Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations
Abstract
1. Introduction
2. Results and Discussion
2.1. Pre-Formulation Studies
2.2. Pre-Compression Parameters
2.3. Bulk Density
2.4. Tapped Density
2.5. Angle of Repose
2.6. Carr’s Index
2.7. Hausner’s Ratio
2.8. Post-Compression Parameters
2.9. Diameter
2.10. Thickness
2.11. Friability
2.12. Average Weight
2.13. Hardness
2.14. Assay and Content Uniformity
2.15. Disintegration Time
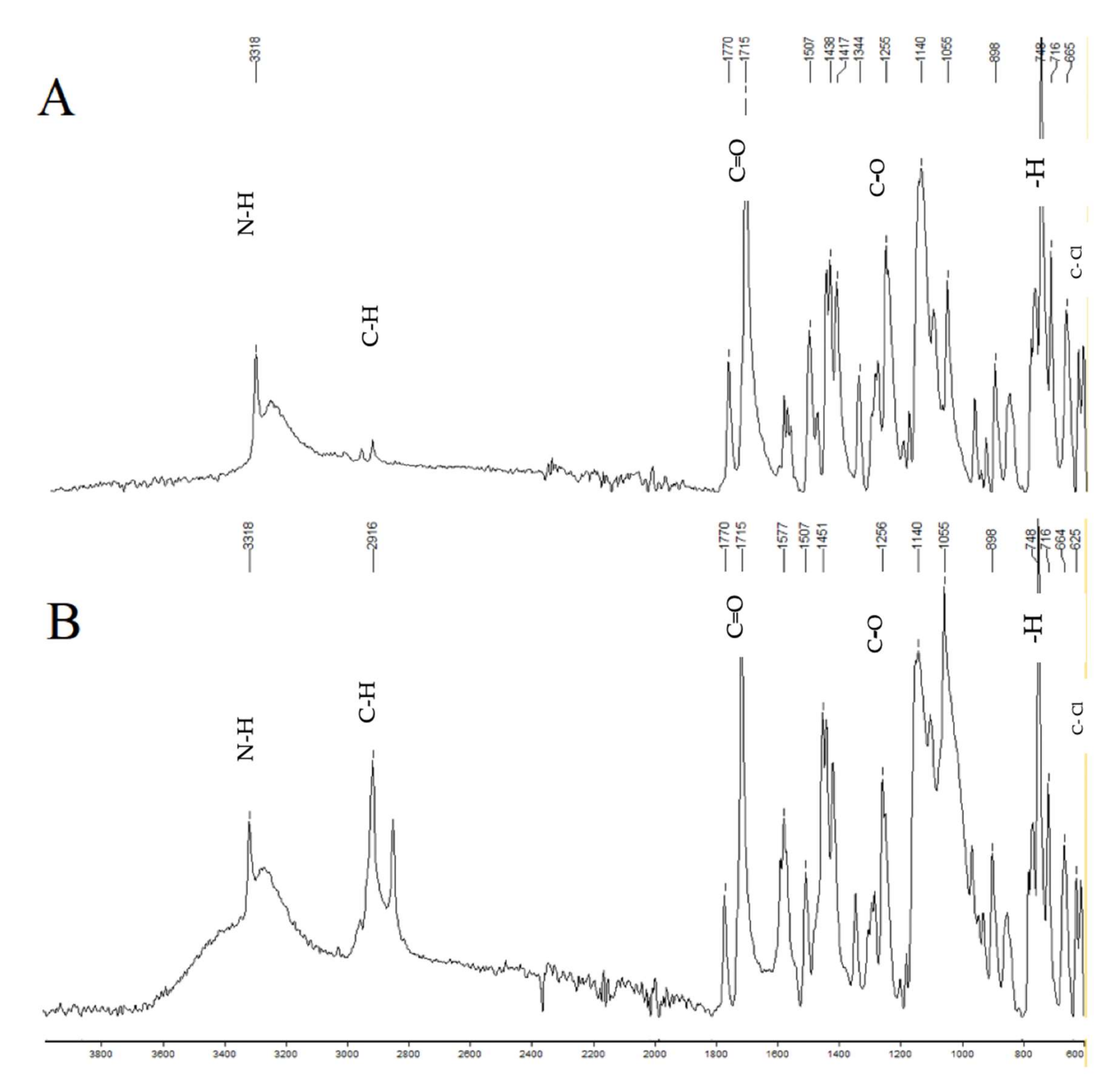
2.16. FTIR Studies
2.17. In Vitro Dissolution Studies
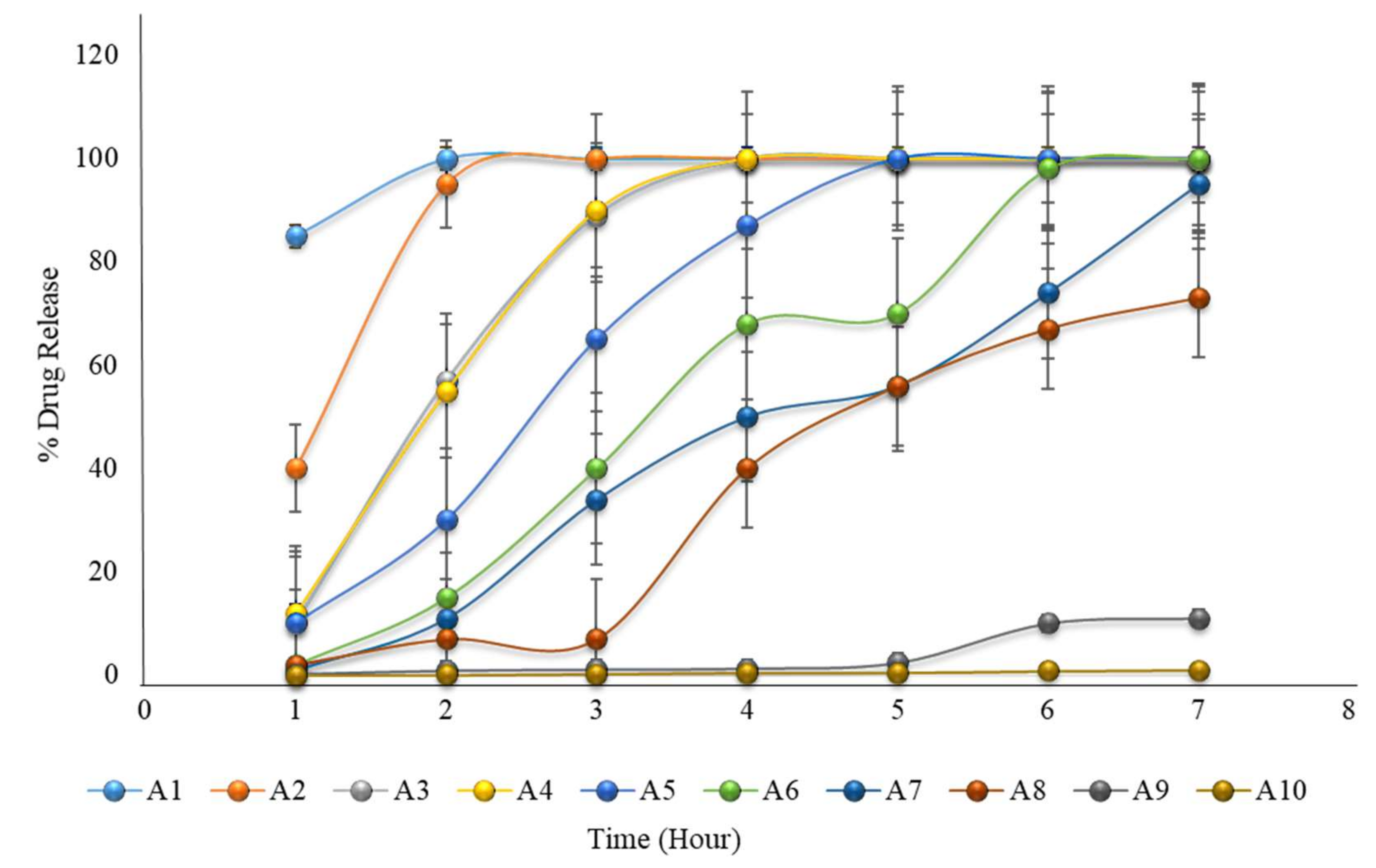
2.18. Formulation with HPMC K100M and Avicel PH102
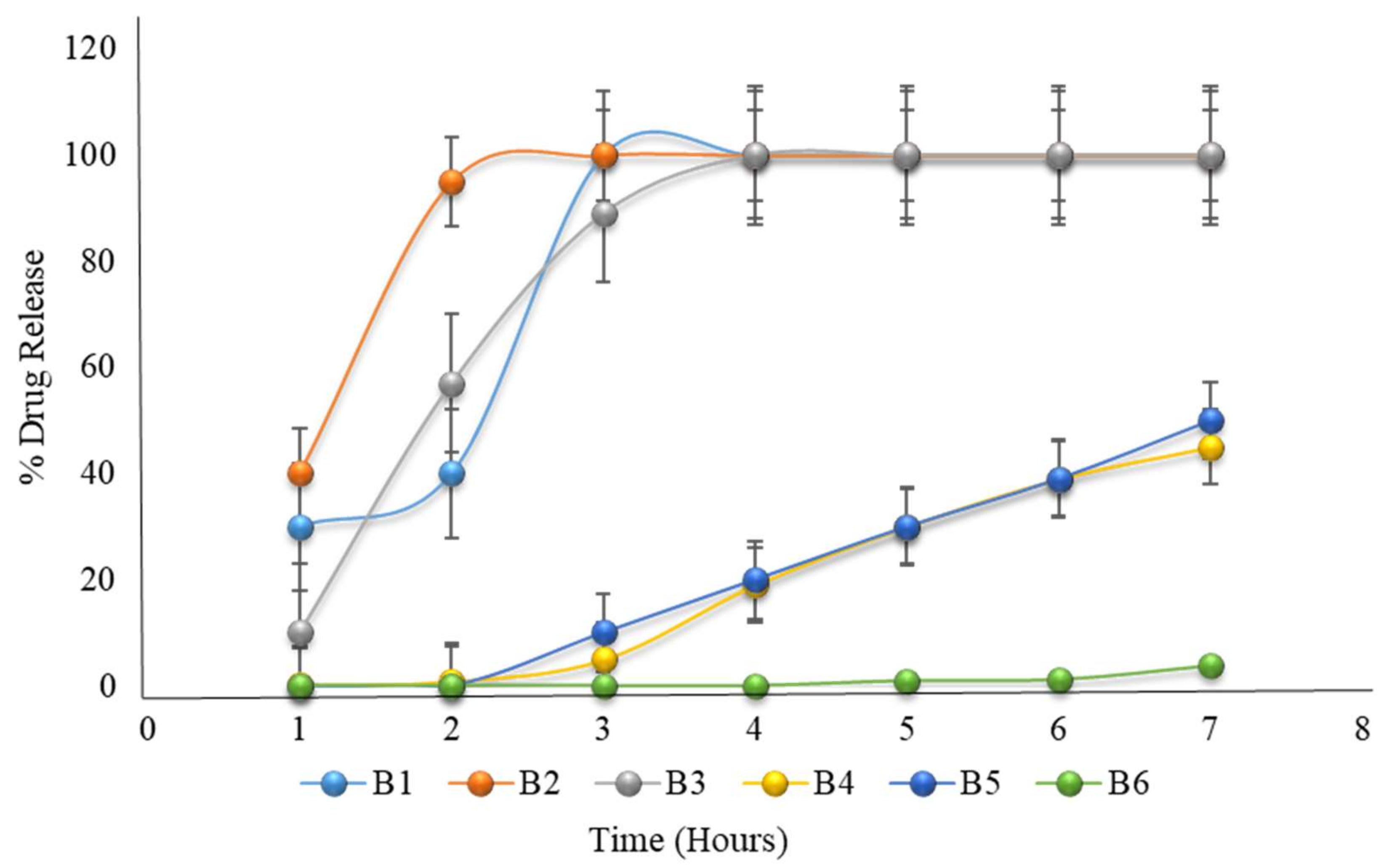
2.19. Formulation with HPMC K100M and Eudragit L100
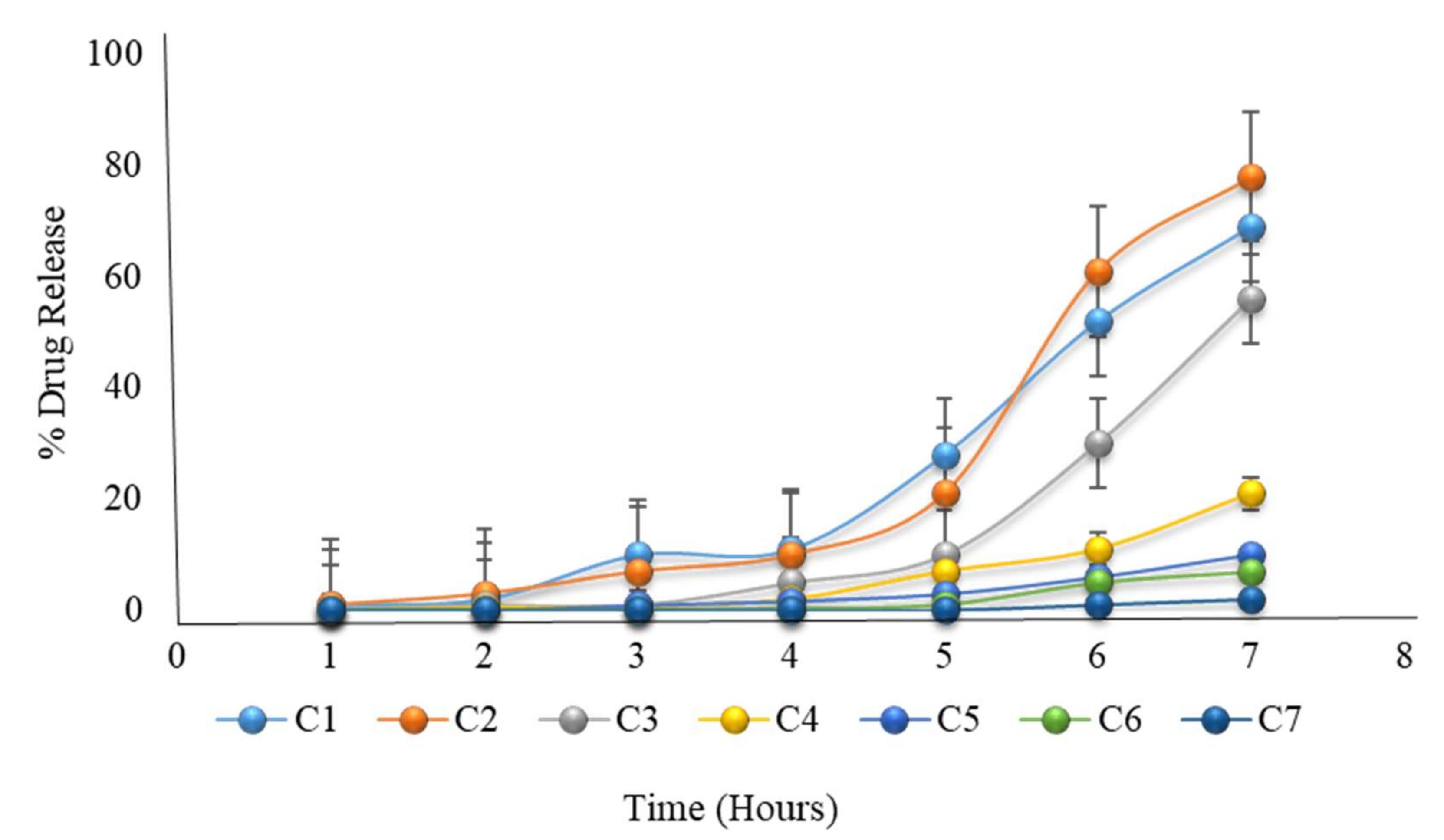
2.20. Formulation with HPMC K100M and Hydroxyethyl Cellulose
2.21. Formulation with HPMC K100M and HPMC E5 with Constant Coating Weight
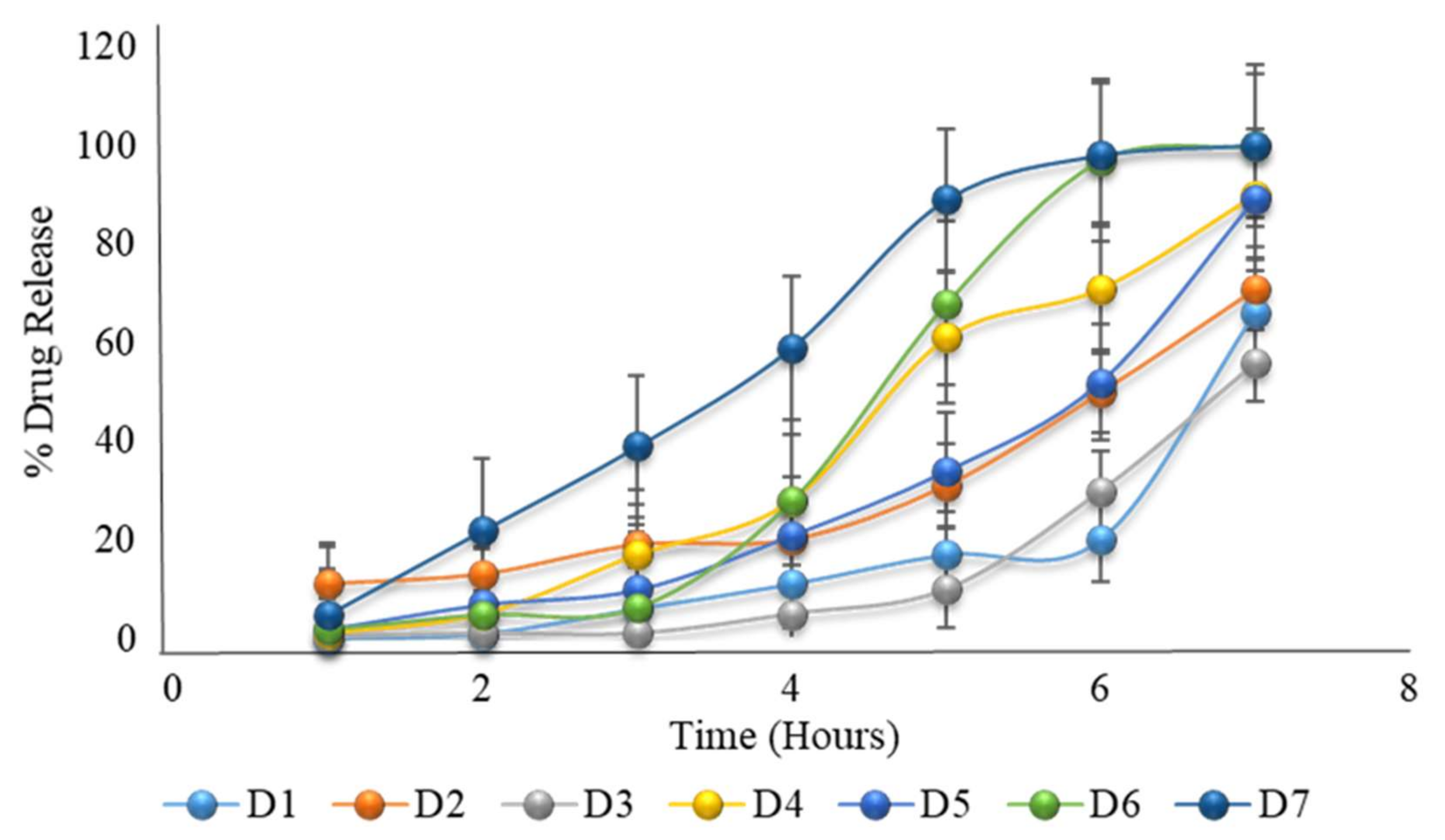
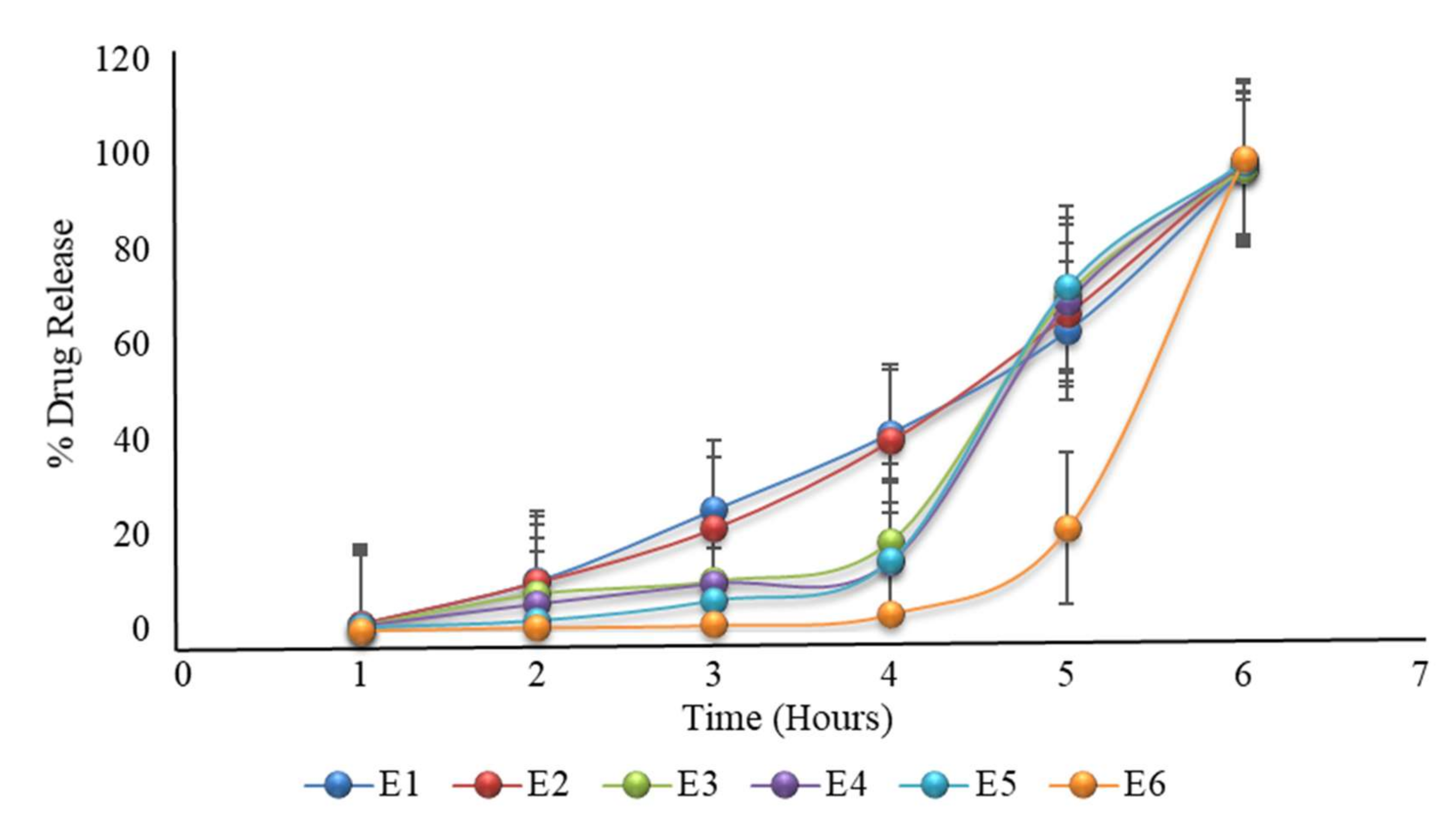
2.22. Formulation with HPMC K100M and HPMC E5 with Variable Coating Weight
2.23. Stability Studies
3. Methodology
3.1. Materials
3.2. Methods
3.3. Preparation of the Core Tablets
3.4. Preparation of the Press-Coated Tablets
3.5. Evaluation of the Powder Blend
3.5.1. Micromeritic Properties
3.5.2. Angle of Repose (θ)
3.5.3. Bulk Density
3.5.4. Tapped Density (Dt)
3.5.5. Carr’s Compressibility Index
3.6. Evaluation of Post-Compression Parameters
3.6.1. Hardness Test
3.6.2. Thickness
3.6.3. Friability Test
3.6.4. Weight Variation Test
3.6.5. Disintegration Time
3.6.6. Assay of Core Tablets
3.6.7. Drug Content Uniformity
3.6.8. In Vitro Dissolution Study
3.6.9. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, A.; Kondawar, M.; Shah, R. Formulation and evaluation of press-coated indomethacin tablets for pulsatile drug delivery system. J. Pharm. Res. 2011, 4, 564–566. [Google Scholar]
- Satani, R.; Chotaliya, M.; Raval, M.; Sheth, N. Review on recent trends in press-coated pulse drug delivery system. Int. Bull. Drug Res. 2014, 4, 60–91. [Google Scholar]
- Prasanth, V.; Mitesh, P.; Sam, T.; Abin, A. Formulation and evaluation of enteric coated time release press coated tablets of theophylline for chronopharmacotherapy. Pharm. Lett. 2012, 4, 599–606. [Google Scholar]
- Arslan, S.A.; Tirnaksiz, F. A nonsteroidal antiinflammatory drug: Aceclofenac. FABA J. Pharm. Sci. 2010, 35, 105–118. [Google Scholar]
- Bushra, R.; Shoaib, M.H.; Naeem, M.I.; Aslam, N. Aceclofenac: A new effective and safe NSAID. Int. J. Drug Deliv. Technol. 2013, 42, 1. [Google Scholar]
- Raza, K.; Kumar, M.; Kumar, P.; Malik, R.; Sharma, G.; Kaur, M.; Katare, O. Topical delivery of aceclofenac: Challenges and promises of novel drug delivery systems. BioMed Res. Int. 2014, 2014, 406731. [Google Scholar] [CrossRef]
- The British Pharmacopoeia Commission Secretariat of the Medicines and Healthcare Products Regulatory Agency (MHRA). British Pharmacopoeia; Appendix XIN; TSO: London, UK, 2015; Volume 336. [Google Scholar]
- Sackey, J.; Olowosulu, A.K.; Abdulsamad, A.; Gwary, S. Design and evaluation of time dependent delayed-release diclofenac sodium tablets for chronopharmaceutical drug delivery. Br. J. Pharm. 2019, 4, 3.1–3.10. [Google Scholar]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Geldart, D.; Abdullah, E.; Hassanpour, A.; Nwoke, L.; Wouters, I. Characterization of powder flowability using measurement of angle of repose. China Particuol. 2006, 4, 104–107. [Google Scholar] [CrossRef]
- The British Pharmacopoeia Commission Secretariat of the Medicines and Healthcare Products Regulatory Agency (MHRA). British Pharmacopoeia; TSO: London, UK, 2016. [Google Scholar]
- Solomon Hambisa, S.B.; Suleman, S. In vitro comparative quality assessment of different brands of norfloxacin tablets available in Jimma, Southwest Ethiopia. Drug Des. Dev. Ther. 2019, 13, 1241. [Google Scholar] [CrossRef]
- Al-anbagi, M.S.; Rajab, N.A.; Khalil, Y.I. Preparation and characterization of timed drug delivery system of sumatriptan using natural polymers. Iraqi J. Pharm. Sci. 2018, 27, 89–99. [Google Scholar] [CrossRef][Green Version]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Chauhan, D.; Shah, S. Formulation and evaluation of pulsatile drug delivery system of aceclofenac for treatment of rheumatoid arthritis. Int. J. Pharm. Pharm. Sci. 2012, 4, 507–512. [Google Scholar]
- Iffat, W.; Shoaib, M.H.; Yousuf, R.I.; Qazi, F.; Mahmood, Z.A.; Muhammad, I.N.; Ahmed, K.; Ahmed, F.R.; Imtiaz, M.S. Use of Eudragit RS PO, HPMC K100M, Ethyl Cellulose, and Their Combination for Controlling Nicorandil Release from the Bilayer Tablets with Atorvastatin as an Immediate-Release Layer. J. Pharm. Innov. 2020, 1–20. [Google Scholar] [CrossRef]
- Sabitha, G.; Punitha, S.; Saikrishna, K.; Uppuluru, A.K.; Anusha, J.; Swapna, R. Formulation and evaluation of aceclofenac Microbeads-A chronotherapeutic approach. J. Pharm. Res. 2011, 4, 4667–4671. [Google Scholar]
- Barzegar Jalali, M.; Siyahi Shadbad, M.R.; Barzegar-Jalai, A.; Adibkia, K.; Mohammadi, G.; Aghai, B.; Zeraati, M. Design and evaluation of delayed-release osmotic capsule of acetaminophen. Iran. J. Pharm. Sci. 2006, 2, 65–72. [Google Scholar]
- Mittal, S.; Sandip, K.; Jobin, J. Design, development and evaluation of pulsatile drug delivery system using tablet in tablet formulation. World J. Pharm. Sci. 2014, 1499–1505. [Google Scholar]
- Malpure, P.S.; Chaudhari, P.D.; Ajab, A.B.; Sanap, D.A.; Bhagat, H.D. Formulation and Evaluation of Enteric Coated Timed-Release Press-Coated Tablets for Colon Targeting. Indian J. Pharm. Educ. Res. 2009, 43, 63–70. [Google Scholar]
- Yasmin, R.; Shoaib, M.H.; Ahmed, F.R.; Qazi, F.; Ali, H.; Zafar, F. Aceclofenac fast dispersible tablet formulations: Effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics. PLoS ONE 2020, 15, e0223201. [Google Scholar]
- Savale, S.K. Formulation and Evalution of Aceclofenac Sustained Released Tablet. World J. Pharm. Pharm. Sci. 2016, 5, 592–598. [Google Scholar]
- Dave, V.; Yadav, S.; Sharma, S.; Vishwakarma, P.; Ali, N. Novel approach of aceclofenac fast dissolving tablet. Pak. J. Pharm. Sci. 2015, 28, 37–41. [Google Scholar] [PubMed]
- Khlebnikova, E. Statistical tools for process qualification. In Process Qualification Demonstrating Process Acceptability Lifecycle Approach to Process; Springer: Berlin/Heidelberg, Germany, 2012; Volume 93. [Google Scholar]
- Cartwright, A.C. The British Pharmacopoeia, 1864 To 2014: Medicines, International Standards and the State; Routledge: London, UK, 2016. [Google Scholar]
- Bhardwaj, S.; Jain, V.; Jat, R.; Mangal, A.; Jain, S. Formulation and evaluation of fast dissolving tablet of aceclofenac. Int. J. Drug Deliv. 2010, 2, 93–97. [Google Scholar] [CrossRef][Green Version]
- Harish, K.H.; Dinesh, M.; Jayanthi, C.; Joshi, H. In vitro dissolution studies of some commercial brands of aceclofenac tablets. World J. Pharm. Res. 2019, 8, 1341–1343. [Google Scholar]
- Kaur, M.; Kaur, G.; Kaur, H.; Sharma, S. Overview on stability studies. Int. J. Pharm. Chem. Biol. Sci. 2013, 3. [Google Scholar]
- Ojha, A.; Bhargava, S. International Council for Harmonisation (ICH) guidelines. In Regulatory Affairs in the Pharmaceutical Industry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–74. [Google Scholar]
| Description | Specification | Observation |
|---|---|---|
| Appearance | A white or off-white crystalline powder | Off-white crystalline powder |
| Identification | FTIR | Complies |
| Appearance of solution | Not more turbid than standard | Complies |
| Loss on drying | ≤0.5% | 0.2% |
| Melting point | 149–151 °C | 151 °C |
| Assay | 99.0–101.0% | 100.1% |
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Bulk Density (gm/cm3) | 0.55 ± 0.015 | 0.56 ± 0.02 | 0.56 ± 0.005 |
| Tapped Density (gm/cm3) | 0.60 ± 0.017 | 0.62 ± 0.05 | 0.61 ± 0.009 |
| Angle of Repose (θ) | 27 ± 0.01 | 27 ± 0.20 | 28 ± 0.02 |
| Carr’s Index (%) | 8.3 ± 0.012 | 9.67 ± 0.04 | 8.19 ± 0.05 |
| Hausner’s Ratio | 1.09 ± 0.02 | 1.11 ± 0.02 | 1.10 ± 0.03 |
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Diameter (mm) | 9.01 ± 0.01 | 9.00 ± 0.01 | 9.01 ± 0.01 |
| Thickness (mm) | 2.91 ± 0.02 | 2.96 ± 0.01 | 2.92 ± 0.011 |
| Hardness (kp) | 5.3 ± 0.17 | 5.56 ± 0.05 | 5.53 ± 0.05 |
| Average weight (mg) | 150.15 ± 1.53 | 150.1 ± 1.41 | 150.15 ± 0.81 |
| Friability (%) | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 |
| Assay (%) | 99.92 ± 0.04 | 99.94 ± 0.13 | 99.98 ± 0.01 |
| Content uniformity (%) | 99.87 ± 0.06 | 101.25 ± 0.07 | 99.97 ± 0.02 |
| Dissolution (%) | 98.91 ± 0.15 | 98.9 ± 0.14 | 99.95 ± 0.03 |
| Formulation | Thickness (mm) | Hardness (kp) | Average Weight (mg) | Friability (%) |
|---|---|---|---|---|
| A1 | 4.38 ± 0.05 | 13.4 ± 0.02 | 502 ± 0.5 | 0.6 ± 0.01 |
| A2 | 4.42 ± 0.07 | 11.8 ± 0.03 | 499 ± 1.1 | 0.6 ± 0.01 |
| A3 | 4.41 ± 0.08 | 13.1 ± 0.06 | 500 ± 0.7 | 0.5 ± 0.02 |
| A4 | 4.50 ± 0.06 | 11.4 ± 0.04 | 501 ± 1.0 | 0.7 ± 0.01 |
| A5 | 4.25 ± 0.11 | 10.1 ± 0.03 | 501 ± 0.4 | 0.4 ± 0.03 |
| A6 | 4.10 ± 0.07 | 12.5 ± 0.01 | 498 ± 0.07 | 0.4 ± 0.02 |
| A7 | 4.27 ± 0.04 | 12.0 ± 0.05 | 499 ± 0.13 | 0.6 ± 0.01 |
| A8 | 4.27 ± 0.05 | 13.7 ± 0.04 | 502 ± 0.6 | 0.6 ± 0.01 |
| A9 | 4.34 ± 0.07 | 13.2 ± 0.04 | 501 ± 0.09 | 0.6 ± 0.01 |
| A10 | 4.55 ± 0.04 | 13.5 ± 0.07 | 503 ± 0.5 | 0.4 ± 0.01 |
| B1 | 4.41 ± 0.02 | 12.1 ± 0.06 | 499 ± 0.42 | 0.5 ± 0.01 |
| B2 | 4.11 ± 0.04 | 12.3 ± 0.05 | 499 ± 0.33 | 0.4 ± 0.02 |
| B3 | 4.47 ± 0.03 | 11.7 ± 0.04 | 501 ± 0.09 | 0.5 ± 0.01 |
| B4 | 4.44 ± 0.03 | 11.5 ± 0.02 | 502 ± 0.11 | 0.7 ± 0.01 |
| B5 | 4.52 ± 0.02 | 11.7 ± 0.04 | 501 ± 0.09 | 0.6 ± 0.01 |
| B6 | 5.45 ± 0.01 | 12.0 ± 0.03 | 498 ± 0.11 | 0.5 ± 0.03 |
| C1 | 4.34 ± 0.01 | 12.4 ± 0.08 | 501 ± 0.13 | 0.5 ± 0.02 |
| C2 | 4.38 ± 0.04 | 13.0 ± 0.12 | 498 ± 1.2 | 0.4 ± 0.03 |
| C3 | 4.42 ± 0.02 | 13.5 ± 0.04 | 500 ± 0.8 | 0.3 ± 0.02 |
| C4 | 4.40 ± 0.05 | 12.7 ± 0.06 | 502 ± 0.17 | 0.6 ± 0.01 |
| C5 | 4.51 ± 0.08 | 11.4 ± 0.06 | 502 ± 1.1 | 0.7 ± 0.01 |
| C6 | 4.49 ± 0.04 | 12.2 ± 0.05 | 499 ± 0.7 | 0.6 ± 0.01 |
| C7 | 4.33 ± 0.07 | 12.5 ± 0.08 | 497 ± 0.14 | 0.5 ± 0.02 |
| D1 | 4.41 ± 0.06 | 12.3 ± 0.08 | 498 ± 0.14 | 0.5 ± 0.02 |
| D2 | 4.5 ± 0.02 | 12.4 ± 0.10 | 501 ± 0.11 | 0.4 ± 0.03 |
| D3 | 4.4 ± 0.02 | 13.4 ± 0.06 | 499 ± 0.09 | 0.6 ± 0.01 |
| D4 | 4.38 ± 0.05 | 11.7 ± 0.09 | 502 ± 0.17 | 0.5 ± 0.01 |
| D5 | 4.42 ± 0.01 | 11.9 ± 0.05 | 501 ± 0.14 | 0.6 ± 0.01 |
| D6 | 4.53 ± 0.04 | 12.5 ± 0.06 | 501 ± 0.09 | 0.5 ± 0.02 |
| D7 | 4.37 ± 0.02 | 12.4 ± 0.09 | 502 ± 1.1 | 0.4 ± 0.01 |
| E1 | 4.29 ± 0.02 | 11.7 ± 0.06 | 473 ± 0.14 | 0.7 ± 0.01 |
| E2 | 4.42 ± 0.04 | 12.4 ± 0.11 | 501 ± 0.23 | 0.6 ± 0.01 |
| E3 | 4.59 ± 0.01 | 12.8 ± 0.09 | 524 ± 0.18 | 0.0.7 ± 0.01 |
| E4 | 4.77 ± 0.01 | 13.1 ± 0.08 | 552 ± 0.09 | 0.5 ± 0.02 |
| E5 | 4.87 ± 0.03 | 13.7 ± 0.06 | 574 ± 0.11 | 0.5 ± 0.02 |
| E6 | 5.08 ± 0.03 | 14 ± 0.06 | 602 ± 0.14 | 0.5 ± 0.01 |
| Time | Percent Drug Release (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hour | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 |
| 1 | 85 | 40 | 10 | 12 | 10 | 2 | 1 | 2 | 0.2 | 0.01 |
| 2 | 100 | 95 | 57 | 55 | 30 | 15 | 11 | 7 | 0.9 | 0.04 |
| 3 | 100 | 100 | 89 | 90 | 65 | 40 | 34 | 7 | 1.1 | 0.24 |
| 4 | 100 | 100 | 100 | 100 | 87 | 68 | 50 | 40 | 1.3 | 0.5 |
| 5 | 100 | 100 | 100 | 100 | 100 | 70 | 56 | 56 | 2.4 | 0.51 |
| 6 | 100 | 100 | 100 | 100 | 100 | 98 | 74 | 67 | 10 | 0.8 |
| 7 | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 73 | 11 | 1 |
| Time | Percent Drug Release (%) | |||||
|---|---|---|---|---|---|---|
| Hour | B1 | B2 | B3 | B4 | B5 | B6 |
| 1 | 30 | 40 | 10 | 0.2 | 0 | 0 |
| 2 | 40 | 95 | 57 | 0.9 | 0 | 0 |
| 3 | 100 | 100 | 89 | 5 | 10 | 0 |
| 4 | 100 | 100 | 100 | 19 | 20 | 0 |
| 5 | 100 | 100 | 100 | 30 | 30 | 1 |
| 6 | 100 | 100 | 100 | 39 | 39 | 1.2 |
| 7 | 100 | 100 | 100 | 45 | 50 | 4 |
| Time | Percent Drug Release (%) | ||||||
|---|---|---|---|---|---|---|---|
| Hour | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
| 1 | 1 | 1 | 0.2 | 0.1 | 0.01 | 0 | 0 |
| 2 | 2 | 3 | 1 | 0.5 | 0.1 | 0 | 0 |
| 3 | 10 | 7 | 1 | 0.7 | 1 | 0 | 0 |
| 4 | 11 | 10 | 5 | 2 | 1.7 | 0.2 | 0 |
| 5 | 28 | 21 | 10 | 7 | 3 | 0.9 | 0 |
| 6 | 52 | 61 | 30 | 11 | 6 | 5 | 1 |
| 7 | 69 | 78 | 56 | 21 | 10 | 7 | 2 |
| Time | Percent Drug Release (%) | ||||||
|---|---|---|---|---|---|---|---|
| Hour | D1 | D2 | D3 | D4 | D5 | D6 | D7 |
| 1 | 0.02 | 11 | 0.2 | 1 | 2 | 2 | 5 |
| 2 | 0.9 | 13 | 1 | 5 | 7 | 5 | 22 |
| 3 | 6 | 19 | 1 | 17 | 10 | 6.4 | 39 |
| 4 | 11 | 20 | 5 | 28 | 21 | 28 | 59 |
| 5 | 17 | 31 | 10 | 61 | 34 | 68 | 89 |
| 6 | 20 | 50 | 30 | 71 | 52 | 97 | 98 |
| 7 | 66 | 71 | 56 | 90 | 89 | 100 | 100 |
| Time | Percent Drug Release (%) | |||||
|---|---|---|---|---|---|---|
| Hour | E1 | E2 | E3 | E4 | E5 | E6 |
| 1 | 1.42 | 1.4 | 1 | 0.9 | 0.75 | 0.08 |
| 2 | 10.4 | 10.1 | 7.7 | 5.5 | 2.2 | 0.71 |
| 3 | 25.4 | 21.4 | 10.4 | 9.8 | 6.4 | 1.2 |
| 4 | 41.5 | 39.8 | 18.7 | 14.49 | 14.8 | 3.5 |
| 5 | 63 | 66.4 | 70.5 | 68.9 | 72.17 | 21.4 |
| 6 | 97.06 | 98.01 | 97.06 | 98.46 | 98.45 | 99.27 |
| Time (Hours) | Dissolution after 1 Month (% Drug Release) | Dissolution after 2 Months (% Drug Release) | Dissolution after 3 Months (% Drug Release) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temp (°C)/ Humidity (%) | 25 °C/60% | 30 °C/65% | 40 °C/75% | 25 °C/60% | 30 °C/65% | 40 °C/75% | 25 °C/60% | 30 °C/65% | 40 °C/75% |
| 1 | 0.09 ± 0.01 | 0.08 ± 0.04 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.04 | 0.09 ± 0.02 | 0.07 ± 0.03 | 0.08 ± 0.01 |
| 2 | 0.69 ± 0.03 | 0.67 ± 0.03 | 0.71 ± 0.03 | 0.68 ± 0.04 | 0.65 ± 0.03 | 0.68 ± 0.12 | 0.70 ± 0.03 | 0.67 ± 0.01 | 0.65 ± 0.04 |
| 3 | 1.3 ± 0.02 | 1.2 ± 0.06 | 1.0 ± 0.03 | 1.3 ± 0.06 | 1.1 ± 0.05 | 1.1 ± 0.07 | 1.3 ± 0.02 | 1.2 ± 0.01 | 1.0 ± 0.04 |
| 4 | 3.6 ± 0.02 | 3.4 ± 0.01 | 3.1 ± 0.07 | 3.4 ± 0.04 | 3.4 ± 0.03 | 3.3 ± 0.04 | 3.6 ± 0.07 | 3.5 ± 0.02 | 3.2 ± 0.02 |
| 5 | 21.1 ± 0.03 | 21.0 ± 0.02 | 20.8 ± 0.05 | 21.5 ± 0.02 | 21.2 ± 0.01 | 20.4 ± 0.02 | 21.5 ± 0.03 | 21.2 ± 0.10 | 20.7 ± 0.03 |
| 6 | 99.34 ± 0.03 | 99.32 ± 0.03 | 99.27 ± 0.03 | 99.38 ± 0.05 | 99.37 ± 0.04 | 99.31 ± 0.03 | 99.33 ± 0.07 | 99.33 ± 0.03 | 99.22 ± 0.02 |
| Assay | Storage Conditions | ||
|---|---|---|---|
| 25 ± 2 °C/60% ± 5% RH | 30 ± 2 °C/65% ± 5% RH | 40 ± 2 °C/75% ± 5% RH | |
| Initial | 99.98% ± 0.01 | 99.98% ± 0.04 | 99.98% ± 0.02 |
| After 1st month | 99.94% ± 0.03 | 99.92% ± 0.06 | 99.92% ± 0.05 |
| After 2nd month | 99.90% ± 0.03 | 99.90% ± 0.05 | 99.89% ± 0.03 |
| After 3rd month | 99.89% ± 0.03 | 99.83% ± 0.03 | 99.82% ± 0.08 |
| Tablet Ingredient * | F1 | F2 | F3 |
|---|---|---|---|
| Aceclofenac | 100 | 100 | 100 |
| Croscarmellose sodium | 0.75 | 1.5 | 3.75 |
| Avicel PH102 | 47.6 | 46.85 | 44.6 |
| Magnesium stearate | 1.5 | 1.5 | 1.5 |
| Sunset yellow lake E100 | 0.15 | 0.15 | 0.15 |
| Total weight of tablets | 150 | 150 | 150 |
| Formulation * | HPMC K100M | Avicel PH102 |
|---|---|---|
| A1 (10%) | 35 | 311.5 |
| A2 (20%) | 70 | 276.5 |
| A3 (25%) | 87.5 | 259 |
| A4 (30%) | 105 | 241.5 |
| A5 (35%) | 122.5 | 224 |
| A6 (40%) | 140 | 206.5 |
| A7 (45%) | 157.5 | 189 |
| A8 (50%) | 175 | 171.5 |
| A9 (60%) | 210 | 136.5 |
| A10 (99%) | 346.5 | 0 |
| Formulation * % (HPMC K100M:Eudragit L100) | HPMC K100M (mg) | Eudragit L100 (mg) | Avicel PH102 (mg) |
|---|---|---|---|
| B1 (10:30) | 35 | 105 | 206.5 |
| B2 (20:20) | 70 | 70 | 206.5 |
| B3 (30:10) | 105 | 35 | 206.5 |
| B4 (40:10) | 140 | 35 | 171.5 |
| B5 (45:15) | 157.5 | 52.5 | 136.5 |
| B6 (50:20) | 175 | 70 | 101.5 |
| Formulation * % (HPMC K100M:HEC) | HPMC K100M (mg) | HEC (mg) |
|---|---|---|
| C1 (100:0) | 346.5 | 0 |
| C2 (87.5:12.5) | 303.19 | 43.31 |
| C3 (75:25) | 260.74 | 85.76 |
| C4 (50:50) | 173.25 | 173.25 |
| C5 (25:75) | 85.76 | 260.74 |
| C6 (12.5:87.5) | 43.31 | 303.19 |
| C7 (0:100) | 0 | 346.5 |
| Formulation * | HPMC K100M | HPMC E5 |
|---|---|---|
| D1 (100:0) | 346.5 | 0 |
| D2 (87.5:12.5) | 303.19 | 43.31 |
| D3 (75:25) | 260.74 | 85.76 |
| D4 (50:50) | 173.25 | 173.25 |
| D5 (25:75) | 85.76 | 260.74 |
| D6 (12.5:87.5) | 43.31 | 303.19 |
| D7 (0:100) | 0 | 346.5 |
| Formulation * HPMC K100M: HPMC E5 (12.5:87.5) | HPMC K100M (mg) | HPMC E5 (mg) | Magnesium Stearate (mg) | Press-Coated Tablet Weight (mg) |
|---|---|---|---|---|
| E1 | 40.22 | 281.53 | 3.25 | 475 |
| E2 | 43.31 | 303.19 | 3.5 | 500 |
| E3 | 46.406 | 324.844 | 3.75 | 525 |
| E4 | 49.5 | 346.5 | 4 | 550 |
| E5 | 52.59 | 368.16 | 4.25 | 575 |
| E6 | 55.69 | 389.81 | 4.5 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, R.; Zaman, M.; Ahmad, M.; Khan, M.A.; Butt, M.H.; Salawi, A.; Almoshari, Y.; Alshamrani, M.; Sarfraz, R.M. Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations. Pharmaceuticals 2022, 15, 326. https://doi.org/10.3390/ph15030326
Rashid R, Zaman M, Ahmad M, Khan MA, Butt MH, Salawi A, Almoshari Y, Alshamrani M, Sarfraz RM. Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations. Pharmaceuticals. 2022; 15(3):326. https://doi.org/10.3390/ph15030326
Chicago/Turabian StyleRashid, Rizwana, Muhammad Zaman, Mahmood Ahmad, Mahtab Ahmad Khan, Muhammad Hammad Butt, Ahmad Salawi, Yosif Almoshari, Meshal Alshamrani, and Rai Muhammad Sarfraz. 2022. "Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations" Pharmaceuticals 15, no. 3: 326. https://doi.org/10.3390/ph15030326
APA StyleRashid, R., Zaman, M., Ahmad, M., Khan, M. A., Butt, M. H., Salawi, A., Almoshari, Y., Alshamrani, M., & Sarfraz, R. M. (2022). Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations. Pharmaceuticals, 15(3), 326. https://doi.org/10.3390/ph15030326









