P.; Wang, X.; Moura, F.A.; Siddiqi, H.K.; Morrow, D.A.; Bohula, E.A. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 2020, 226, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ertas, Y.N.; Mahmoodi, M.; Shahabipour, F.; Jahed, V.; Diltemiz, S.E.; Tutar, R.; Ashammakhi, N. Role of biomaterials in the diagnosis, prevention, treatment, and study of corona virus disease 2019 (COVID-19). Emergent Mater. 2021, 4, 1–21. [Google Scholar] [CrossRef]
- Shpichka, A.; Bikmulina, P.; Peshkova, M.; Kosheleva, N.; Zurina, I.; Zahmatkesh, E.; Khoshdel-Rad, N.; Lipina, M.; Golubeva, E.; Butnaru, D. Engineering a model to study viral infections: Bioprinting, microfluidics, and organoids to defeat coronavirus disease 2019 (COVID-19). Int. J. Bioprint. 2020, 6, 302. [Google Scholar] [CrossRef]
- Bhowmick, R.; Derakhshan, T.; Liang, Y.; Ritchey, J.; Liu, L.; Gappa-Fahlenkamp, H. A Three-Dimensional Human Tissue-Engineered Lung Model to Study Influenza A Infection. Tissue Eng. Part A 2018, 24, 1468–1480. [Google Scholar] [CrossRef]
- Mastromarinoa, P.; Petruzzielloa, R.; Macchiaa, S.; Rietia, S.; Nicolettib, R.; Orsi, N. Antiviral activity of natural and semisynthetic polysaccharides on the early steps of rubella virus infection. J. Antimicrob. Chemother. 1997, 33, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Elfiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- El-Sikaily, A.; Helal, M.; Saad, A. Enhancement of immune tolerance of COVID-19 patients might be achieved with alginate supplemented therapy. Int. J. Cancer Biomed. Res. (IJCBR) 2020, 4, 21–26. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020, 18, 784–790. [Google Scholar] [CrossRef]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Cheema, S.U.R.; Rehman, M.S.; Hussain, G.; Cheema, S.S.; Gilani, N. Efficacy and tolerability of sofosbuvir and daclatasvir for treatment of hepatitis C genotype 1 3 in patients undergoing hemodialysis—A prospective interventional clinical trial. BMC Nephrol 2019, 20, 438. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Huang, B.; Ling, R.; Cheng, Y.; Wen, J.; Dai, Y.; Huang, W.; Zhang, S.; Lu, X.; Luo, Y.; Jiang, Y.Z. Characteristics of the Coronavirus Disease 2019 and related Therapeutic Options. Mol. Methods Clin. Dev. 2020, 18, 367–375. [Google Scholar] [CrossRef]
- He, Q.; Cui, Y.; Li, J. Molecular assembly and application of biomimetic microcapsules. Chem. Soc. Rev. 2009, 38, 2292–2303. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, eaau0810. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Chellappan, D.K.; Dua, K.; Mehta, M.; Satija, S.; Singh, I. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. Expert Opin. Pat. 2020, 30, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Lazaridis, C.; Vlachogiannis, N.I.; Bakogiannis, C.; Spyridopoulos, I.; Stamatelopoulos, K.; Kanakakis, I.; Vassilikos, V.; Stellos, K. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hell. J. Cardiol. 2020, 61, 381–395. [Google Scholar] [CrossRef]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7–11. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Ganatra, S.; Dani, S.S.; Shah, S.; Asnani, A.; Neilan, T.G.; Lenihan, D.; Ky, B.; Barac, A.; Hayek, S.S.; Leja, M.; et al. Management of Cardiovascular Disease During Coronavirus Disease (COVID-19) Pandemic. Trends Cardiovasc. Med. 2020, 30, 315–325. [Google Scholar] [CrossRef]
- Mahajan, K.; Chandra, K.S. Cardiovascular comorbidities and complications associated with coronavirus disease 2019. Med. J. Armed Forces India 2020, 76, 253–260. [Google Scholar] [CrossRef]
- Kunal, S.; Sharma, S.M.; Sharma, S.K.; Gautam, D.; Bhatia, H.; Mahla, H.; Sharma, S.; Bhandari, S. Cardiovascular complications and its impact on outcomes in COVID-19. Indian Heart J. 2020, 72, 593–598. [Google Scholar] [CrossRef]
- Boukhris, M.; Hillani, A.; Moroni, F.; Annabi, M.S.; Addad, F.; Ribeiro, M.H.; Mansour, S.; Zhao, X.; Ybarra, L.F.; Abbate, A.; et al. Cardiovascular Implications of the COVID-19 Pandemic: A Global Perspective. Can. J. Cardiol. 2020, 36, 1068–1080. [Google Scholar] [CrossRef] [PubMed]

| No. | Alginate-Based Materials | Potential Applications | Findings | References |
|---|---|---|---|---|
| 1 | Alginate | Pharmaceutical | The development of novel polymers is useful for medical drugs. | [5,29,66,67] |
| 2 | Alginate gel | Cardiovascular | Alginate can be applied in the design of solutions for the treatment of cardiovascular diseases and the creation of heart valves, blood vessels, and drug and stem cell delivery systems. | [6] |
| 3 | Alginate hydrogels | Wound healing dressings | Composite hydrogels were developed as a wound dressing for healing wounds. | [7,8] |
| 4 | Alginate hydrogels | Cardiac tissue engineering | Alginate hydrogels were used for the fabrication of heart valve tissue engineering. | [9,66] |
| 5 | Alginate hydrogels | Tissue engineering | Injectable alginate hydrogels were used for cell delivery in tissue engineering. | [10] |
| 6 | Alginate–reduced graphene oxide | Cardiac repair | The prepared alginate–reduced GO electroactive hydrogel was used as a platform for stem cell therapy. | [11] |
| 7 | Alginate matrices | Drug delivery | Alginate polymeric matrices were developed for drug delivery applications. | [12] |
| 8 | Alginate polymers | Biomedical | Pseudomonads cultured from several cases of cystic fibrosis were described. | [13,18] |
| 9 | Alginate films | Food | Biodegradable polysaccharides have the potential for food packaging applications. | [14,24] |
| 10 | Alginate matrices | Regenerative | Alginate formulations of porous scaffolding matrices of cell culture were developed for regenerative medicine applications. | [30,49] |
| 11 | Alginate matrices | Tissue engineering | Alginate polymers can serve as matrix delivery vehicles for gene carriers and tissue engineering scaffolds. | [31] |
| 12 | Alginate hydrogel | Drug delivery | Alginate-based polymers can function as oral delivery matrices for proteins. | [31,32] |
| 13 | Lysozyme chitosan–alginate microspheres | Pharmaceutical | Lysozyme-containing chitosan-coated alginate microspheres systems can be applied for oral immunization with microencapsulated antigens. | [39] |
| 14 | Alginate scaffolds | Myocardial tissue engineering | Alginate scaffolds were designed for myocardial tissue engineering. | [44] |
| 15 | Alginate hydrogels as scaffolds | Tissue engineering | Alginate gels and gel/cell systems were formulated for tissue engineering applications. | [45] |
| 16 | Alginate hydrogels as drug delivery carriers | Biomedical | Alginate hydrogels were studied as drug and cell carriers and as tissue engineering matrices. | [46] |
| 17 | Injectable calcium phosphate–alginate hydrogel–umbilical cord mesenchymal stem cell paste | Bone tissue engineering | The injectable stem cell construct is based on calcium phosphate–alginate hydrogel for bone tissue engineering. | [47] |
| 18 | Alginate–ceramic composite materials | Bone tissue engineering | Alginate encapsulated murine-derived adipose-tissue stromal cells may be suitable as injectable bone graft substitutes. | [50] |
| 19 | Core–shell fibrous collagen–alginate hydrogel | Bone tissue engineering | The newly designed core–shell collagen–alginate fibrous carrier enables the encapsulation of tissue cells and their delivery into damaged targeted tissue to promote bone tissue engineering. | [54] |
| 20 | Gallium 3D alginate-coated bioglass scaffolds | Bone tissue engineering | Novel gallium-loaded 45S5 bioglass-based scaffolds coated with alginate are a candidate for bone tissue engineering. | [56] |
| 21 | Polycaprolactone–alginate–chondrocyte scaffolds | Cartilage tissue engineering | Polycaprolactone–alginate–chondrocyte scaffold is an innovative cell-printed scaffold for cartilage regeneration fabricated by advanced bioprinting technology. | [58] |
| 22 | Nanocellulose–alginate | Cartilage tissue engineering | Nanocellulose–alginate bioink is a suitable hydrogel for 3D bioprinting of living tissues and organs. | [59] |
| 23 | Alginate scaffolds | Liver tissue engineering | Alginate scaffolds provide a favorable microenvironment for new liver tissue creation and regeneration. | [62] |
| 24 | Alginate–gelatin hydrogels | Muscle tissue engineering | Oxidized alginate–gelatin hydrogels could be a suitable candidate for muscle tissue engineering. | [63] |
| 25 | Fibrinogen-modified sodium alginate scaffolds | Skin tissue engineering | Thrombin-modified alginate sponges can be successfully used as a grafting material to promote skin healing and regeneration. | [64] |
| 26 | Alginate hydrogels | Adipose tissue engineering | Alginate hydrogel has promising applications in soft tissue engineering. | [65] |
| 27 | Alginate beads immobilized on a polyurethane matrix | Biomedical | Alginate-based polyurethane can modernize the food and biomedical industries. | [67] |
| 28 | Graphene mesh loaded with netrin-1 supported by alginate | Peripheral nerve regeneration | The hydrogel nerve scaffold can significantly promote the regeneration of peripheral nerves and restoration of denervated muscles. | [68] |
| 29 | Polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel | Peripheral nerve regeneration | The design and development of hydrogel scaffolds provide an important experimental basis for nerve tissue engineering applications. | [69] |
| 30 | Alginate hydrogels in regenerative and therapeutic medicine | Biomedical | Alginate hydrogels are solutions for creating heart valves, blood vessels, and drug/stem cell delivery vehicles. | [6] |
| 31 | Montmorillonite–alginate nanocomposite as drug delivery systems in chemotherapy | Biomedical | Nanocomposite beads based on montmorillonite–alginate may be a promising drug delivery system. | [70] |
| 32 | Alginate-based matrix | Pharmaceutical | Alginate-based matrix tablets were used in modified drug delivery formulations using metronidazole as a model drug. | [71] |
| 33 | Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles | Biomedical (clinical) | Heart and liver cells surrounding the tumor were not affected by drug intake. | [72] |
| 34 | Calcium alginate | Wound healing | Calcium alginate is more appropriate for topical treatment of diabetic foot lesions. | [73,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19. Pharmaceuticals 2022, 15, 318. https://doi.org/10.3390/ph15030318
Spoială A, Ilie C-I, Ficai D, Ficai A, Andronescu E. From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19. Pharmaceuticals. 2022; 15(3):318. https://doi.org/10.3390/ph15030318
Chicago/Turabian StyleSpoială, Angela, Cornelia-Ioana Ilie, Denisa Ficai, Anton Ficai, and Ecaterina Andronescu. 2022. "From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19" Pharmaceuticals 15, no. 3: 318. https://doi.org/10.3390/ph15030318
APA StyleSpoială, A., Ilie, C.-I., Ficai, D., Ficai, A., & Andronescu, E. (2022). From Biomedical Applications of Alginate towards CVD Implications Linked to COVID-19. Pharmaceuticals, 15(3), 318. https://doi.org/10.3390/ph15030318